TNF-α-Induced VEGF and MMP-9 Expression Promotes Hemorrhagic Transformation in Pituitary Adenomas
Abstract
:1. Introduction
2. Results and Discussion
2.1. Clinical Features of Patients
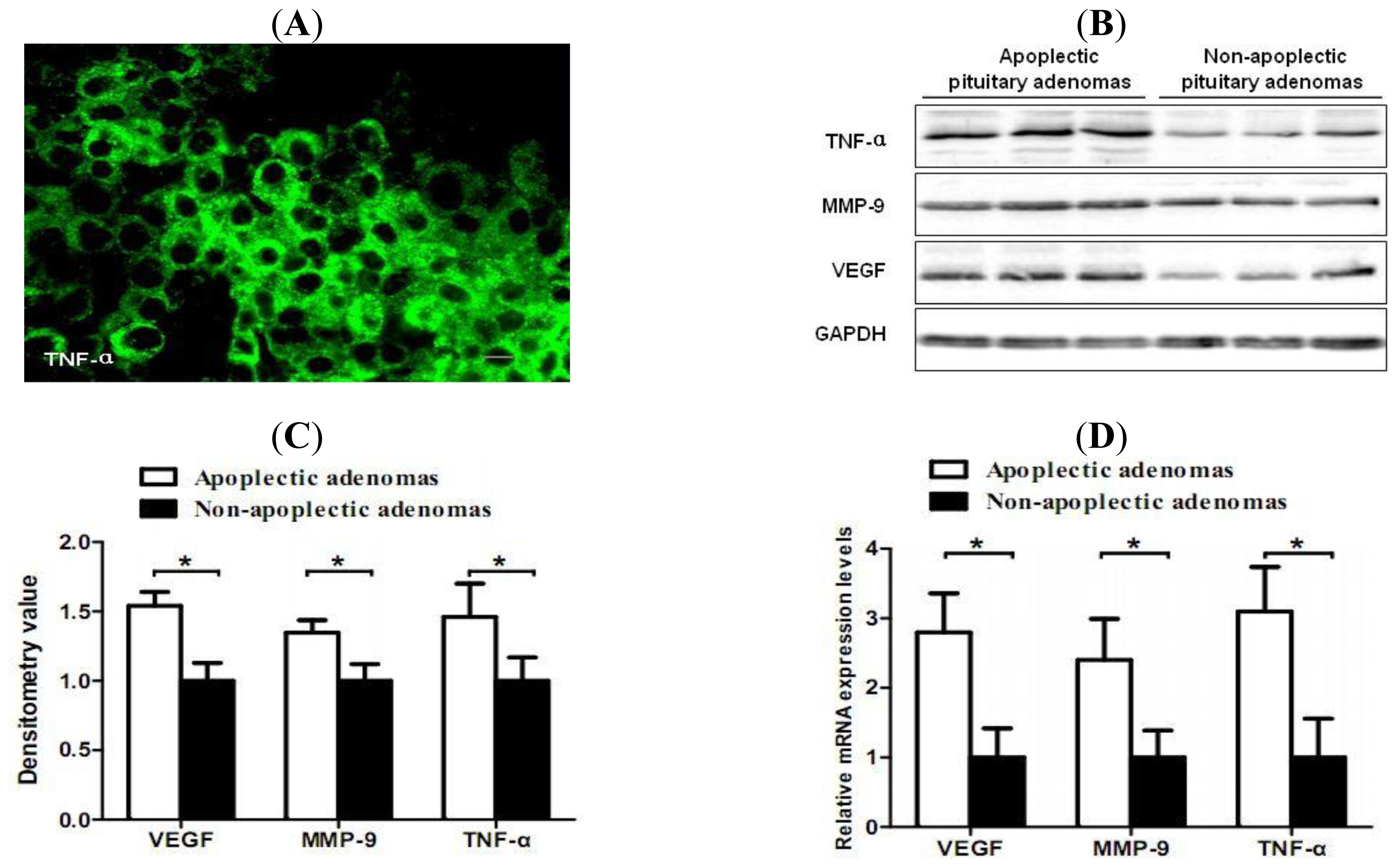
2.2. Increased Expression of TNF-α, VEGF and MMP-9 in Hemorrhagic Pituitary Adenoma Tissues
2.3. Correlation of TNF-α and Hemorrhage-Associated Genes Expression in Hemorrhagic and Non-Hemorrhagic Pituitary Adenoma Tissues
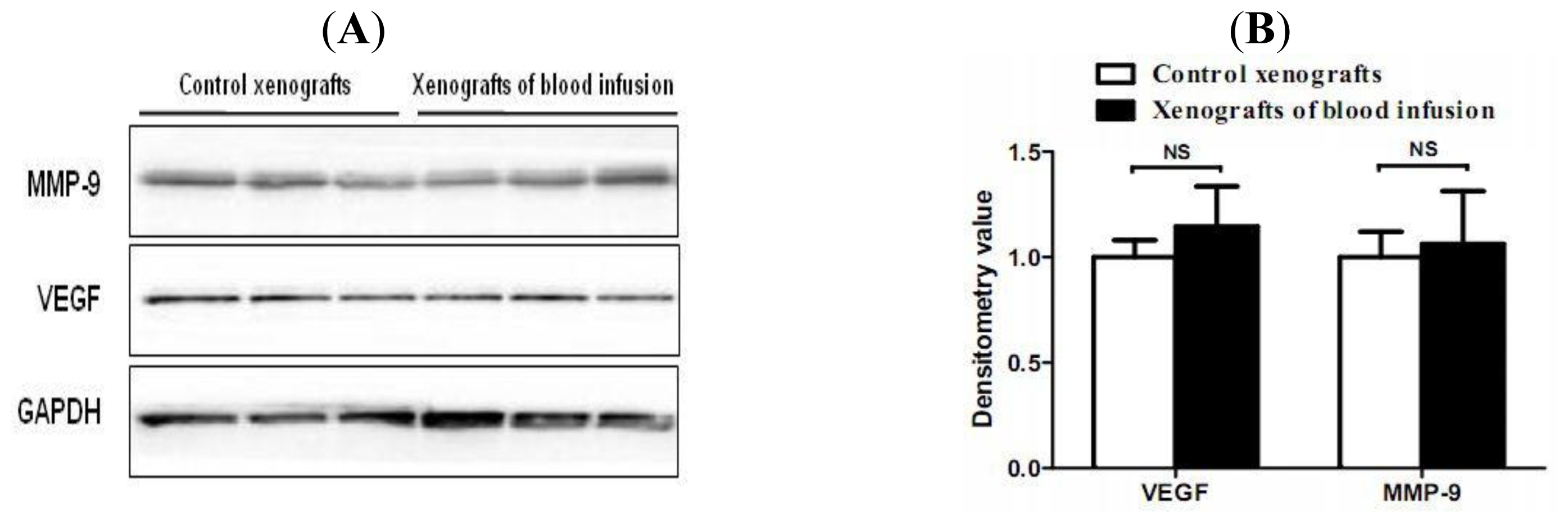
2.4. Hemorrhagic Incite Has no Effect on MMP-9 and VEGF Expression
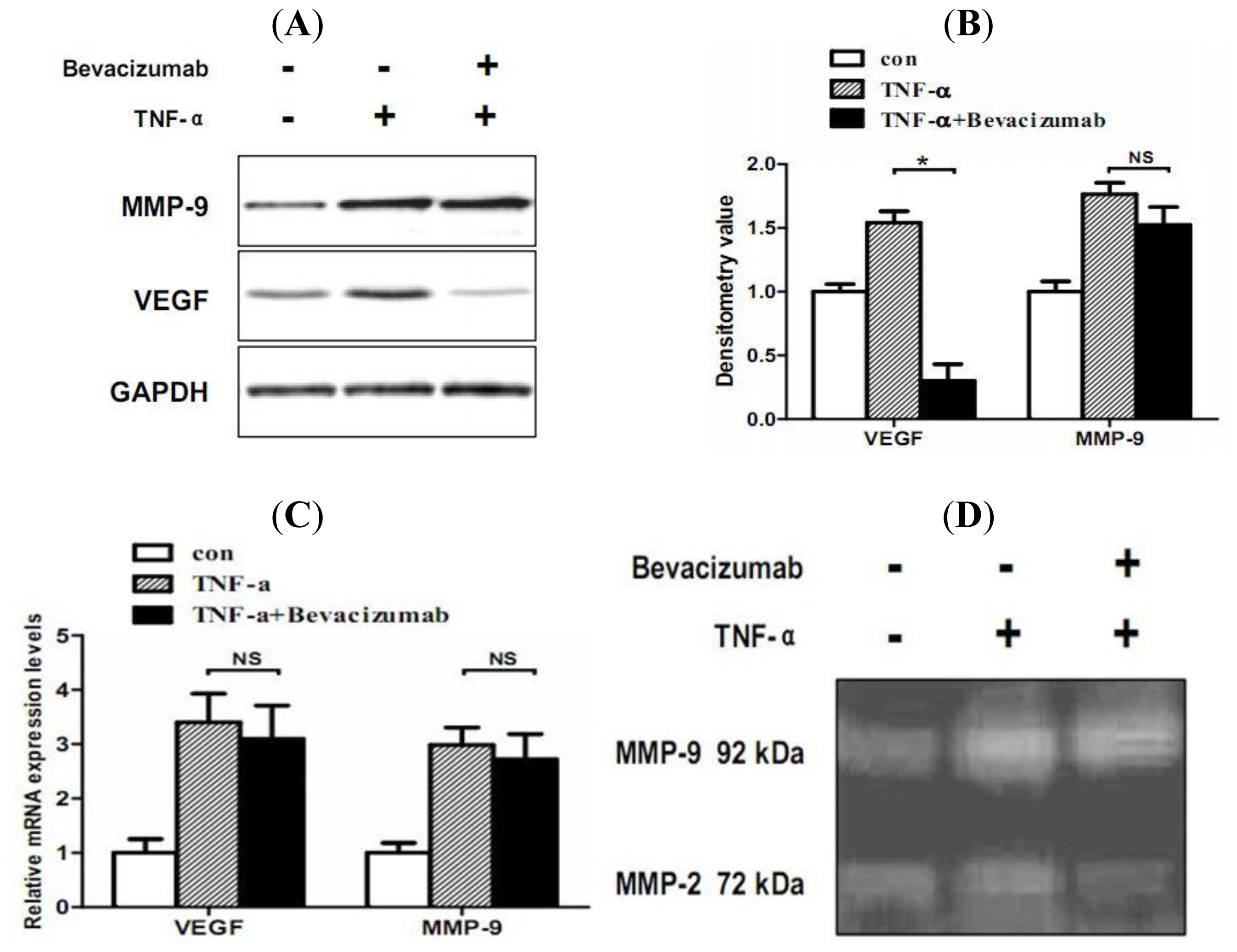
2.5. TNF-α Inhibits Proliferation and Increases Hemorrhage-Associated Genes Expression of MMQ Cells in Vitro
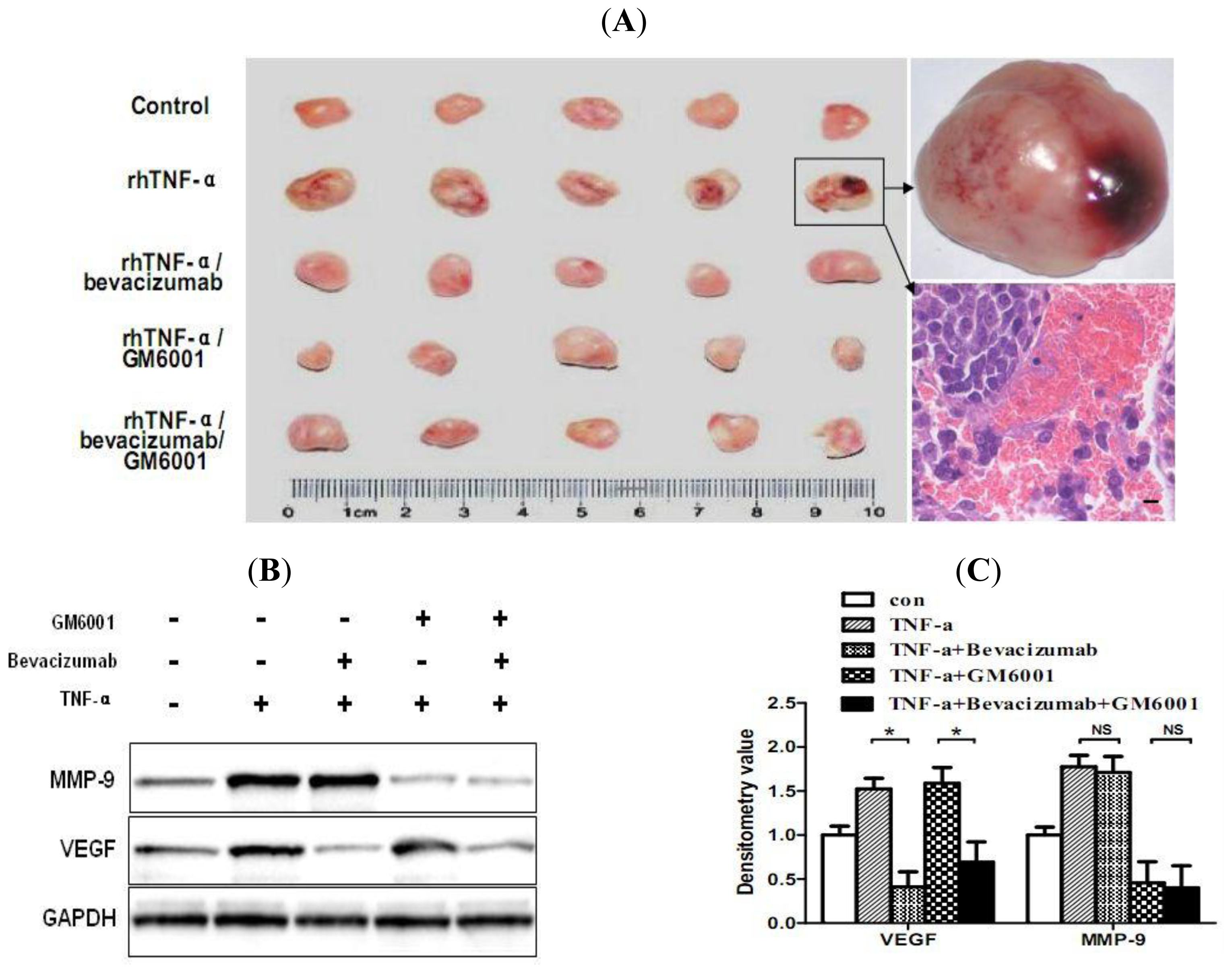
2.6. TNF-α Causes Hemorrhagic Transformation and Increases Hemorrhage-Associated Genes Expression in MMQ Cell Xenografts in Mice
2.7. Discussion
3. Materials and Methods
3.1. Patients and Samples
3.2. Histology and Immunofluorescence
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. Western Blot Analysis
3.6. Zymography
3.7. Real-Time RT-PCR
3.8. Animal Xenografts
3.9. Blood Infusion in Xenograft Tumors
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Reid, RL; Quigley, ME; Yen, SS. Pituitary apoplexy. A review. Arch. Neurol 1985, 42, 712–719. [Google Scholar]
- Nawar, RN; AbdelMannan, D; Selman, WR; Arafah, BM. Pituitary tumor apoplexy: A review. J. Intensive Care Med 2008, 23, 75–90. [Google Scholar]
- Kleinschmidt-DeMasters, BK; Lillehei, KO. Pathological correlates of pituitary adenomas presenting with apoplexy. Hum. Pathol 1998, 29, 1255–1265. [Google Scholar]
- Jayaraman, T; Berenstein, V; Li, X; Mayer, J; Silane, M; Shin, YS; Niimi, Y; Kilic, T; Gunel, M; Berenstein, A. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms. Neurosurgery 2005, 57, 558–564, discussion 558–564. [Google Scholar]
- Achrol, AS; Pawlikowska, L; McCulloch, CE; Poon, KY; Ha, C; Zaroff, JG; Johnston, SC; Lee, C; Lawton, MT; Sidney, S; et al. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke 2006, 37, 231–234. [Google Scholar]
- Jayaraman, T; Paget, A; Shin, YS; Li, X; Mayer, J; Chaudhry, H; Niimi, Y; Silane, M; Berenstein, A. TNF-alpha-mediated inflammation in cerebral aneurysms: A potential link to growth and rupture. Vasc. Health Risk Manag 2008, 4, 805–817. [Google Scholar]
- Hofmann, S; Grasberger, H; Jung, P; Bidlingmaier, M; Vlotides, J; Janssen, OE; Landgraf, R. The tumour necrosis factor-alpha induced vascular permeability is associated with a reduction of VE-cadherin expression. Eur. J. Med. Res 2002, 7, 171–176. [Google Scholar]
- Jung, S; Moon, KS; Jung, TY; Kim, IY; Lee, YH; Rhu, HH; Sun, HS; Jeong, YI; Kim, KK; Kang, SS. Possible pathophysiological role of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in metastatic brain tumor-associated intracerebral hemorrhage. J. Neurooncol 2006, 76, 257–263. [Google Scholar]
- Chen, Y; Pawlikowska, L; Yao, JS; Shen, F; Zhai, W; Achrol, AS; Lawton, MT; Kwok, PY; Yang, GY; Young, WL. Interleukin-6 involvement in brain arteriovenous malformations. Ann. Neurol 2006, 59, 72–80. [Google Scholar]
- Ryuto, M; Ono, M; Izumi, H; Yoshida, S; Weich, HA; Kohno, K; Kuwano, M. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J. Biol. Chem 1996, 271, 28220–28228. [Google Scholar]
- Tsuge, M; Yasui, K; Ichiyawa, T; Saito, Y; Nagaoka, Y; Yashiro, M; Yamashita, N; Morishima, T. Increase of tumor necrosis factor-alpha in the blood induces early activation of matrix metalloproteinase-9 in the brain. Microbiol. Immunol 2010, 54, 417–424. [Google Scholar]
- Nagy, JA; Benjamin, L; Zeng, H; Dvorak, AM; Dvorak, HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008, 11, 109–119. [Google Scholar]
- Cheng, SY; Nagane, M; Huang, HS; Cavenee, WK. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc. Natl. Acad. Sci. USA 1997, 94, 12081–12087. [Google Scholar]
- Ke, LD; Shi, YX; Yung, WK. VEGF(121), VEGF(165) overexpression enhances tumorigenicity in U251 MG but not in NG-1 glioma cells. Cancer Res 2002, 62, 1854–1861. [Google Scholar]
- Abumiya, T; Yokota, C; Kuge, Y; Minematsu, K. Aggravation of hemorrhagic transformation by early intraarterial infusion of low-dose vascular endothelial growth factor after transient focal cerebral ischemia in rats. Brain Res 2005, 1049, 95–103. [Google Scholar]
- Arita, K; Kurisu, K; Tominaga, A; Sugiyama, K; Eguchi, K; Hama, S; Yoshioka, H; Yamasaki, F; Kanou, Y. Relationship between intratumoral hemorrhage and overexpression of vascular endothelial growth factor (VEGF) in pituitary adenoma. Hiroshima J. Med. Sci 2004, 53, 23–27. [Google Scholar]
- Fukui, S; Otani, N; Nawashiro, H; Yano, A; Nomura, N; Tokumaru, AM; Miyazawa, T; Ohnuki, A; Tsuzuki, N; Katoh, H; et al. The association of the expression of vascular endothelial growth factor with the cystic component and haemorrhage in pituitary adenoma. J. Clin. Neurosci 2003, 10, 320–324. [Google Scholar]
- Takada, K; Yamada, S; Teramoto, A. Correlation between tumor vascularity and clinical findings in patients with pituitary adenomas. Endocr. Pathol 2004, 15, 131–139. [Google Scholar]
- Fukui, S; Nawashiro, H; Otani, N; Ooigawa, H; Yano, A; Nomura, N; Tokumaru, AM; Miyazawa, T; Ohnuki, A; Tsuzuki, N; et al. Vascular endothelial growth factor expression in pituitary adenomas. Acta Neurochir. (Suppl) 2003, 86, 519–521. [Google Scholar]
- Reyes, R; Guo, M; Swann, K; Shetgeri, SU; Sprague, SM; Jimenez, DF; Barone, CM; Ding, Y. Role of tumor necrosis factor-alpha and matrix metalloproteinase-9 in blood-brain barrier disruption after peripheral thermal injury in rats. J. Neurosurg 2009, 110, 1218–1226. [Google Scholar]
- McColl, BW; Rose, N; Robson, FH; Rothwell, NJ; Lawrence, CB. Increased brain microvascular MMP-9 and incidence of haemorrhagic transformation in obese mice after experimental stroke. J. Cereb. Blood Flow Metab 2010, 30, 267–272. [Google Scholar]
- Ballabh, P. Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatr. Res 2010, 67, 1–8. [Google Scholar]
- Zhang, X; Qi, C; Lin, J. Enhanced expressions of matrix metalloproteinase (MMP)-2 and -9 and vascular endothelial growth factors (VEGF) and increased microvascular density in the endometrial hyperplasia of women with anovulatory dysfunctional uterine bleeding. Fertil. Steril 2010, 93, 2362–2367. [Google Scholar]
- Mou, C; Han, T; Zhao, H; Wang, S; Qu, Y. Clinical features and immunohistochemical changes of pituitary apoplexy. J. Clin. Neurosci 2009, 16, 64–68. [Google Scholar]
- Lee, CZ; Xue, Z; Zhu, Y; Yang, GY; Young, WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke 2007, 38, 2563–2568. [Google Scholar]
- Green, VL; Atkin, SL; Speirs, V; Jeffreys, RV; Landolt, AM; Mathew, B; Hipkin, L; White, MC. Cytokine expression in human anterior pituitary adenomas. Clin. Endocrinol. (Oxf.) 1996, 45, 179–185. [Google Scholar]
- Candolfi, M; Jaita, G; Pisera, D; Ferrari, L; Barcia, C; Liu, C; Yu, J; Liu, G; Castro, MG; Seilicovich, A. Adenoviral vectors encoding tumor necrosis factor-alpha and FasL induce apoptosis of normal and tumoral anterior pituitary cells. J. Endocrinol 2006, 189, 681–690. [Google Scholar]
- Pawlikowska, L; Tran, MN; Achrol, AS; McCulloch, CE; Ha, C; Lind, DL; Hashimoto, T; Zaroff, J; Lawton, MT; Marchuk, DA; et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke 2004, 35, 2294–2300. [Google Scholar]
- Ishimoto, O; Saijo, Y; Narumi, K; Kimura, Y; Ebina, M; Matsubara, N; Asou, N; Nakai, Y; Nukiwa, T. High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology 2002, 63, 70–75. [Google Scholar]
- Aiello, LP; Avery, RL; Arrigg, PG; Keyt, BA; Jampel, HD; Shah, ST; Pasquale, LR; Thieme, H; Iwamoto, MA; Park, JE; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med 1994, 331, 1480–1487. [Google Scholar]
- Kovacs, Z; Ikezaki, K; Samoto, K; Inamura, T; Fukui, M. VEGF and flt. Expression time kinetics in rat brain infarct. Stroke 1996, 27, 1865–1872, discussion 1872–1873. [Google Scholar]
- Wizigmann-Voos, S; Plate, KH. Pathology, genetics and cell biology of hemangioblastomas. Histol. Histopathol 1996, 11, 1049–1061. [Google Scholar]
- Polavarapu, R; Gongora, MC; Winkles, JA; Yepes, M. Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappa B pathway activation. J. Neurosci 2005, 25, 10094–10100. [Google Scholar]
- Power, C; Henry, S; Del, BMR; Larsen, PH; Corbett, D; Imai, Y; Yong, VW; Peeling, J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann. Neurol 2003, 53, 731–742. [Google Scholar]
- Belayev, L; Saul, I; Curbelo, K; Busto, R; Belayev, A; Zhang, Y; Riyamongkol, P; Zhao, W; Ginsberg, MD. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke 2003, 34, 2221–2227. [Google Scholar]
- Liu, ZH; Chang, CN; Pai, PC; Wei, KC; Jung, SM; Chen, NY; Chuang, CC. Clinical features and surgical outcome of clinical and subclinical pituitary apoplexy. J. Clin. Neurosci 2010, 17, 694–699. [Google Scholar]
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xiao, Z.; Liu, Q.; Mao, F.; Wu, J.; Lei, T. TNF-α-Induced VEGF and MMP-9 Expression Promotes Hemorrhagic Transformation in Pituitary Adenomas. Int. J. Mol. Sci. 2011, 12, 4165-4179. https://doi.org/10.3390/ijms12064165
Xiao Z, Liu Q, Mao F, Wu J, Lei T. TNF-α-Induced VEGF and MMP-9 Expression Promotes Hemorrhagic Transformation in Pituitary Adenomas. International Journal of Molecular Sciences. 2011; 12(6):4165-4179. https://doi.org/10.3390/ijms12064165
Chicago/Turabian StyleXiao, Zhengzheng, Qin Liu, Feng Mao, Jun Wu, and Ting Lei. 2011. "TNF-α-Induced VEGF and MMP-9 Expression Promotes Hemorrhagic Transformation in Pituitary Adenomas" International Journal of Molecular Sciences 12, no. 6: 4165-4179. https://doi.org/10.3390/ijms12064165



