Wheat Drought-Responsive Grain Proteome Analysis by Linear and Nonlinear 2-DE and MALDI-TOF Mass Spectrometry
Abstract
:1. Introduction
2. Results
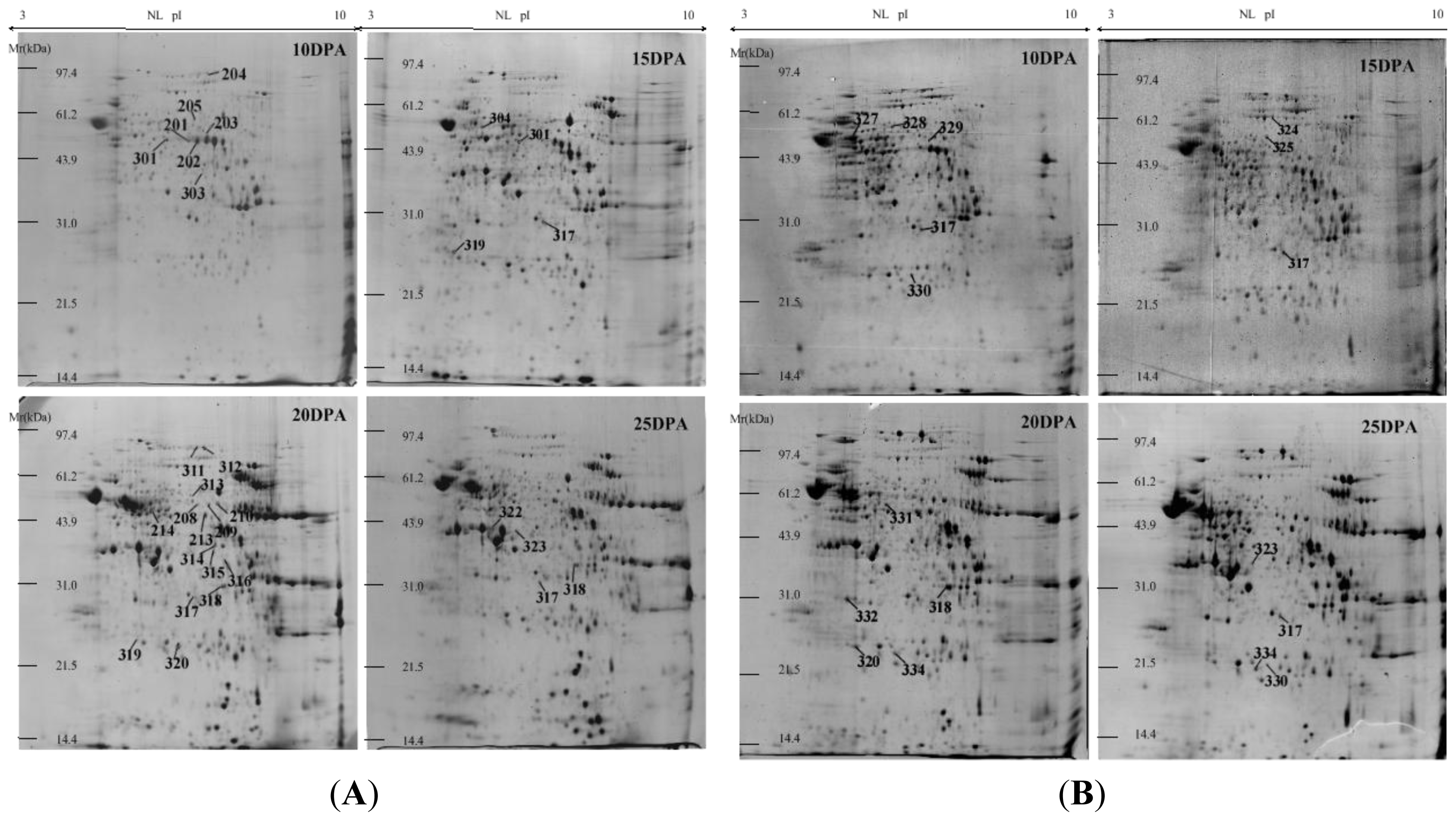
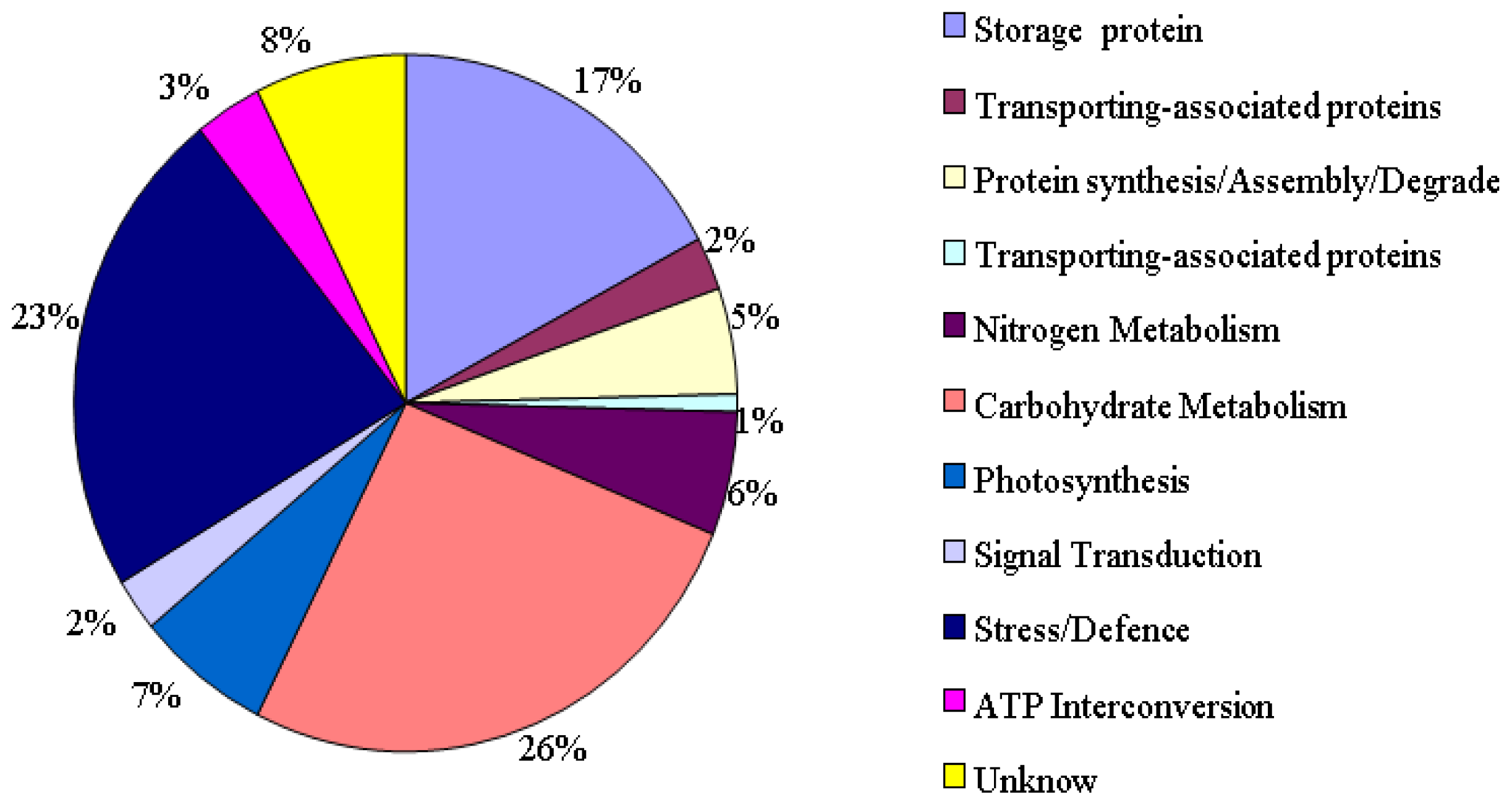
2.1. Identification and Functional Classification of Drought-Responsive Proteins through Linear and Nonlinear 2-DE and MALDI-TOF Mass Spectrometry
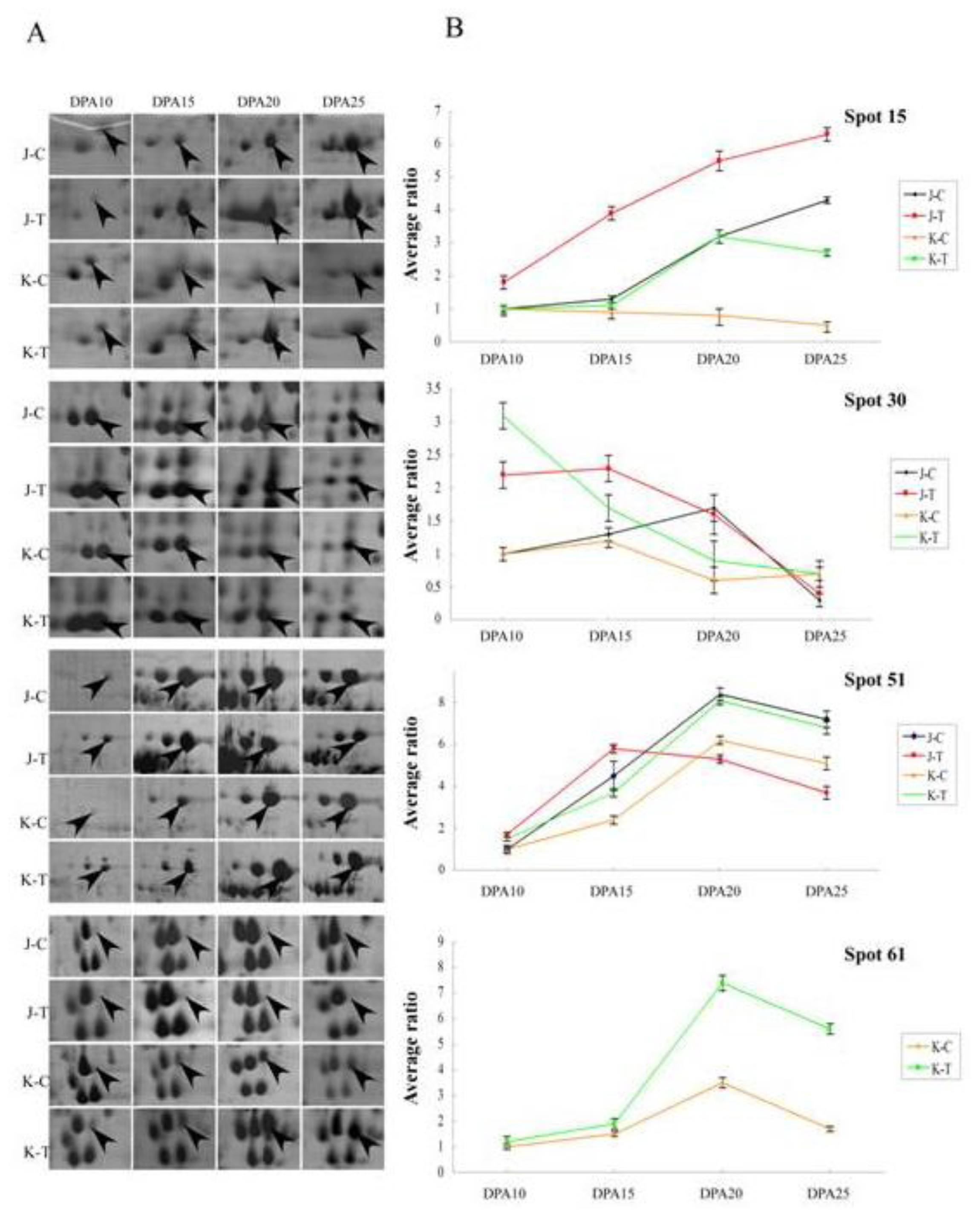
2.2. Protein-Expression Profiles under Well-Watered and Drought-Stress Conditions
2.3. Comparative Proteomic Analysis between Janz and Kauz under Drought Stress
3. Discussion
3.1. Linear and Nonlinear 2-DE for Wheat Protein Maps
3.2. Detoxification and Defense Proteins
3.3. Carbohydrate Metabolism Proteins
3.4. Signal Transduction Related Proteins
3.5. Photosynthetic Proteins
3.6. Storage Proteins
3.7. A Putative Basis of Drought Response and Tolerance in Respect to Wheat Grain Development
4. Experimental Section
4.1. Plant Materials, Treatments and Sample Collection
4.2. Protein Extraction and Two-Dimensional Electrophoresis
4.3. Gel Scanning and Image Analysis
4.4. Proteolytic Digestion and MS Analysis
5. Conclusions
Supplementary Information
ijms-13-16065-s001.pdfAcknowledgments
References
- Reynolds, M.P.; Ortiz-Monasterio, J.I.; McNab, A. Traits to Improve Yield in Dry Environments; Richards, R.A., Condon, A.G., Rebetzke, G.J., Eds.; CIMMYT: Texcoco, Mexico, 2011; pp. 88–100. [Google Scholar]
- Stone, P.J.; Nicholas, M.E. Effect of timing of heat stress during grain filling on two wheat varieties differing in heat tolerance. I. Grain Growth. Aust. J. Plant Physiol 1995, 22, 927–934. [Google Scholar]
- Yang, J.; Zhang, J.; Huang, Z.; Zhu, Q.; Wang, L. Remobilization of carbon reserves is improved by controlled soil-drying during grain filling of wheat. Crop Sci 2000, 40, 1645–1655. [Google Scholar]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Water deficit induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agron. J 2001, 93, 196–206. [Google Scholar]
- Singh, N.K.; Shepherd, K.W.; Langridge, P.; Gruen, L.C. Purification and biochemical characterization of triticin, a legumin-like protein in wheat endosperm. J. Cereal Sci 1991, 13, 207–219. [Google Scholar]
- Gupta, R.B.; Shepherd, K.W.; MacRitchie, F. Genetic control and biochemical properties of some high molecular weight albumins in bread wheat. J. Cereal Sci 1991, 13, 221–235. [Google Scholar]
- Gupta, R.B.; Batey, I.L.; MacRitchie, F. Relationships between protein composition and functional properties of wheat flours. Cereal Chem 1992, 69, 125–131. [Google Scholar]
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 2008, 31, 11–38. [Google Scholar]
- Yan, S.H.; Yin, Y.P.; Li, W.Y.; Li, Y.; Liang, T.B.; Wu, Y.H.; Geng, Q.H.; Wang, Z.L. Effect of high temperature after anthesis on starch formation of two wheat cultivars differing in heat tolerance. Acta Ecol. Sin 2008, 28, 6138–6147. [Google Scholar]
- Lalonde, S.; Morse, D.; Saini, H.S. Expression of a wheat ADP-glucose pyrophosphorylase gene during development of normal and water-stress-affected anthers. Plant Mol. Biol 1997, 34, 445–453. [Google Scholar]
- Saeedipour, S.; Moradi, F. Comparison of the drought stress responses of tolerant and sensitive wheat cultivars during grain filling: Impact of invertase activity on carbon metabolism during kernel development. J. Agric. Sci 2011, 3, 32–44. [Google Scholar]
- Gao, L.Y.; Wang, A.L.; Li, X.H.; Dong, K.; Wang, K.; Appels, R.; Ma, W.J.; Yan, Y.M. Wheat quality related differential expressions of albumins and globulins revealed by two-dimensional difference gel electrophoresis (2-D DIGE). J. Proteomics 2009, 73, 279–296. [Google Scholar]
- Nadaud, I.; Girousse, C.; Debiton, C.; Chambon, C.; Bouzidi, M.F.; Martre, P.; Branlard, G. Proteomic and morphological analysis of early stages of wheat grain development. Proteomics 2010, 10, 2901–2910. [Google Scholar]
- Tasleem-Tahir, A.; Nadaud, I.; Girousse, C.; Martre, P.; Marion, D.; Branlard, G. Proteomic analysis of peripheral layers during wheat (Triticum aestivum L.) grain development. Proteomics 2011, 11, 371–379. [Google Scholar]
- Wang, Y.; Qian, Y.; Hu, H.; Xu, Y.; Zhang, H. Comparative proteomic analysis of Cd-responsive proteins in wheat roots. Acta Physiol. Planta 2011, 33, 349–357. [Google Scholar]
- Gao, L.Y.; Yan, X.; Li, X.H.; Guo, G.F.; Hu, Y.; Ma, W.J.; Yan, Y.M. Proteome analysis of wheat leaf under salt stress by two-dimensional difference gel electrophoresis (2D-DIGE). Phytochemistry 2011, 72, 1180–1191. [Google Scholar]
- Rajaram, S.; Borlaug, N.E.; van Ginkel, M. Bread Wheat Improvement and Production; Curtis, B.C., Rajaram, S., Gómez-Macpherson, H., Eds.; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; pp. 103–117. [Google Scholar]
- Zhang, J.J.; Dell, B.; Conocono, E.; Waters, I.; Setter, T.; Appels, R. Water deficits in wheat: Fructan exohydrolase (1-FEH) mRNA expression and relationship to soluble carbohydrate concentrations in two varieties. New Phytol 2009, 181, 843–850. [Google Scholar]
- Wang, J.; Wang, E.L.; Luo, Q.Y.; Kirby, M. Modelling the sensitivity of wheat growth and water balance to climate change in Southeast Australia. Clim. Change 2009, 96, 79–96. [Google Scholar]
- Littlewood, N.; Garlinge, J. Crop Variety Sowing Guide for Western Australia 2002; Crop Variety Testing, Department of Agriculture: Perth, Australia, 2001. [Google Scholar]
- Bennett, M.D.; Smith, J.B. Nuclear DNA amounts in angiosperms. Philos. Trans. R. Soc. B 1976, 274, 227–274. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol 2004, 55, 373–399. [Google Scholar]
- Nagy, N.E.; Fossdal, C.G.; Dalen, L.S.; Lonneborg, A.; Heldala, I.; Johnsen, Ø. Effects of Rhizoctonia infection and drought on peroxidase and chitinase activity in Norway spruce (Picea abies). Plant Physiol. 2004, 120, 465–473. [Google Scholar]
- Veljovic-Jovanovic, S.; Kukavica, B.; Stevanovic, B.; Navari-Izzo, F. Senescence- and drought-related changes in peroxidase and superoxide dismutase isoforms in leaves of Ramonda serbica. J. Exp. Bot 2006, 57, 1759–1768. [Google Scholar]
- Zhang, J.X.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 1994, 35, 785–791. [Google Scholar]
- Mittler, R.; Zilinskas, B.A. Regulation of pea cytssolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J 1994, 5, 397–405. [Google Scholar]
- Koiwa, H.; Bressan, R.A.; Hasegawa, P.M. Regulation of protease inhibitors and plant defense. Trends Plant Sci 1997, 2, 379–383. [Google Scholar]
- Van Dam, N.M.; Horn, M.; Mares, M.; Baldwin, I.T. Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuate. J. Chem. Ecol 2001, 27, 547–568. [Google Scholar]
- Dombrowski, J.E. Salt stress activation of wound-related genes in tomato plants. Plant Physiol 2003, 132, 2098–2107. [Google Scholar]
- Hajheidari, M.; Eivazi, A.; Buchanan, B.B.; Wong, J.H.; Majidi, I.; Salekdeh, G.H. Proteomics uncovers a role for redox in drought tolerance in wheat. J. Proteome Res 2007, 6, 1451–1460. [Google Scholar]
- Gibbs, B.F.; Alli, I. Characterization of a purified α-amylase inhibitor from white kidney beans (Phaseolus vulganis). Food Res. Int 1998, 31, 217–225. [Google Scholar]
- Sanchez-Hernandez, C.; Martınez-Gallardo, N.; Guerrero-Rangel, A.; Valdes-Rodriguez, S.; Delano-Frier, J. Trypsin and a-amylase inhibitors are differentially induced in leaves of amaranth (Amaranthus hypochondriacus) in response to biotic and abiotic stress. Physiol. Planta 2004, 122, 254–264. [Google Scholar]
- Rawlings, N.D.; Tolle, D.P.; Barrett, A.J. Evolutionary families of peptidase inhibitors. Biochem. J 2004, 378, 705–716. [Google Scholar]
- Rosenkrands, I.; Hejgaard, J.; Rasmussen, S.K.; Bjorn, S.E. Serpins from wheat grain. FEBS Lett 1994, 343, 75–80. [Google Scholar]
- Roberts, T.H.; Hejgaard, J. Serpins in plants and green algae. Funct. Integr. Genomics 2007, 8, 1–27. [Google Scholar]
- Moons, A.; Bauw, G.; Prinsen, E.; Vanmontagu, M.; Vanderstraeten, D. Molecular and physiological-responses to abscisic-acid and salts in roots of salt-sensitive and salt-tolerant Indica rice varieties. Plant Physiol 1995, 107, 177–186. [Google Scholar]
- Moons, A.; Gielen, J.; Vandekerckhove, J.; Vanderstraeten, D.; Gheysen, G.; Vanmontagu, M. An abscisic-acid- and salt-stress-responsive rice cDNA from a novel plant gene family. Planta 1997, 202, 443–454. [Google Scholar]
- Carpentier, S.C.; Witters, E.; Laukens, K.; Onckelen, H.V.; Swennen, R.; Panis, B. Banana (Musa spp.) as a model to study the meristem proteome: Acclimation to osmotic stress. Proteomics 2007, 7, 92–105. [Google Scholar]
- Riccardi, F.; Gazeau, P.; Jacquemot, M.P.; Vincent, D.; Zivy, M. Deciphering genetic variations of proteome responses to water deficit in maize leaves. Plant Physiol. Biochem 2004, 42, 1003–1011. [Google Scholar]
- Chopra, J.; Kaur, N.; Gupta, A.K. Ontogenic changes in enzymes of carbon metabolism in relation to carbohydrate status in developing mungbean reproductive structures. Phytochemistry 2000, 53, 539–548. [Google Scholar]
- Dai, Z.M. Activities of enzymes involved in starch synthesis in wheat grains differing in starch content. Russ. J. Plant Physiol 2010, 57, 74–78. [Google Scholar]
- Cross, J.M.; Clancy, M.; Shaw, J.R.; Greene, T.W.; Schmidt, R.R.; Okita, T.W.; Hannah, L.C. Both subunits of ADP-glucose pyrophosphorylase are regulatory. Plant Physiol 2004, 135, 137–144. [Google Scholar]
- Elling, L. Effect of metal ions on sucrose synthase from rice grains—A study on enzyme inhibition and enzyme topography. Glycobiol 1995, 5, 201–206. [Google Scholar]
- Keeling, P.L.; Wood, J.R.; Tyson, R.H.; Bridges, I.G. Starch biosynthesis in developing wheat grain. Plant Physiol 1988, 87, 311–319. [Google Scholar]
- Quick, W.P.; Schaffer, A.A. Photoassimilate Distribution in Plants and Crops; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 115–159. [Google Scholar]
- Obata-Sasamoto, H.; Suzuki, H. Activities of enzymes relating starch synthesis and endogenous levels of growth regulators in potato stolen tips during tuberization. Physiol. Planta 1979, 45, 320–324. [Google Scholar]
- Roncarati, R.; Salamini, F.; Bartels, D. An aldose reductase homologous gene from barley: Regulation and function. Plant J 1995, 7, 809–822. [Google Scholar]
- Bartels, D.; Engelhardt, K.; Roncarati, R.; Schneider, K.; Rotter, M.; Salamini, F. An ABA and GA modulated gene expressed in the barley embryo encodes an aldose reductase related protein. EMBO J 1991, 10, 1037–1043. [Google Scholar]
- Li, B.; Foley, M.E. Cloning and characterization of differentially expressed genes in imbibed dormant and after ripened Avena fatua. Plant Mol. Biol 1995, 29, 823–831. [Google Scholar]
- Veeranagamallaiah, G.; Ranganayakulu, G.S.; Thippeswamy, M.; Pandurangaiah, M.; Sridevi, V.; Sudhakar, C. Aldose reductase expression contributes in sorbitol accumulation and 4-hydroxynon-2-enal detoxification in two foxtail millet (Setaria italica L.) cultivars with different salt stress tolerance. Plant Growth Regul 2009, 59, 137–143. [Google Scholar]
- Zhu, J. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 2002, 53, 247–273. [Google Scholar]
- Sondek, J.; Bohm, A.; Lambright, D.G.; Hamm, H.E.; Sigler, P.B. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature 1996, 379, 369–374. [Google Scholar]
- Neer, E.J.; Schmidt, C.J.; Nambudripad, R.; Smith, T.F. The ancient regulatoryprotein family of WD-repeat proteins. Nature 1994, 371, 297–300. [Google Scholar]
- Smith, T.F.; Gaitatzes, C.; Saxena, K.; Neer, E.J. The WD repeat: A common architecture for diverse functions. Trends Biochem. Sci 1999, 24, 81–185. [Google Scholar]
- Reddy, P.C.O.; Sairanganayakulu, G.; Thippeswamy, M.; Reddy, P.S.; Reddy, M.K.; Sudhakar, S. Identification of stress-induced genes from the drought tolerant semi-arid legumecrop horsegram (Macrotyloma uniflorum (Lam.) Verdc.) through analysis of subtracted expressed sequence tags. Plant Sci 2008, 175, 372–384. [Google Scholar]
- Huang, J.; Wang, M.M.; Bao, Y.M.; Sun, S.J.; Pan, L.J.; Zhang, H.S. SRWD: A novel WD40 protein subfamily regulated by salt stress in rice (Oryza sativa L.). Gene 2008, 424, 71–79. [Google Scholar]
- Assmann, S.M. G proteins Go green: A plant G protein signaling FAQ sheet. Science 2005, 310, 71–73. [Google Scholar]
- Xiong, L.M.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14, 165–183. [Google Scholar]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci 2001, 6, 66–71. [Google Scholar]
- Tschiersch, H.; Borisjuk, L.; Rutten, T.; Rolletschek, H. Gradients of seed photosynthesis and its role for oxygen balancing. Biosystems 2010, 103, 302–308. [Google Scholar]
- Seidler, A.; Ruflerford, A.W.; Michel, H. On the role of the N-terminus of the extrinsic 33 kDa protein in photosystem II. Plant Mol. Biol 1996, 31, 183–188. [Google Scholar]
- Mizobuchi, A.; Mizobuchi, Y. Assembly of photosystem II polypeptides and expression of oxygen evolution activity in the chloroplasts of Euglena gracilis Z. during the dark-light transition. Biochim. Biophys. Acta 1989, 977, 26–32. [Google Scholar]
- Sugihara, K.; Hanagata, N.; Dubinsky, Z.; Baba, S.; Karube, I. Molecular characterization of cDNA encoding oxygen evolving enhancer protein 1 increased by salt Treatment in the mangrove Bruguiera gymnorrhiza. Plant Cell Physiol 2000, 41, 1279–1285. [Google Scholar]
- Guttieri, M.J.; Ahmad, R.; Stark, J.C.; Souza, E. End-use quality of six hard red spring wheat cultivars at different irrigation levels. Crop Sci 2000, 40, 631–635. [Google Scholar]
- Wiesner, H.; Seilmeier, W.; Belitz, H.D. Vergleichende untersuchungen uber partielle aminosauresequenzen von prolaminen und glutelinen verschriedener getreidearten, Z. Lebensm. Unters. Forsch 1980, 170, 17. [Google Scholar]
- Hendry, G.A.F. Evolutionary origins and natural functions of fructans-a climatological, biogeographic and mechanistic appraisal. New Phytol 1993, 123, 3–14. [Google Scholar]
- Pilon-Smits, E.A.H.; Ebskamp, M.J.M.; Paul, M.J.; Jeuken, M.J.W.; Weisbeek, P.J.; Smeekens, S.C.M. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol 1995, 107, 25–130. [Google Scholar]
- Van der Meer, I.M.; Ebskamp, M.J.M.; Visser, R.G.F.; Weisbeek, P.J.; Smeekens, S.C.M. Fructan as a new carbohydrate sink in transgenic potato plants. Plant Cell 1994, 6, 561–570. [Google Scholar]
- Chen, P.; Wang, C.; Li, K.; Chang, J.; Wang, Y.; Yang, G.; Shewry, P.R.; He, G. Cloning, expression and characterization of novel avenin-like genes in wheat and related species. J. Cereal Sci 2008, 48, 734–740. [Google Scholar]
- Orth, R.A.; Bushuk, W. A comparative study of the proteins of wheat of diverse baking qualities. Cereal Chem 1972, 49, 268–275. [Google Scholar]
- Carceller, J.L.; Aussenac, T. Accumulation and changes in molecular size distribution of polymeric proteins in developing grains of hexaploid wheats: Role of the desiccation phase. Aust. J. Plant Physiol 1999, 26, 301–310. [Google Scholar]
- De Caro, S.; Ferranti, P.; Addeo, F.; Mamone, G. Isolation and characterization of Avenin-like protein type-B from durum wheat. J. Cereal Sci 2010, 52, 426–431. [Google Scholar]
- Ganeshan, S.; Drinkwater, J.M.; Repellin, A.; Chibbar, R.N. Selected carbohydrate metabolism genes show coincident expression peaks in grains of in vitro-cultured immature spikes of wheat (Triticum aestivum L.). J. Agric. Food Chem 2010, 58, 4193–4201. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, S.-S.; Liang, X.-N.; Li, X.; Wang, S.-L.; Lv, D.-W.; Ma, C.-Y.; Li, X.-H.; Ma, W.-J.; Yan, Y.-M. Wheat Drought-Responsive Grain Proteome Analysis by Linear and Nonlinear 2-DE and MALDI-TOF Mass Spectrometry. Int. J. Mol. Sci. 2012, 13, 16065-16083. https://doi.org/10.3390/ijms131216065
Jiang S-S, Liang X-N, Li X, Wang S-L, Lv D-W, Ma C-Y, Li X-H, Ma W-J, Yan Y-M. Wheat Drought-Responsive Grain Proteome Analysis by Linear and Nonlinear 2-DE and MALDI-TOF Mass Spectrometry. International Journal of Molecular Sciences. 2012; 13(12):16065-16083. https://doi.org/10.3390/ijms131216065
Chicago/Turabian StyleJiang, Shan-Shan, Xiao-Na Liang, Xin Li, Shun-Li Wang, Dong-Wen Lv, Chao-Ying Ma, Xiao-Hui Li, Wu-Jun Ma, and Yue-Ming Yan. 2012. "Wheat Drought-Responsive Grain Proteome Analysis by Linear and Nonlinear 2-DE and MALDI-TOF Mass Spectrometry" International Journal of Molecular Sciences 13, no. 12: 16065-16083. https://doi.org/10.3390/ijms131216065



