Types, Causes, Detection and Repair of DNA Fragmentation in Animal and Human Sperm Cells
Abstract
:1. Introduction
2. Detection of DNA Damage: Techniques
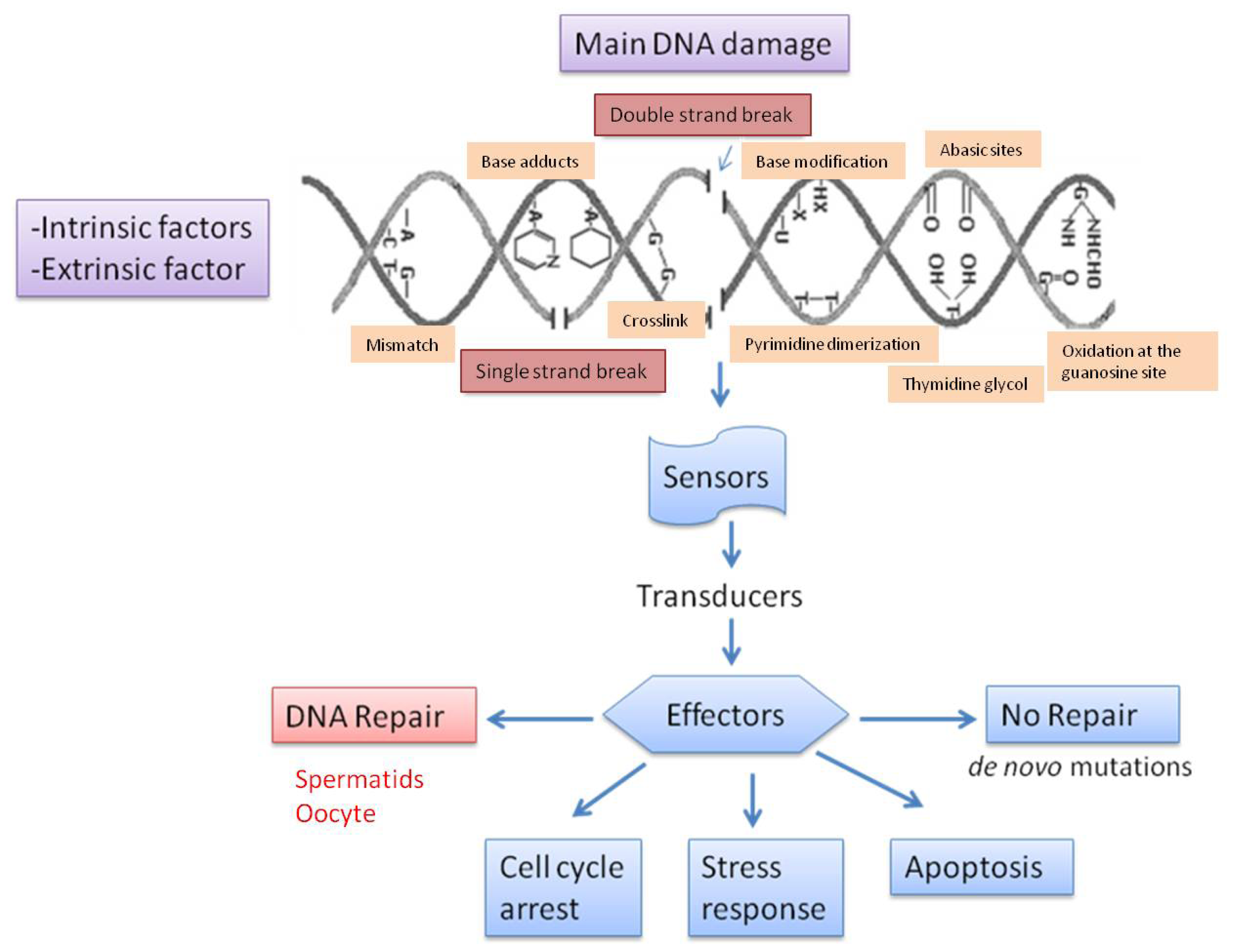
3. Types of DNA Damage and Base Modification
4. Causes of DNA Fragmentation
4.1. Intrinsic Causes
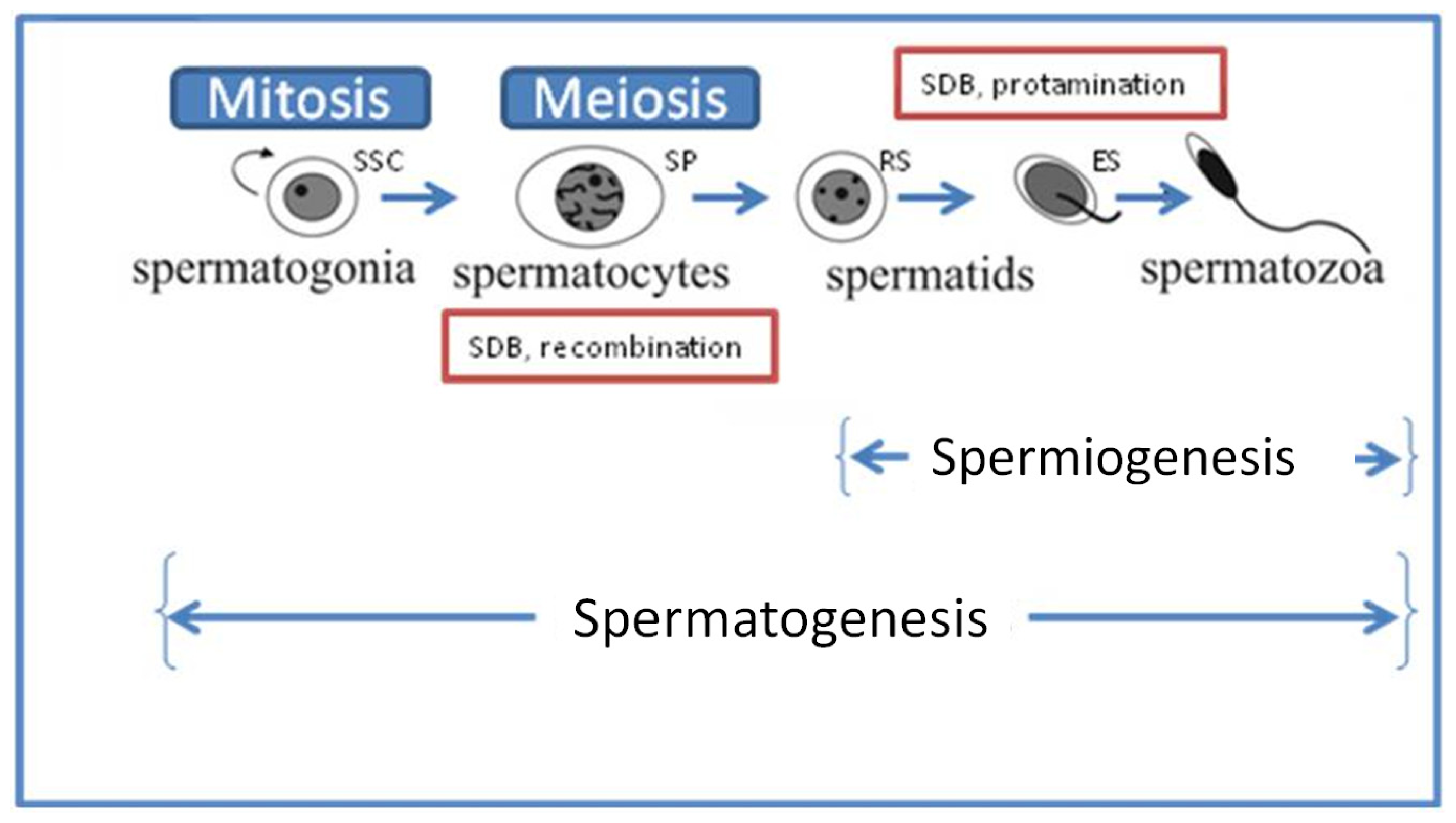
4.1.1. Deficiencies in Recombination during Spermatogenesis
4.1.2. Abnormal Spermatid Maturation
4.1.3. Protamine 1 and 2 Ratios
4.1.4. Abortive Apoptosis
4.1.5. Oxidative Stress
4.2. Extrinsic Causes
4.2.1. Lapse of Time from the Ejaculation
4.2.2. Collection Methods, Extenders and Post-Ejaculation Treatments
4.2.3. Sperm Preparation Techniques for ART
4.2.4. Storage Temperature and Cryopreservation
4.2.5. Mechanical Conditions—Sex-Sorting
4.2.6. Post-Testicular Oxidative Stress
4.2.7. Varicocele
4.2.8. Bacterial Infections
4.2.9. Age
4.2.10. Abstinence
4.2.11. Temperature of Testis
4.2.12. Reaction to Clinical Procedures, Medicines or Vaccines
4.2.13. Exposure to Environmental Chemicals
5. Repair of Sperm DNA Damage
5.1. Repair during Spermiogenesis
5.2. Repair in the Fertilized Oocyte and during Early Embryonic Development
5.2.1. Sperm DNA Fragmentation Repair in the Fertilized Oocyte
5.2.2. Sperm DNA Fragmentation Repair in the Zygote
5.2.3. Sperm DNA Fragmentation Repair in during/after Implantation Embryo
6. Conclusions and Future Directions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Evenson, D.P.; Darzynkiewicz, Z.; Melamed, M.R. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 1980, 210, 1131–1133. [Google Scholar]
- Sun, J.G.; Jurisicova, A.; Casper, R.F. Detection of deoxyribonucleic acid fragmentation in human sperm: Correlation with fertilization in vitro. Biol. Reprod 1997, 56, 602–607. [Google Scholar]
- Evenson, D.P.; Jost, L.K.; Marshall, D.; Zinaman, M.J.; Clegg, E.; Purvis, K.; de Angelis, P.; Claussen, O.P. Utility of the sperm chromatin assay as a diagnostic and prognostic tool in the human fertility clinic. Hum. Reprod 1999, 14, 1039–1049. [Google Scholar]
- Spanò, M.; Bonde, J.P.; Hjøllund, H.I.; Kolstad, H.A.; Cordelli, E.; Leter, G. Sperm chromatin damage impairs human fertility. The Danish first pregnancy planner study team. Fertil. Steril 2000, 73, 43–50. [Google Scholar]
- Larson, K.L.; DeJonge, C.J.; Barnes, A.M.; Jost, L.K.; Evenson, D.P. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum. Reprod 2000, 15, 1717–1722. [Google Scholar]
- Fernandez-Gonzalez, R.; Moreira, P.N.; Perez-Crespo, M.; Sanchez-Martin, M.; Ramirez, M.A.; Pericuesta, E.; Bilbao, A.; Bermejo-Alvarez, P.; de Dios Hourcade, J.; de Fonseca, F.R. Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol. Reprod 2008, 78, 761–772. [Google Scholar]
- Delbès, G.; Hales, B.F.; Robaire, B. Toxicants and human sperm chromatin integrity. Mol. Hum. Reprod 2010, 16, 14–22. [Google Scholar]
- Conwell, C.C.; Vilfan, I.D.; Hud, N.V. Controlling the size of nanoscale toroidal DNA condensates with static curvature and ionic strength. Proc. Natl. Acad. Sci. USA 2003, 100, 9296–9301. [Google Scholar]
- Barratt, C.L.; Aitken, R.J.; Björndahl, L.; Carrell, D.T.; de Boer, P.; Kvist, U.; Lewis, S.E.; Perreault, S.D.; Perry, M.J.; Ramos, L.; et al. Sperm DNA: Organization, protection and vulnerability: From basic science to clinical applications—A position report. Hum. Reprod 2010, 25, 824–838. [Google Scholar]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril 2010, 93, 1027–1036. [Google Scholar]
- Gosálvez, J.; López-Fernández, C.; Fernández, J.L.; Gouraud, A.; Holt, W.V. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol. Reprod. Dev 2011, 78, 951–961. [Google Scholar]
- Smith, A.; Haaf, T. DNA nicks and increased sensitivity of DNA to fluorescence in situ end labeling during functional spermiogenesis. Biotechniques 1998, 25, 496–502. [Google Scholar]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm chromatin structure assay: Its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J. Androl 2002, 23, 25–43. [Google Scholar]
- Gorczyca, W.; Gong, J.; Darzynkiewicz, Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res 1993, 53, 1945–1951. [Google Scholar]
- Fernández, J.L.; Gosálvez, J. Application of FISH to detect DNA damage. DNA breakage detection-FISH (DBD-FISH). Methods Mol. Biol 2002, 203, 203–216. [Google Scholar]
- Fernández, J.L.; Muriel, L.; Rivero, M.T.; Goyanes, V.; Vázquez, R.; Alvarez, J.G. The sperm chromatin dispersion test: A simple method for the determination of sperm DNA fragmentation. J. Androl 2003, 24, 59–66. [Google Scholar]
- Kaneko, S.; Yoshida, J.; Ishikawa, H.; Takamatsu, K. Single-cell pulsed-field gel electrophoresis to detect the early stage of DNA fragmentation in human sperm nuclei. PLoS One 2012, 7, e42257. [Google Scholar]
- Lewis, S.E.; Agbaje, I.M. Using the alkaline comet assay in prognostic tests for male infertility and assisted reproductive technology outcomes. Mutagenesis 2008, 23, 163–170. [Google Scholar]
- Gosálvez, J.; Gosálbez, A.; Arroyo, F.; Fernández, J.L.; López-Fernández, C. Assessing sperm DNA fragmentation in the field: An adaptation of sperm chromatin dispersion technology. Biotech. Histochem 2008, 83, 247–252. [Google Scholar]
- Henkel, R.; Hajimohammad, M.; Stalf, T.; Hoogendijk, C.; Mehnert, C.; Menkveld, R.; Gips, H.; Schill, W.B.; Kruger, T.F. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil. Steril 2004, 81, 965–972. [Google Scholar]
- Benchaib, M.; Braun, V.; Lornage, J.; Hadj, S.; Salle, B.; Lejeune, H.; Guérin, J.F. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum. Reprod 2003, 18, 1023–1028. [Google Scholar]
- Bakos, H.W.; Thompson, J.G.; Feil, D.; Lane, M. Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int. J. Androl 2008, 31, 518–526. [Google Scholar]
- Gandini, L.; Lombardo, F.; Paoli, D.; Caruso, F.; Eleuteri, P.; Lete, G.; Ciriminna, R.; Culasso, F.; Dondero, F.; Lenzi, A.; et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum. Reprod 2004, 19, 1409–1417. [Google Scholar]
- Virro, M.R.; Kjersten, L.; Larson-Cook, K.L.; Evenson, D.P. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplamic sperm injection cycles. Fertil. Steril 2004, 81, 1289–1295. [Google Scholar]
- Payne, J.F.; Raburn, D.J.; Couchman, G.M.; Price, T.M.; Jamison, M.G.; Walmer, D.K. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil. Steril 2005, 84, 356–364. [Google Scholar]
- Nijs, M.; Creemers, E.; Cox, A.; Franssen, K.; Janssen, M.; Vanheusden, E.; de Jonge, C.; Ombelet, W. Chromomycin A3 staining, sperm chromatin structure assay and hyaluronic acid binding assay as predictors for assisted reproductive outcome. Reprod. Biomed. Online 2009, 19, 671–684. [Google Scholar]
- Li, Z.; Wang, L.; Cai, J.; Huang, H. Correlation of sperm DNA damage with IVF and ICSI outcomes: A systematic review and meta-analysis. J. Assist. Reprod. Genet 2006, 23, 367–376. [Google Scholar]
- Evenson, D.P.; Kasperson, K.; Wixon, R.L. Analysis of sperm DNA fragmentation using flow cytometry and other techniques. Soc. Reprod. Fertil. Suppl 2007, 65, 93–113. [Google Scholar]
- Zini, A.; Sigman, M. Are tests of sperm DNA damage clinically useful? Pros and cons. J. Androl 2009, 3, 219–229. [Google Scholar]
- Zini, A. Are sperm chromatin and DNA defects relevant in the clinic? Syst. Biol. Reprod. Med 2011, 57, 78–85. [Google Scholar]
- Lin, M.-H.; Lee, R.K.-K.; Li, S.-H.; Lu, C.-H.; Sun, F.-J.; Hwu, Y.-M. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil. Steril 2008, 90, 352–359. [Google Scholar]
- Greco, E.; Scarselli, F.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Franco, G.; Anniballo, N.; Mendoza, C.; Tesarik, J. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum. Reprod 2005, 20, 226–230. [Google Scholar]
- Enciso, M.; Sarasa, J.; Agarwal, A.; Fernández, J.L.; Gosálvez, J. A two-tailed Comet assay for assessing DNA damage in spermatozoa. Reprod. Biomed. Online 2009, 18, 609–616. [Google Scholar]
- Badouard, C.; Ménézo, Y.; Panteix, G.; Ravanat, J.L.; Douki, T.; Cadet, J.; Favier, A. Determination of new types of DNA lesions in human sperm. Zygote 2008, 16, 9–13. [Google Scholar]
- Garcia-Diaz, M.; Dominguez, O.; Lopez-Fernandez, L.A.; de Lera, L.T.; Saniger, M.L.; Ruiz, J.F.; Párraga, M.; García-Ortiz, M.J.; Kirchhoff, T.; del Mazo, J.; et al. DNA polymerase lambda (Pol lambda), a novel eukaryotic DNA polymerase with a potential role in meiosis. J. Mol. Biol 2000, 301, 851–867. [Google Scholar]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes. Dev 2011, 25, 409–433. [Google Scholar]
- Wang, J.; Fan, H.C.; Behr, B.; Quake, S.R. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell 2012, 150, 402–412. [Google Scholar]
- Menezo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar]
- Bennetts, L.E.; de Iuliis, G.N.; Nixon, B.; Kime, M.; Zelski, K.; McVicar, C.M.; Lewis, S.E.; Aitken, R.J. Impact of estrogenic compounds on DNA integrity in human spermatozoa: Evidence for cross-linking and redox cycling activities. Mutat. Res 2008, 641, 1–11. [Google Scholar]
- Windt, M.L.; de Beer, P.M.; Franken, D.R.; Rhemrev, J.; Menkveld, R.; Lombard, C.J.; Kruger, T.F. Sperm decondensation and semen parameters: Utilization of a simple staining technique for the evaluation of human sperm decondensation. Andrologia 1994, 26, 67–72. [Google Scholar]
- Mengual, L.; Ballescá, J.L.; Ascaso, C.; Oliva, R. Marked differences in protamine content and P1/P2 ratios in sperm cells from percoll fractions between patients and controls. J. Androl 2003, 24, 438–447. [Google Scholar]
- Oliva, R. Protamines and male infertility. Hum. Reprod. Update 2006, 12, 417–435. [Google Scholar]
- Carrell, D.T.; Liu, L. Altered protamine 2 expression is uncommon in donors of known fertility, but common among men with poor fertilizing capacity, and may reflect other abnormalities of spermiogenesis. J. Androl 2001, 22, 604–610. [Google Scholar]
- Simon, L.; Castillo, J.; Oliva, R.; Lewis, S.E. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod. Biomed. Online 2011, 23, 724–734. [Google Scholar]
- García-Peiró, A.; Martínez-Heredia, J.; Oliver-Bonet, M.; Abad, C.; Amengual, M.J.; Navarro, J.; Jones, C.; Coward, K.; Gosálvez, J.; Benet, J. Protamine 1 to protamine 2 ratio correlates with dynamic aspects of DNA fragmentation in human sperm. Fertil. Steril 2011, 95, 105–109. [Google Scholar]
- Sakkas, D.; Seli, E.; Manicardi, G.C.; Nijs, M.; Ombelet, W.; Bizzaro, D. The presence of abnormal spermatozoa in the ejaculate: Did apoptosis fail? Hum. Fertil 2004, 7, 99–103. [Google Scholar]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function—In sickness and in health. J. Androl 2012. in print. [Google Scholar]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox. Signal 2011, 14, 367–381. [Google Scholar]
- De Iuliis, G.N.; Thomson, L.K.; Mitchell, L.A.; Finnie, J.M.; Koppers, A.J.; Hedges, A.; Nixon, B.; Aitken, R.J. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-20-deoxyguanosine, a marker of oxidative stress. Biol. Reprod 2009, 81, 517–524. [Google Scholar]
- Gosálvez, J.; López-Fernández, C.; Fernández, J.L.; Gouraud, A.; Holt, W.V. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol. Reprod. Dev 2011, 78, 951–961. [Google Scholar]
- Parrilla, I.; del Olmo, D.; Sijses, L.; Martinez-Alborcia, M.J.; Cuello, C.; Vazquez, J.M.; Martinez, E.A.; Roca, J. Differences in the ability of spermatozoa from individual boar ejaculates to withstand different semen-processing techniques. Anim. Reprod. Sci 2012, 132, 66–73. [Google Scholar]
- Jiménez-Rabadán, P.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; del Olmo, E.; Pérez-Guzmán, M.D.; Bisbal, A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of Blanca-Celtibérica buck ejaculates. Anim. Reprod. Sci 2012, 132, 88–95. [Google Scholar]
- De Lamirande, E.; San Gabriel, M.C.; Zini, A. Human sperm chromatin undergoes physiological remodeling during in vitro capacitation and acrosome reaction. J. Androl 2012, 33, 1025–1035. [Google Scholar]
- Bormann, C.; Rocha, A.; Hassun, P.; Motta, E.; Serafini, P.; Smith, G. The effect of sperm separation on sperm chromatin decondensation and motility at 0 and 24 hours of culture. Fertil. Steril 2008, 90, 452–453. [Google Scholar]
- Jayaraman, V.; Upadhya, D.; Narayan, P.K.; Adiga, S.K. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J. Assist. Reprod. Genet 2012, 29, 557–563. [Google Scholar]
- Lo, C.C.; Thompson, J.A.; Lowry, V.K.; Varner, D.D. Effect of storage time and temperature on stallion sperm DNA and fertility. Theriogenology 2002, 57, 1135–1142. [Google Scholar]
- Prinosilova, P.; Rybar, R.; Zajicova, A.; Hlavicova, J. DNA integrity in fresh, chilled and frozen-thawed canine spermatozoa. Veterinarni Med 2012, 57, 133–142. [Google Scholar]
- Imrat, P.; Mahasawangkul, S.; Gosálvez, J.; Suthanmapinanth, P.; Sombutputorn, P.; Jansittiwate, S.; Thongtip, N.; Pinyopummin, A.; Colenbrander, B.; Holt, W.V.; et al. Effect of cooled storage on quality and DNA integrity of Asian elephant (Elephas maximus) spermatozoa. Reprod. Fertil. Dev 2012, 24, 1105–1116. [Google Scholar]
- Jackson, R.E.; Bormann, C.L.; Hassun, P.A.; Rocha, A.M.; Motta, E.L.; Serafini, P.C.; Smith, G.D. Effects of semen storage and separation techniques on sperm DNA fragmentation. Fertil. Steril 2010, 94, 2626–2630. [Google Scholar]
- Gosálvez, J.; de la Torre, J.; López-Fernández, C.; Pérez-Gutiérrez, L.; Ortega, L.; Caballero, P.; Nuñez, R. DNA fragmentation dynamics in fresh versus frozen thawed 1 plus gradient-isolated human spermatozoa. Sys. Biol. Rep. Med 2010, 56, 27–36. [Google Scholar]
- Kjelland, M.E.; González-Marín, C.; Gosálvez, J.; López-Fernández, C.; Lenz, R.W.; Evans, K.M.; Moreno, J.F. DNA fragmentation kinetics and postthaw motility of flow cytometric-sorted white-tailed deer sperm. J. Anim. Sci 2011, 89, 3996–4006. [Google Scholar]
- De Ambrogi, M.; Spinaci, M.; Galeati, G.; Tamanini, C. Viability and DNA fragmentation in differently sorted boar spermatozoa. Theriogenology 2006, 66, 1994–2000. [Google Scholar]
- Gosálvez, J.; Ramirez, M.A.; López-Fernández, C.; Crespo, F.; Evans, K.M.; Kjelland, M.E.; Moreno, J.F. Sex-sorted bovine spermatozoa and DNA damage: II. Dynamic features. Theriogenology 2011, 75, 206–211. [Google Scholar]
- Gil-Guzman, E.; Ollero, M.; Lopez, M.C.; Sharma, R.K.; Alvarez, J.G.; Thomas, A.J.; Agarwal, A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod 2001, 16, 1922–1930. [Google Scholar]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod 1998, 59, 1037–1046. [Google Scholar]
- Britan, A.; Maffre, V.; Tone, S.; Drevet, J.R. Quantitative and spatial differences in the expression of tryptophan-metabolizing enzymes in mouse epididymis. Cell Tissue Res 2006, 324, 301–310. [Google Scholar]
- Banks, S.; King, S.A.; Irvine, D.S.; Saunders, P.T. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction 2005, 129, 505–514. [Google Scholar]
- Menezo, Y.J.; Hazout, A.; Panteix, G.; Robert, F.; Rollet, J.; Cohen-Bacrie, P.; Chapuis, F.; Clément, P.; Benkhalifa, M. Antioxidants to reduce sperm DNA fragmentation: An unexpected adverse effect. Reprod. Biomed. Online 2007, 14, 418–421. [Google Scholar]
- Zini, A.; Dohle, G. Are varicoceles associated with increased deoxyribonucleic acid fragmentation? Fertil. Steril 2011, 96, 1283–1287. [Google Scholar]
- Talebi, A.R.; Moein, M.R.; Tabibnejad, N.; Ghasemzadeh, J. Effect of varicocele on chromatin condensation and DNA integrity of ejaculated spermatozoa using cytochemical tests. Andrologia 2008, 40, 245–251. [Google Scholar]
- Gallegos, G.; Ramos, B.; Santiso, R.; Goyanes, V.; Gosálvez, J.; Fernández, J.L. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil. Steril 2008, 90, 328–334. [Google Scholar]
- González-Marín, C.; Roy, R.; López-Fernández, C.; Diez, B.; Carabaño, M.J.; Fernández, J.L.; Kjelland, M.E.; Moreno, J.F.; Gosálvez, J. Bacteria in bovine semen can increase sperm DNA fragmentation rates: A kinetic experimental approach. Anim. Reprod. Sci 2011, 123, 139–148. [Google Scholar]
- Winkle, T.; Rosenbusch, B.; Gagsteiger, F.; Paiss, T.; Zoller, N. The correlation between male age, sperm quality and sperm DNA fragmentation in 320 men attending a fertility center. J. Assist. Reprod. Genet 2009, 26, 41–46. [Google Scholar]
- Nijs, M.; de Jonge, C.; Cox, A.; Janssen, M.; Bosmans, E.; Ombelet, W. Correlation between male age, WHO sperm parameters, DNA fragmentation, chromatin packaging and outcome in assisted reproduction technology. Andrologia 2011, 43, 174–179. [Google Scholar]
- Belloc, S.; Benkhalifa, M.; Junca, A.M.; Dumont, M.; Bacrie, P.C.; Ménézo, Y. Paternal age and sperm DNA decay: Discrepancy between chromomycin and aniline blue staining. Reprod. Biomed. Online 2009, 19, 264–269. [Google Scholar]
- Vagnini, L.; Baruffi, R.L.R.; Mauri, A.L.; Petersen, C.G.; Massaro, F.C.; Pontes, A.; Oliveira, J.B.A.; Franco, J.G. The effects of male age on sperm DNA damage in an infertile population. Reprod. Biomed. Online 2007, 15, 514–519. [Google Scholar]
- De Jonge, C.; LaFromboise, M.; Bosmans, E.; Ombelet, W.; Cox, A.; Nijs, M. Influence of the abstinence period on human sperm quality. Fertil. Steril 2004, 82, 57–65. [Google Scholar]
- Gosálvez, J.; González-Martínez, M.; López-Fernández, C.; Fernández, J.L.; Sánchez-Martín, P. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil. Steril 2011, 96, 1083–1086. [Google Scholar]
- Paul, C.; Murray, A.A.; Spears, N.; Saunders, P.T.K. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 2008, 136, 73–84. [Google Scholar]
- Morris, I.D. Sperm DNA damage and cancer treatment. Int. J. Androl 2002, 25, 255–261. [Google Scholar]
- Tanrikut, C.; Feldman, A.S.; Altemus, M.; Paduch, D.A.; Schlegel, P.N. Adverse effect of paroxetine on sperm. Fertil. Steril 2010, 94, 1021–1026. [Google Scholar]
- Gosálvez, J.; Vázquez, J.M.; Enciso, M.; Fernández, J.L.; Gosálbez, A.; Bridle, J.R.; López-Fernández, C. Sperm DNA fragmentation in rams vaccinated with miloxan. Open Vet. Sci. J 2008, 2, 7–10. [Google Scholar]
- Evenson, D.P.; Wixon, R. Environmental toxicants cause sperm DNA fragmentation as detected by the Sperm Chromatin Structure Assay (SCSA). Toxicol. Appl. Pharmacol 2005, 207, 532–537. [Google Scholar]
- Specht, I.O.; Hougaard, K.S.; Spanò, M.; Bizzaro, D.; Manicardi, G.C.; Lindh, C.H.; Toft, G.; Jönsson, B.A.; Giwercman, A.; Bonde, J.P. Sperm DNA integrity in relation to exposure to environmental perfluoroalkyl substances—A study of spouses of pregnant women in three geographical regions. Reprod. Toxicol 2012, 33, 577–583. [Google Scholar]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev 2008, 1, 15–24. [Google Scholar]
- Rubes, J.; Selevan, S.G.; Sram, R.J.; Evenson, D.P.; Perreault, S.D. GSTM1 genotype influences the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat. Res 2007, 625, 20–28. [Google Scholar]
- Jurisicova, A.; Acton, B.M. Deadly decisions: The role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction 2004, 128, 281–291. [Google Scholar]
- Brugmans, L.; Kanaar, R.; Essers, J. Analysis of DNA double-strand break repair pathways in mice. Mutat. Res 2007, 614, 95–108. [Google Scholar]
- Kimmins, S.; Sassone-Corsi, P. Chromatin remodelling and epigenetic features of germ cells. Nature 2005, 434, 583–589. [Google Scholar]
- Carrell, D.T.; Hammoud, S.S. The human sperm epigenome and its potential role in embryonic development. Mol. Hum. Reprod 2010, 16, 37–47. [Google Scholar]
- Zhang, X.; San Gabriel, M.; Zini, A. Sperm nuclear histone to protamine ratio in fertile and infertile men: Evidence of heterogeneous subpopulations of spermatozoa in the ejaculate. J. Androl 2006, 27, 414–420. [Google Scholar]
- Singleton, S.; Zalensky, A.; Doncel, G.F.; Morshedi, M.; Zalenskaya, I.A. Testis/sperm-specific histone 2B in the sperm of donors and subfertile patients: Variability and relation to chromatin packaging. Hum. Reprod 2007, 22, 743–750. [Google Scholar]
- Zini, A.; Gabriel, M.S.; Zhang, X. The histone to protamine ratio in human spermatozoa: Comparative study of whole and processed semen. Fertil. Steril 2007, 87, 217–219. [Google Scholar]
- Marcon, L.; Boissonneault, G. Transient DNA strand breaks during mouse and human spermiogenesis: New insights in stage specificity and link to chromatin remodeling. Biol. Reprod 2004, 70, 910–918. [Google Scholar]
- Meyer-Ficca, M.L.; Scherthan, H.; Burkle, A.; Meyer, R.G. Poly(ADPribosyl)ation during chromatin remodeling steps in rat spermiogenesis. Chromosoma 2005, 114, 67–74. [Google Scholar]
- Laberge, R.M.; Boissonneault, G. Chromatin remodeling in spermatids: A sensitive step for the genetic integrity of the male gamete. Arch. Androl 2005, 51, 125–133. [Google Scholar]
- Leduc, F.; Maquennehan, V.; Nkoma, G.B.; Boissonneault, G. DNA damage response during chromatin remodeling in elongating spermatids of mice. Biol. Reprod 2008, 78, 324–332. [Google Scholar]
- Riballo, E.; Kühne, M.; Rief, N.; Doherty, A.; Smith, G.C.; Recio, M.J.; Reis, C.; Dahm, K.; Fricke, A.; Krempler, A.; et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol. Cell 2004, 16, 715–724. [Google Scholar]
- Baarends, W.M.; van der Laan, R.; Grootegoed, J.A. DNA repair mechanisms and gametogenesis. Reproduction 2001, 121, 31–39. [Google Scholar]
- Neal, J.A.; Meek, K. Choosing the right path: Does DNA-PK help make the decision? Mutat. Res 2011, 711, 73–86. [Google Scholar]
- Britton, S.; Froment, C.; Frit, P.; Monsarrat, B.; Salles, B.; Calsou, P. Cell nonhomologous end joining capacity controls SAF-A phosphorylation by DNA-PK in response to DNA double-strand breaks inducers. Cell Cycle 2009, 8, 3717–3722. [Google Scholar]
- Rube, C.E.; Zhang, S.; Miebach, N.; Fricke, A.; Rube, C. Protecting the heritable genome: DNA damage response mechanisms in spermatogonial stem cells. DNA Repair 2011, 10, 159–168. [Google Scholar]
- Nussenzweig, A.; Nussenzweig, M.C. A backup DNA repair pathway moves to the forefront. Cell 2007, 131, 223–225. [Google Scholar]
- Iliakis, G. Backup pathways of NHEJ in cells of higher eukaryotes: Cell cycle dependence. Radiother. Oncol 2009, 92, 310–315. [Google Scholar]
- Audebert, M.; Salles, B.; Calsou, P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNAligase III in an alternative route for DNA double-strand breaks rejoining. J. Biol. Chem 2004, 279, 55117–55126. [Google Scholar]
- Ahmed, E.A.; de Boer, P.; Philippens, M.E.; Kal, H.B.; de Rooij, D.G. Parp1-XRCC1 and the repair of DNA double strand breaks in mouse round spermatids. Mutat. Res 2010, 683, 84–90. [Google Scholar]
- Pandita, T.K.; Richardson, C. Chromatin remodeling finds its place in the DNA double-strand break response. Nucleic Acids Res 2009, 37, 1363–1377. [Google Scholar]
- Goodarzi, A.A.; Noon, A.T.; Deckbar, D.; Ziv, Y.; Shiloh, Y.; Lobrich, M.; Jeggo, P.A. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol. Cell 2008, 31, 167–177. [Google Scholar]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res 2008, 36, 5678–5694. [Google Scholar]
- Nakamura, A.J.; Rao, V.A.; Pommier, Y.; Bonner, W.M. The complexity of phosphorylated H2AX foci formation and DNA repair assembly at DNA double-strand breaks. Cell Cycle 2010, 9, 389–397. [Google Scholar]
- Cho, C.; Jung-Ha, H.; Willis, W.D.; Goulding, E.H.; Stein, P.; Xu, Z.; Schultz, R.M.; Hecht, N.B.; Eddy, E.M. Protamine-2 deficiency leads to sperm DNA damage and embryo death in mice. Biol. Reprod 2003, 69, 211–217. [Google Scholar]
- Zhao, M.; Shirley, C.R.; Yu, Y.E.; Mohapatra, B.; Zhang, Y.; Unni, E.; Deng, J.M.; Arango, N.A.; Terry, N.H.; Weil, M.M.; et al. Targeted disruption of the transition protein 2 gene affects sperm chromatin structure and reduces fertility in mice. Mol. Cell Biol 2001, 21, 7243–7255. [Google Scholar]
- Gorczyca, W.; Traganos, F.; Jesionowska, H.; Darzynkiewicz, Z. Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: Analogy to apoptosis of somatic cells. Exp. Cell Res 1993, 207, 202–205. [Google Scholar]
- Manicardi, G.C.; Tombacco, A.; Bizzaro, D.; Bianchi, U.; Bianchi, P.G.; Sakkas, D. DNA strand breaks in ejaculated human spermatozoa: Comparison of susceptibility to the nick translation and terminal transferase assays. Histochem. J 1998, 30, 33–39. [Google Scholar]
- Aravindan, G.R.; Moudgal, N.R. Susceptibility of sperm chromatin to acid denaturation in situ: A study in endogenous FSH-deprived adult male bonnet monkeys (Macaca radiate). Arch. Androl 1998, 40, 29–41. [Google Scholar]
- Bianchi, P.G.; Manicardi, G.C.; Bizzaro, D.; Bianchi, U.; Sakkas, D. Effect of deoxyribonucleic acid protamination on fluorochrome staining and in situ nick-translation of murine and human mature spermatozoa. Biol. Reprod. 1993, 49, 1083–1088. [Google Scholar]
- Meistrich, M.L.; Mohapatra, B.; Shirley, C.R.; Zhao, M. Roles of transition nuclear proteins in spermiogenesis. Chromosoma 2003, 111, 483–488. [Google Scholar]
- Suganuma, R.; Yanagimachi, R.; Meistrich, M.L. Decline in fertility of mouse sperm with abnormal chromatin during epididymal passage as revealed by ICSI. Hum. Reprod 2005, 20, 3101–3108. [Google Scholar]
- Tomar, D.M.E.; Chamberlin, J.; Allen, L.; Olson, S.; Donlon, T.; Barton, S.; Sheehy, R.; Waggonner, D. Preferential paternal origin of de novo structural chromosome rearrangements. Am. J. Hum. Genet 1984, 36, 115. [Google Scholar]
- Brandriff, B.; Pedersen, R.A. Repair of the ultraviolet-irradiated male genome in fertilized mouse eggs. Science 1981, 211, 1431–1433. [Google Scholar]
- Ashwood-Smith, M.J.; Edwards, R.G. DNA repair by oocytes. Mol. Hum. Reprod 1996, 2, 46–51. [Google Scholar]
- Jurisicova, A.; Latham, K.E.; Casper, R.F.; Casper, R.F.; Varmuza, S.L. Expression and regulation of genes associated with cell death during murine preimplantation embryo development. Mol. Reprod. Dev 1998, 51, 243–253. [Google Scholar]
- Menezo, Y., Jr; Russo, G.; Tosti, E.; El Mouatassim, S.; Benkhalifa, M. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays and with a special focus on ROS linked decays. J. Assist. Reprod. Genet. 2007, 24, 513–520. [Google Scholar]
- Mac, A.A.; Werb, Z.; Mirkes, P.E. Characterization of the unusually rapid cell cycles during rat gastrulation. Development 1993, 117, 873–883. [Google Scholar]
- Derijck, A.; van der Heijden, G.; Giele, M.; Philippens, M.; de Boer, P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum. Mol. Genet 2008, 17, 1922–1937. [Google Scholar]
- Barton, T.S.; Robaire, B.; Hales, B.F. DNA damage recognition in the rat zygote following chronic paternal cyclophosphamide exposure. Toxicol. Sci 2007, 100, 495–503. [Google Scholar]
- Zenzes, M.T.; Puy, L.A.; Bielecki, R.; Reed, T.E. Detection of benzo(a)pyrene diol epoxide-DNA adducts in embryos from smoking couples: Evidence for transmission by spermatozoa. Mol. Hum. Reprod 1999, 5, 125–131. [Google Scholar]
- Zheng, P.; Schramm, R.D.; Latham, K.E. Developmental regulation and in vitro culture effects on expression of DNA repair and cell cycle checkpoint control genes in rhesus monkey oocytes and embryos. Biol. Reprod 2005, 72, 1359–1369. [Google Scholar]
- Genesca, A.; Caballin, M.R.; Miro, R.; Benet, J.; Germa, J.R.; Egozcue, J. Repair of human sperm chromosome aberrations in the hamster egg. Hum. Genet 1992, 89, 181–186. [Google Scholar]
- Ahmadi, A.; Ng, S.C. Fertilizing ability of DNA-damaged spermatozoa. J. Exp. Zool 1999, 284, 696–704. [Google Scholar]
- Jaroudi, S.; Kakourou, G.; Cawood, S.; Doshi, A.; Ranieri, D.M.; Serhal, P.; Harper, J.C.; SenGupta, S.B. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum. Reprod 2009, 24, 2649–2655. [Google Scholar]
- Hamatani, T.; Falco, G.; Carter, M.G.; Akutsu, H.; Stagg, C.A.; Sharov, A.A.; Dudekula, D.B.; VanBuren, V.; Ko, M.S. Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet 2004, 13, 2263–2278. [Google Scholar]
- Bultman, S.J.; Gebuhr, T.C.; Pan, H.; Svoboda, P.; Schultz, R.M.; Magnuson, T. Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev 2006, 20, 1744–1754. [Google Scholar]
- Marchetti, F.; Essers, J.; Kanaar, R.; Wyrobek, A.J. Disruption of maternal DNA repair increases sperm-derived chromosomal aberrations. Proc. Natl. Acad. Sci. USA 2007, 104, 17725–17729. [Google Scholar]
- Gurtu, V.E.; Verma, S.; Grossmannm, A.H.; Liskaym, R.M.; Skarnesm, W.C.; Bakerm, S.M. Maternal effect for DNA mismatch repair in the mouse. Genetics 2002, 160, 271–277. [Google Scholar]
- Matsuda, Y.; Tobari, I.; Maemori, M.; Seki, N. Mechanism of chromosome aberration induction in the mouse egg fertilized with sperm recovered from postmeiotic germ cells treated with methyl methanesulfonate. Mutat. Res 1989, 214, 165–180. [Google Scholar]
- Rothkamm, K.; Krüger, I.; Thompson, L.H.; Löbrich, M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell Biol 2003, 23, 5706–5715. [Google Scholar]
- Arnaudeau, C.; Lundin, C.; Helleday, T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol 2001, 307, 1235–1245. [Google Scholar]
- Tesarik, J.; Greco, E.; Mendoza, C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum. Reprod 2004, 19, 611–615. [Google Scholar]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.A.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod 2006, 21, 2876–2881. [Google Scholar]
- Seli, E.; Gardner, D.K.; Schoolcraft, W.B.; Moffatt, O.; Sakkas, D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil. Steril 2004, 82, 378–383. [Google Scholar]
- Shoukir, Y.; Chardonnens, D.; Campana, A.; Sakkas, D. Blastocyst development from supernumerary embryos after intracytoplasmic sperm injection: A paternal influence? Hum. Reprod 1998, 13, 1632–1637. [Google Scholar]
- Dumoulin, J.C.; Coonen, E.; Bras, M.; van Wissen, L.C.; Ignoul-Vanvuchelen, R.; Bergers-Jansen, J.M.; Derhaag, J.G.; Geraedts, J.P.; Evers, J.L. Comparison of in-vitro development of embryos originating from either conventional in-vitro fertilization or intracytoplasmic sperm injection. Hum. Reprod 2000, 15, 402–409. [Google Scholar]
- Van Dyck, E.; Stasiak, A.Z.; Stasiak, A.; West, S.C. Binding of double-strand breaks in DNA by human Rad52 protein. Nature 1999, 398, 728–731. [Google Scholar]
- Bassing, C.H.; Alt, F.W. The cellular response to general and programmed DNA double strand breaks. DNA Repair 2004, 3, 781–796. [Google Scholar]
- Vinson, R.K.; Hales, B.F. DNA repair during organogenesis. Mutat. Res 2002, 509, 79–91. [Google Scholar]
- Jaroudi, S.; SenGupta, S. DNA repair in mammalian embryos. Mutat. Res 2007, 635, 53–77. [Google Scholar]
- Matzuk, M.M.; Lamb, D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med 2008, 14, 1197–1213. [Google Scholar]
- Bartkova, J.; Rajpert-De Meyts, E.; Skakkebaek, N.E.; Lukas, J.; Bartek, J. DNA damage response in human testes and testicular germ cell tumours: Biology and implications for therapy. Int. J. Androl 2007, 30, 282–291. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
González-Marín, C.; Gosálvez, J.; Roy, R. Types, Causes, Detection and Repair of DNA Fragmentation in Animal and Human Sperm Cells. Int. J. Mol. Sci. 2012, 13, 14026-14052. https://doi.org/10.3390/ijms131114026
González-Marín C, Gosálvez J, Roy R. Types, Causes, Detection and Repair of DNA Fragmentation in Animal and Human Sperm Cells. International Journal of Molecular Sciences. 2012; 13(11):14026-14052. https://doi.org/10.3390/ijms131114026
Chicago/Turabian StyleGonzález-Marín, Clara, Jaime Gosálvez, and Rosa Roy. 2012. "Types, Causes, Detection and Repair of DNA Fragmentation in Animal and Human Sperm Cells" International Journal of Molecular Sciences 13, no. 11: 14026-14052. https://doi.org/10.3390/ijms131114026



