Molecular Systematics of Genus Atractylodes (Compositae, Cardueae): Evidence from Internal Transcribed Spacer (ITS) and trnL-F Sequences
Abstract
:1. Introduciton
2. Results and Discussion
2.1. The Features of Alignments in cpDNA (trnL-F) and nrDNA (ITS)
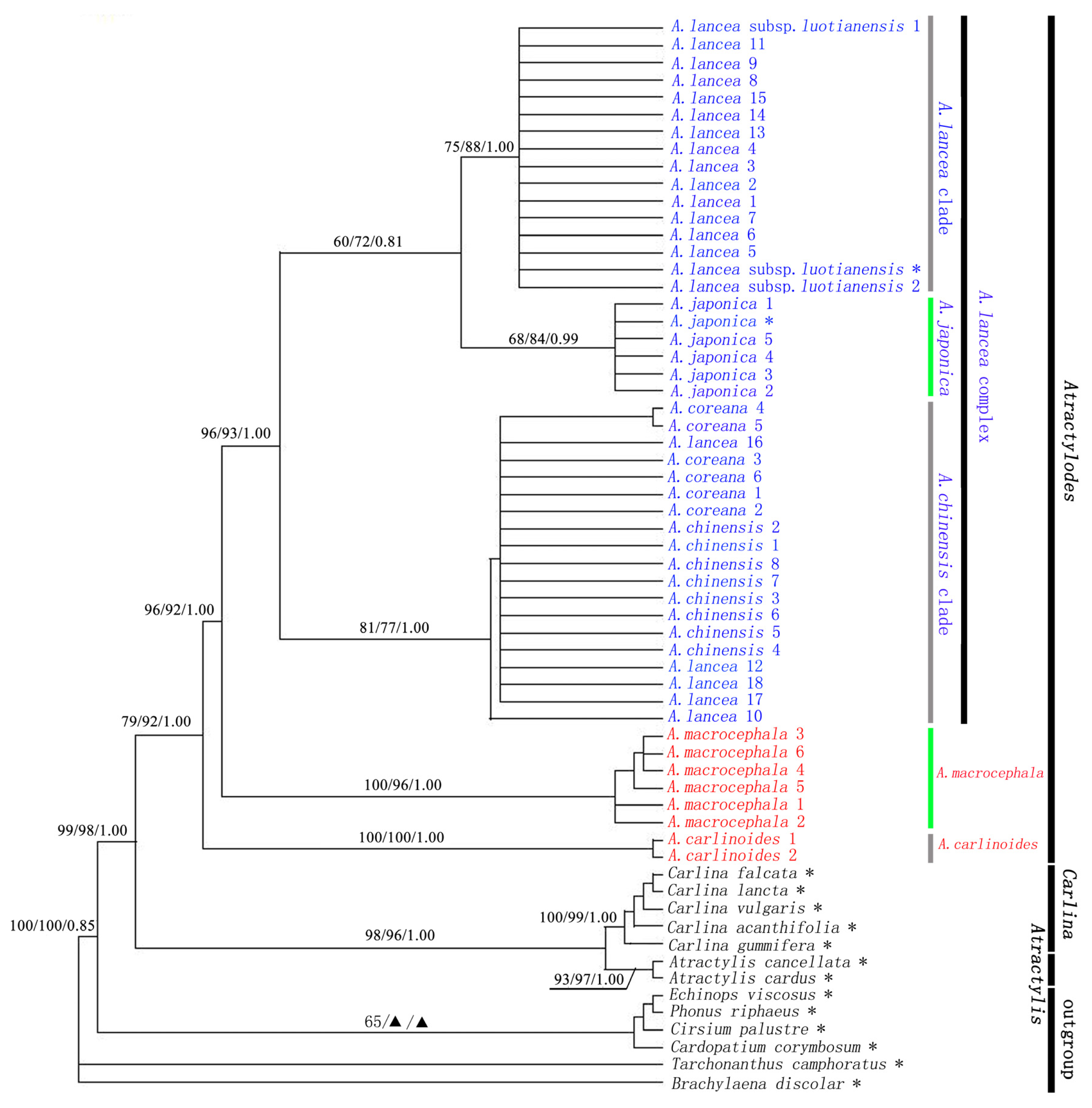
2.2. The Relationship between Atractylodes and Related Genera
2.3. Atractylodes is a Monophyletic Group
2.4. Atractylodes carlinoides is Sister Species of the Rest of Atractylodes Species
2.5. Genetic Relationship of the Atractylodes lancea Complex
3. Experimental Section
3.1. Selection of Taxa and Outgroups
3.2. DNA Extraction, Amplification, and Sequencing
3.3. Sequence Alignment and Phylogenetic Analysis
4. Conclusions
Acknowledgements
References
- Peng, H.S.; Wang, D.Q. The history and differentiation of raw Atractylodes commodity in successive ages. Chin. J. Med. Hist 2007, 37, 15–18. [Google Scholar]
- De Candolle, A.P. Prodromus Systematis Naturalis Regni Vegetabilis, Pars VI; Treuttel & Würtz: Paris, France, 1838. [Google Scholar]
- Ling, Y. Les Composees Chinoises De L’herbier De L’academie De Peiping. Contr. Inst. Bot. Nat. Acad. Peip. 1935, III, 132–134. [Google Scholar]
- Hu, S.Y. The Compositaeof China (II). Quart J. Taiwan. Mus 1965, XVIII, 233–334. [Google Scholar]
- Fu, S.M.; Fang, H.J.; Liu, G.S.; Xiao, P.G. A study on the medicinal plants of the genus Atractylodes. Acta Phytotaxon. Sin 1981, 19, 195–201. [Google Scholar]
- Shi, Z. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1987; Volume 78. [Google Scholar]
- Peng, H.S.; Wang, D.Q. Studies on population biology of transitional types of genus Atractylodes in Anhui Province. Chin. J.Chin. Mater. Med 2007, 32, 793–797. [Google Scholar]
- Xu, G.F.; Yu, Y.L.; Liu, J.; Wang, L.X. Observations on the morphological charcter of Atractylodes japonica Koidz. Et Kitam. J. Jiamusi Med. Coll 1993, 16, 15–17. [Google Scholar]
- Petit, D.P. Generic interrelationships of theCardueae (Compositae): A cladistic analysis of morphological data. Plant Syst. Evol 1997, 207, 173–203. [Google Scholar]
- Shi, Z. On the nomenclature of Chinese drug “Cangzhu”. Acta Phytotaxon. Sin 1981, 19, 318–322. [Google Scholar]
- Kunio, I.; Takasi, Y.; David, E.B.; Hideaki, O. Flora of Japan; Kodansha: Tokyo, Japan, 1997; Volume III. [Google Scholar]
- Bobrov, E.; Bochantsev, V.; Iljin, M.; Linchevskii, I.; Lipshits, S.; Yu, S.E.V.; Cherepanov, S.; Cherneva, O.; Yuzepchuk, S. Flora of the USSR; Bishen Singh Mahendrapal Singh: Dehradun, Moscow, Russia, 1997; Volume 27. [Google Scholar]
- Zou, X.X.; Huang, L.Q.; Cui, G.H.; Yuan, Q.J.; Peng, Y.; Liu, Y.; Xiao, P.G. Genetic relationship of Atractylodes plants. Acta. Pharm. Sin 2009, 44, 680–686. [Google Scholar]
- Hu, S.L.; Feng, X.F.; Wang, J.; Ge, X.G. A new subspecies of Atractylodes from China. Acta Phytotaxon. Sin 2001, 39, 84–86. [Google Scholar]
- Wu, Z.Y. Flora of China; Science Press: Beijing, China, 2011; Volume 20. [Google Scholar]
- Committee, J.P.E. The Japanese Pharmacopoeia, 16th ed; Hirohawa Press: Tokyo, Japan, 2011. [Google Scholar]
- Susanna, A.; Garcia-Jacas, N.; Hidalgo, O.; Vilatersana, R.; Garnatje, T. The Cardueae (Compositae) revisited: Insights from ITS, trnL-trnF, and matK nuclear and chloroplast DNA analysis. Ann. Mo. Bot. Gard 2006, 93, 150–171. [Google Scholar]
- Panero, J.L.; Funk, V.A. The value of sampling anomalous taxa in phylogenetic studies: Major clades of the Asteraceae revealed. Mol. Phylogenet. Evol 2008, 47, 757–782. [Google Scholar]
- Mizukami, H.; Shimizu, R.; Kohjyouma, M.; Kohda, H.; Kawanishi, F.; Hiraoka, N. Phylogenetic analysis of Atractylodes plants based on chloroplast trnK sequence. Biol. Pharm. Bull 1998, 21, 474–478. [Google Scholar]
- Ge, Y.F.; Hang, Y.Y.; Xia, B.; Wei, Y.L. Sequencing of trnL-F and analysis of interspecific genetic relationship of five medicinal species in Atractylodes DC. J. Plant Resour. Environ 2007, 16, 12–16. [Google Scholar]
- Guo, Y.P.; Ehrendorfer, F.; Samuel, R. Phylogeny and systematics of Achillea (Asteraceae-Anthemideae) inferred from nrITS and plastid trnL-F DNA sequences. Taxon 2004, 53, 657–672. [Google Scholar]
- Fernández, I.Á.; Aguilar, J.F.; Panero, J.L.; Feliner, G.N. A phylogenetic analysis of Doronicum (Asteraceae, Senecioneae) based on morphological, nuclear ribosomal (ITS), and chloroplast (trnL-F) evidence. Mol. Phylogenet. Evol 2001, 20, 41–64. [Google Scholar]
- Sánchez-Jiménez, I.; Lazkov, G.A.; Hidalgo, O.; Garnatje, T. Molecular systematics of Echinops L.(Asteraceae, Cynareae): A phylogeny based on ITS and trnL-trnF sequences with emphasis on sectional delimitation. Taxon 2010, 59, 698–708. [Google Scholar]
- Shiba, M.; Kondo, K.; Miki, E.; Yamaji, H.; Morota, T.; Terabayashi, S.; Takeda, S.; Sasaki, H.; Miyamoto, K.; Aburada, M. Identification of medicinal Atractylodes based on ITS sequences of nrDNA. Biol. Pharm. Bull 2006, 29, 315–320. [Google Scholar]
- Liu, S.E. Claves Plantarum Chinae Boreali-Olientalis; Science Press: Beijing, China, 1959. [Google Scholar]
- Guo, Y.Z.; Chen, F.K.; Zhao, J.F.; Zhou, Y.; Yao, N.X.; Zhang, Y.Y. Studies on Cangzhu. Species Systematization and Quality Evaluation of Commonly Used Chinese Traditional Drugs 2003, 3, 743–778. [Google Scholar]
- Guo, L.P.; Huang, L.Q.; Wang, M.; Feng, X.F.; Fu, G.F.; Yan, Y.N. A preliminary study on relationship between Atractylodes lancea and A. chinensis as analyzed by RAPD. Zhongguo Zhong Yao Za Zhi 2001, 26, 156–158. [Google Scholar]
- Garcia-Jacas, N.; Garnatje, T.; Susanna, A.; Vilatersana, R. Tribal and subtribal delimitation and phylogeny of the Cardueae (Asteraceae): A combined nuclear and chloroplast DNA analysis. Mol. Phylogenet. Evol 2002, 22, 51–64. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull 1987, 19, 11–15. [Google Scholar]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol 1991, 17, 1105–1109. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols A Guide to Methods and Applications 1990, 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994, 22, 4673–4680. [Google Scholar]
- Farris, J.S.; Källersjö, M.; Kluge, A.G.; Bult, C. Testing significance of incongruence. Cladistics 1994, 10, 315–319. [Google Scholar]
- Swofford, D.L. Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4; Sinauer Associates: Sunderland, MA, USA; p. 2000.
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar]
- Nylander, J. MrModeltest v2. In Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol 2003, 52, 696–704. [Google Scholar]

| Parameter | ITS | trnL-F | Combines |
|---|---|---|---|
| Number of accession | 49 | 49 | 49 |
| Range of sequence length (bp) | 642–643 | 799–801 | 1442–1443 |
| Characters of data matrix | 643 | 800 | 1443 |
| Number of constant sites (%) | 584 (90.82%) | 793 (99.13%) | 1377 (96.09%) |
| Number of parsimony-information sites (%) | 55 (8.55%) | 7 (0.88%) | 62 (4.32%) |
| Number of autopomorphic-information sites (%) | 4 (0.62%) | 0 (0.00%) | 4 (0.28%) |
| Consistency index (CI) | 0.892 | 0.969 | 0.891 |
| Retention index (RI) | 0.955 | 0.958 | 0.947 |
| Rescaled consistency index (RC) | 0.852 | 0.928 | 0.844 |
| Homoplasy index (HI) | 0.108 | 0.031 | 0.109 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, H.-S.; Yuan, Q.-J.; Li, Q.-Q.; Huang, L.-Q. Molecular Systematics of Genus Atractylodes (Compositae, Cardueae): Evidence from Internal Transcribed Spacer (ITS) and trnL-F Sequences. Int. J. Mol. Sci. 2012, 13, 14623-14633. https://doi.org/10.3390/ijms131114623
Peng H-S, Yuan Q-J, Li Q-Q, Huang L-Q. Molecular Systematics of Genus Atractylodes (Compositae, Cardueae): Evidence from Internal Transcribed Spacer (ITS) and trnL-F Sequences. International Journal of Molecular Sciences. 2012; 13(11):14623-14633. https://doi.org/10.3390/ijms131114623
Chicago/Turabian StylePeng, Hua-Sheng, Qing-Jun Yuan, Qian-Quan Li, and Lu-Qi Huang. 2012. "Molecular Systematics of Genus Atractylodes (Compositae, Cardueae): Evidence from Internal Transcribed Spacer (ITS) and trnL-F Sequences" International Journal of Molecular Sciences 13, no. 11: 14623-14633. https://doi.org/10.3390/ijms131114623




