Metathesis of Fatty Acid Ester Derivatives in 1,1-Dialkyl and 1,2,3-Trialkyl Imidazolium Type Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
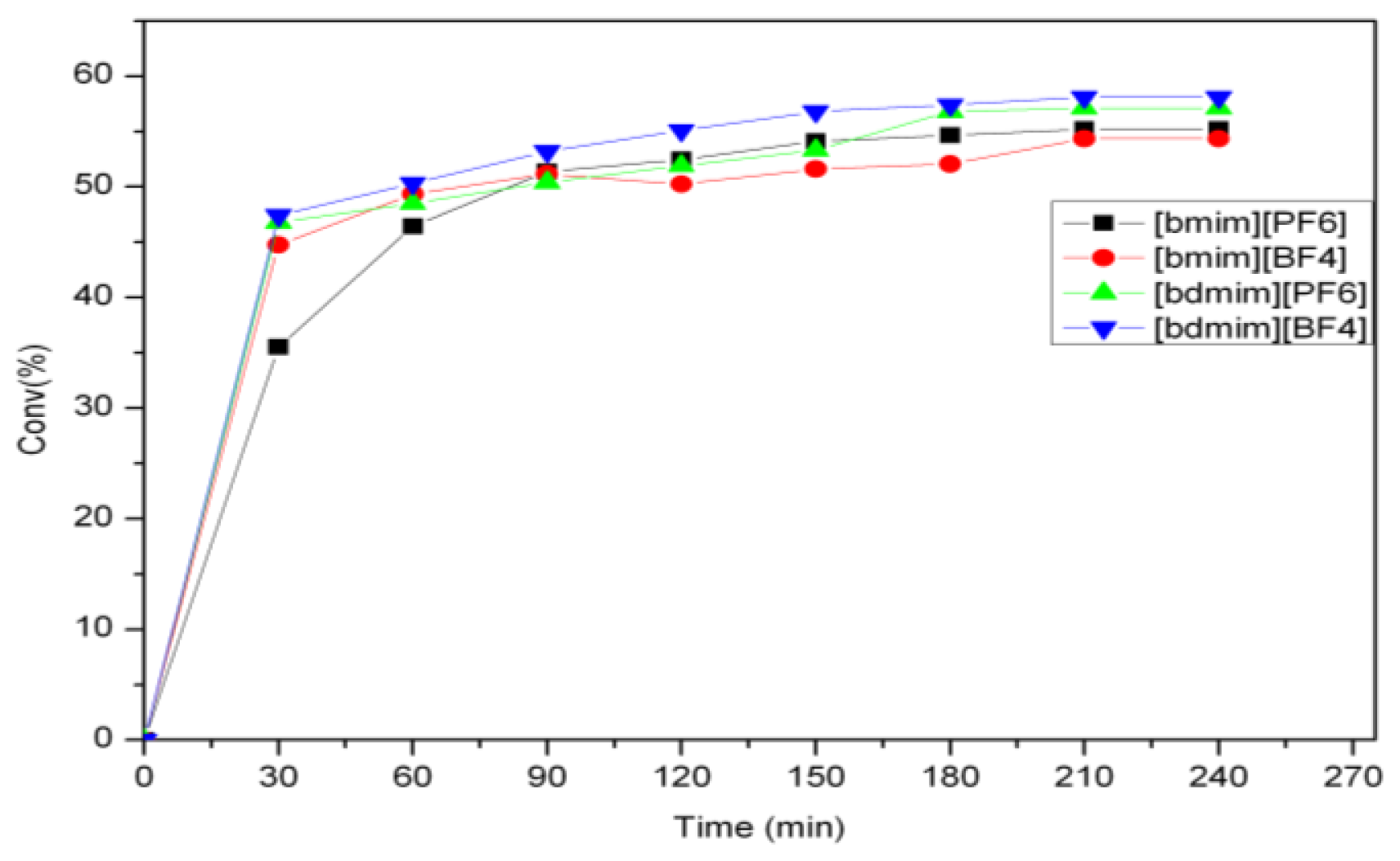
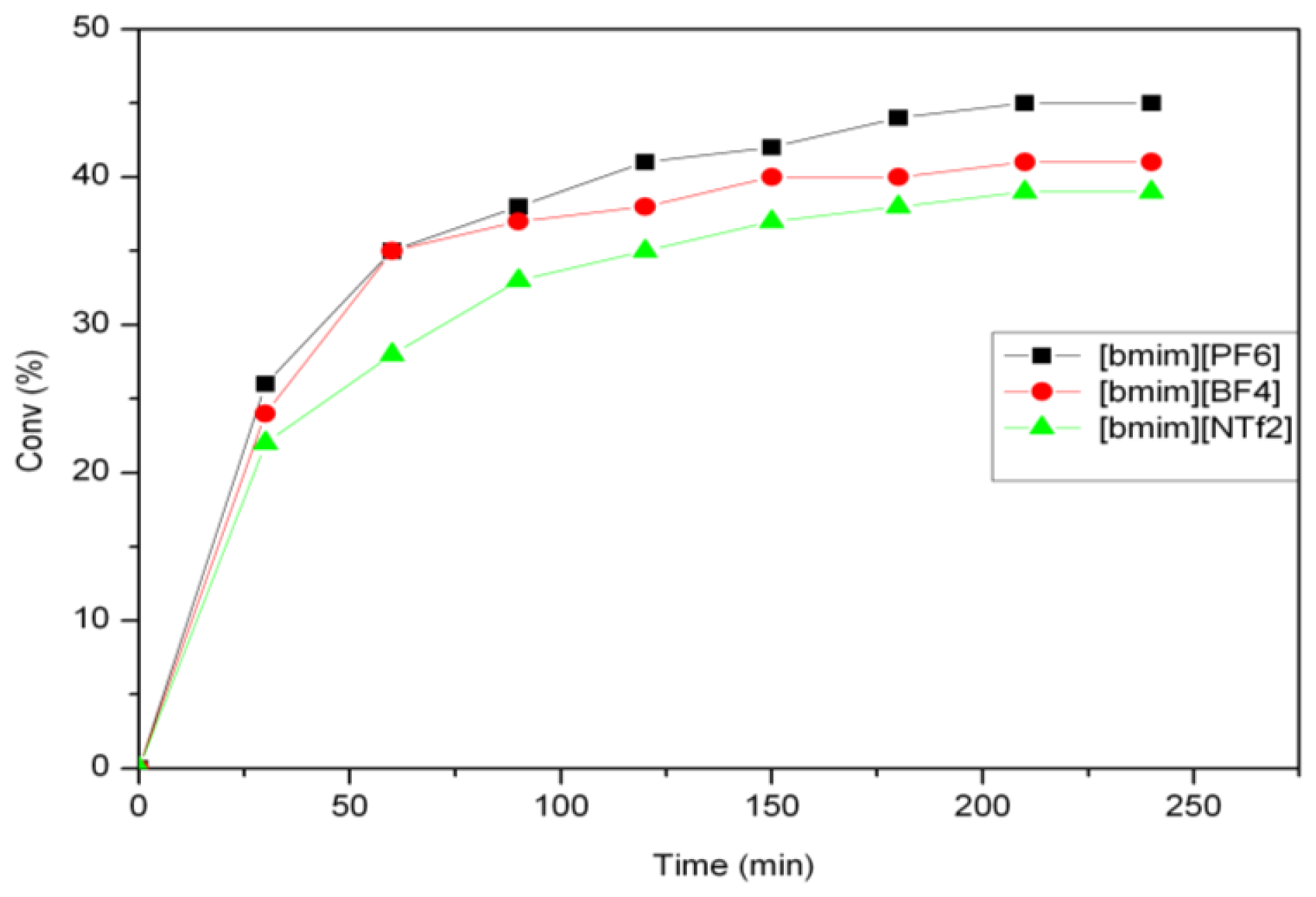
2.1. Self-Metathesis of Methyl Oleate
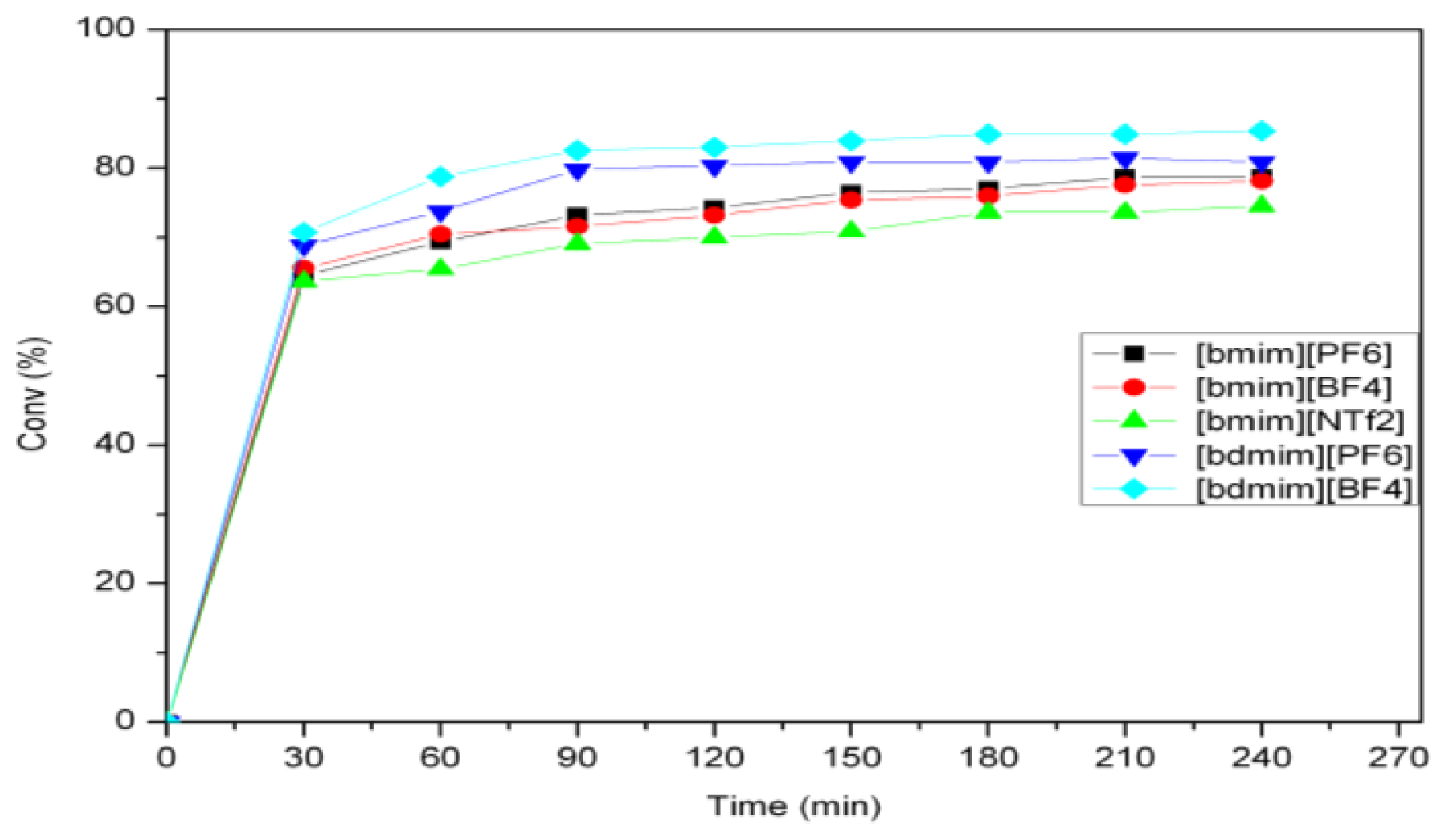
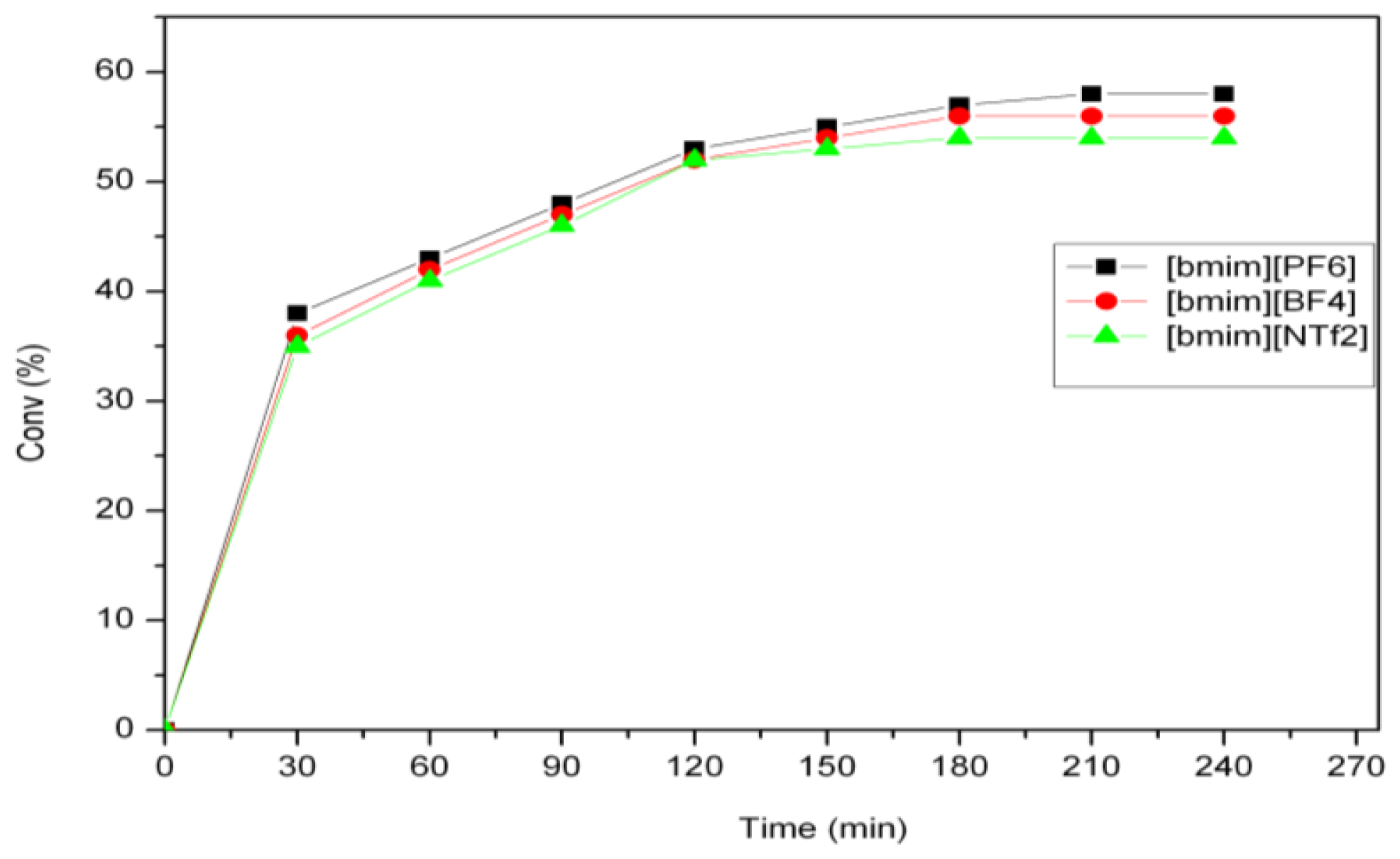
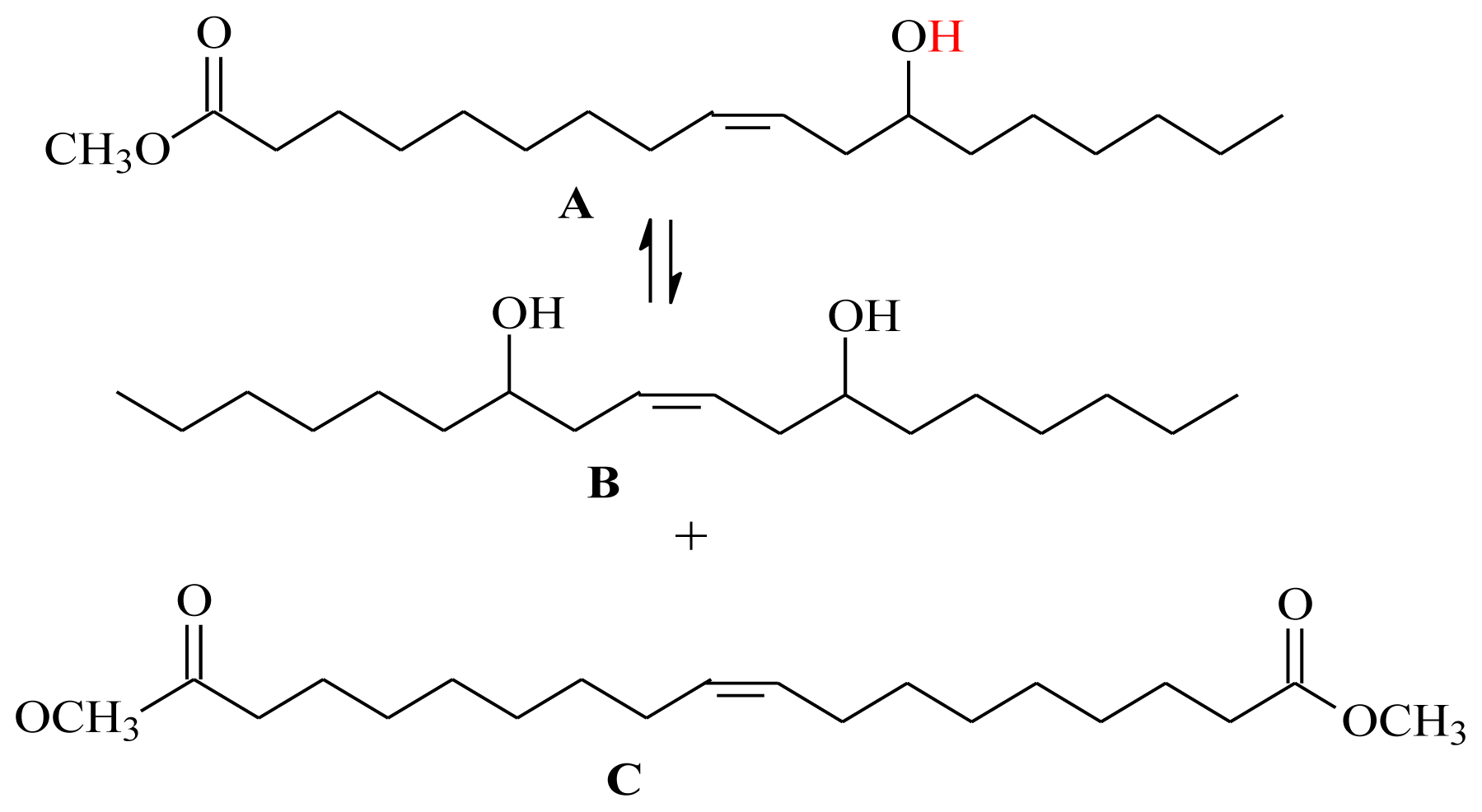
2.2. Self-Metathesis of Methyl Ricinoleate
2.3. Catalyst Recycling in Ionic Liquids
3. Experimental Section
3.1. Materials and Apparatus
3.2. Metathesis Experiments
4. Conclusions
References
- Olivier-Bourbigou, H; Magna, L. Ionic liquids: Perspectives for organic and catalytic reactions. J. Mol. Catal. A: Chem 2002, 182, 419–437. [Google Scholar]
- Sledz, P; Mauduit, M; Grela, K. Olefin metathesis in ionic liquids. Chem. Soc. Rev 2008, 37, 2433–2442. [Google Scholar]
- Buijsman, RC; van Vuuren, E; Sterrenburg, JG. Ruthenium-catalyzed olefin metathesis in ionic liquids. Org. Lett 2001, 3, 3785–3787. [Google Scholar]
- Csihony, S; Fischmeister, C; Bruneau, C; Horvath, IT; Dixneuf, PH. First ring-opening metathesis polymerization in an ionic liquid. Efficient recycling of a catalyst generated from a cationic ruthenium allenylidene complex. New J. Chem 2002, 26, 1667–1670. [Google Scholar]
- Semeril, D; Olivier-Bourbigou, H; Bruneau, C; Dixneuf, PH. Alkene metathesis catalysis in ionic liquids with ruthenium allenylidene salts. Chem. Commun 146–147.
- Mayo, KG; Nearhoof, EH; Kiddle, JJ. Microwave accelerated ruthenium catalyzed olefin metathesis. Org. Lett 2002, 4, 1567–1570. [Google Scholar]
- Ding, X; Lu, X; Hui, B; Chen, Z; Xiao, M; Guo, B; Tang, W. Olefin self-cross-metathesis catalyzed by the second generation Grubbs carbene complex in room temperature ionic liquids. Tetrahedron Lett 2006, 47, 2921–2924. [Google Scholar]
- Williams, DBG; Ajam, M; Ranwell, A. Highly selective metathesis of 1-octene in ionic liquids. Organometallics 2006, 25, 3088–3090. [Google Scholar]
- Audic, N; Clavier, H; Mauduit, M; Guillemin, JC. An ionic liquid-supported ruthenium carbene complexe: A robust and recyclable catalyst for ring-closing olefin metathesis in ionic liquids. J. Am. Chem. Soc 2003, 125, 9248–9249. [Google Scholar]
- Clavier, H; Audic, N; Mauduit, M; Guillemin, JC. Ring-closing metathesis in biphasic BMI.PF6 ionic liquid/toluene medium: A powerful recyclable and environmentally friendly process. Chem. Commun 2282–2283.
- Yao, Q; Sheets, M. An ionic-liquid tagged second generation Hoveyda-Grubbs ruthenium carbene complex as highly reactive and recyclable catalyst for ring-closing metathesis of di-, triand tetrasubstituted dienes. Cheminform 2005, 36. [Google Scholar] [CrossRef]
- Liu, G; Zhang, J; Wu, B; Wang, J. Carborane as a tunable tag for Ru catalysts: Generating an anion appended recyclable and robust catalyst suitable for the non-covalent binding concept. Org. Lett 2007, 9, 4263–4266. [Google Scholar]
- Thurier, C; Fischmeister, C; Bruneau, C; Olivier-Bourbigou, H; Dixneuf, PH. Ionic imidazolium containing ruthenium complexes and olefin metathesis in ionic liquids. J. Mol. Catal. A Chem 2007, 268, 127–133. [Google Scholar]
- Rix, D; Clavier, H; Coutard, Y; Gulajski, L; Grela, K; Mauduit, M. Activated pyridiniumtagged ruthenium complexes as efficient catalysts for ring-closing metathesis. J. Organomet. Chem 2006, 691, 5397–5405. [Google Scholar]
- Thomas, PA; Marvey, BB. C18:1 methyl ester metathesis in [bmim][X] type ionic liquids. Int. J. Mol. Sci 2009, 10, 5020–5030. [Google Scholar]
- Thurier, C; Fischmeister, C; Bruneau, C; Olivier-Bourbigou, H; Dixneuf, PH. Ethenolysis of methyl oleate in room temperature ionic liquids. ChemSusChem 2008, 1, 118–122. [Google Scholar]
- Marvey, BB; Segakweng, CK; Vosloo, HCM. Ruthenium carbene mediated metathesis of oleate-type fatty compounds. Int. J. Mol. Sci 2008, 9, 615–625. [Google Scholar]
- Sanford, MS; Love, JA; Grubbs, RH. Mechanism and activity of ruthenium olefin metathesis catalysts. J. Am. Chem. Soc 2001, 123, 6543–6554. [Google Scholar]
- Hoveyda, AH; Gillingham, DG; Van Veldhuizen, JJ; Kataoka, O; Garber, SB; Kingsbury, JS; Harrity, JPA. Ru complexes bearing bidentate carbenes: From innocent curiosity to uniquely effective catalysts for olefin metathesis. Org. Biomol. Chem 2004, 2, 8–23. [Google Scholar]
- Thurier, C; Fischmeister, C; Bruneau, C; Olivier-Bourbigou, H; Dixneuf, PH. Ethenolysis of methyl oleate in room-temperature ionic liquids. ChemSusChem 2008, 1, 118–122. [Google Scholar]
- Segakweng, CK. Olefin metathesis in oleochemistry-application of ruthenium catalysts to vegetable oil-derived olefins. MS Thesis, July 2007; pp. 66–67. [Google Scholar]
- Buchowicz, W; Mol, J. Catalytic activity and selectivity of Ru(=CHPh)Cl2(PCy3)2 in the metathesis of linear olefins. J. Mol. Catal. A Chem 1999, 148, 97–103. [Google Scholar]
- Hsu, J; Yen, Y; Chu, Y. Baylis-Hillman reaction in [bdmim][PF6] ionic liquid. Tetrahedron Lett 2004, 45, 4673–4676. [Google Scholar]
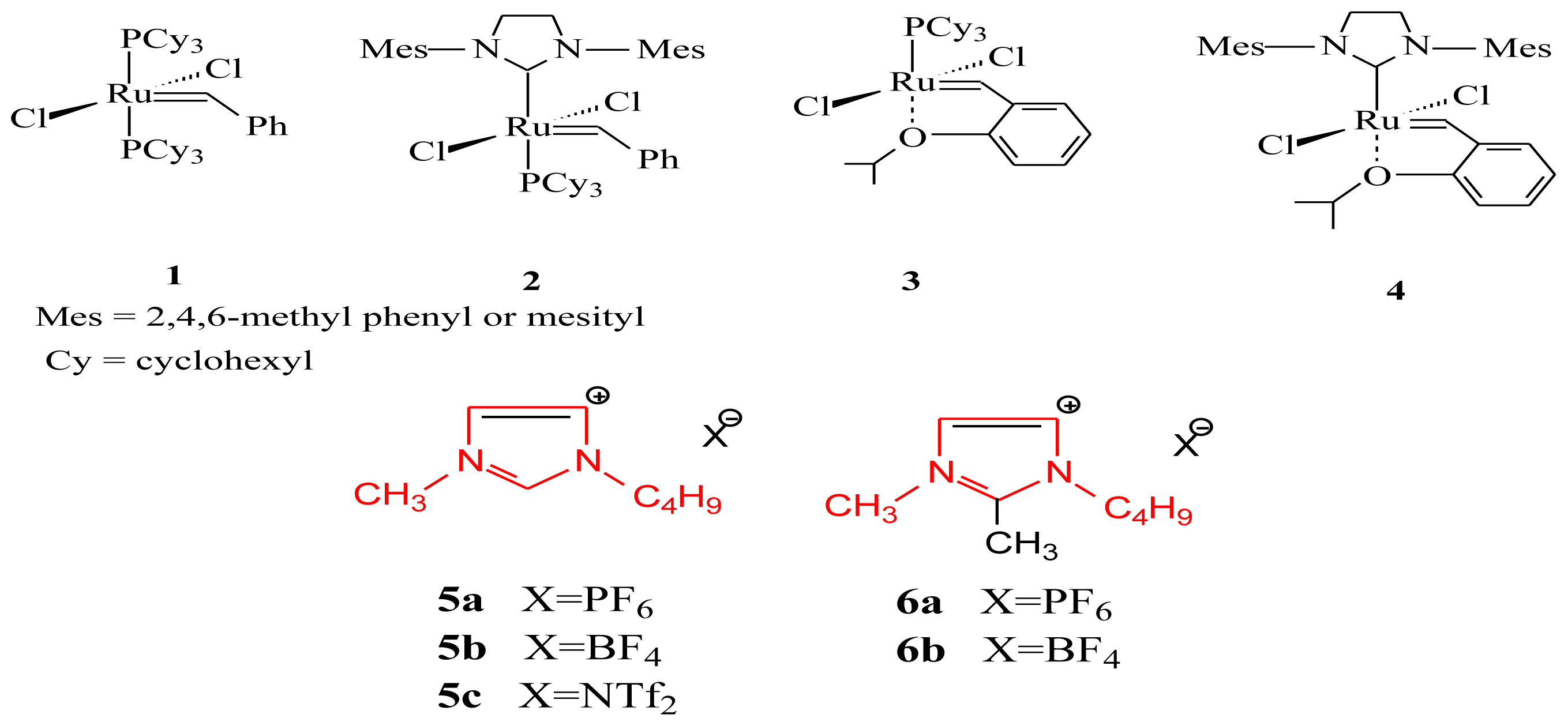
| RTIL | Catalyst | Temperature (°C) | Conversion (%) | Selectivity a (%) |
|---|---|---|---|---|
| 5a | 1 | 40 | 57 | 99 |
| 2 | 65 | 97 | ||
| 3 | 55 | 99 | ||
| 4 | 78 | 96 | ||
| 1 | 60 | 59 | 99 | |
| 2 | 75 | 94 | ||
| 3 | 57 | 98 | ||
| 4 | 87 | 93 | ||
| 5b | 1 | 40 | 58 | 99 |
| 2 | 67 | 96 | ||
| 3 | 56 | 99 | ||
| 4 | 79 | 96 | ||
| 1 | 60 | 61 | 99 | |
| 2 | 78 | 94 | ||
| 3 | 59 | 97 | ||
| 4 | 87 | 95 | ||
| 5c | 1 | 40 | 57 | 99 |
| 2 | 64 | 96 | ||
| 3 | 54 | 99 | ||
| 4 | 74 | 97 | ||
| 1 | 60 | 60 | 99 | |
| 2 | 73 | 96 | ||
| 3 | 59 | 99 | ||
| 4 | 85 | 95 | ||
| 6a | 3 | 40 | 57 | 99 |
| 4 | 81 | 98 | ||
| 3 | 60 | 60 | 97 | |
| 4 | 90 | 95 | ||
| 6b | 3 | 40 | 58 | 99 |
| 4 | 85 | 99 | ||
| 3 | 60 | 60 | 97 | |
| 4 | 40 | 92 | 95 |
| RTIL | Catalyst | Temperature (°C) | Conversion (%) | Selectivity a (%) |
|---|---|---|---|---|
| 5a | 1 | 60 | 45 | 99 |
| 2 | 58 | 99 | ||
| 5b | 1 | 60 | 41 | 99 |
| 2 | 56 | 99 | ||
| 5c | 1 | 60 | 38 | 99 |
| 2 | 60 | 55 | 99 |
| Catalyst | Conversion (%) | Selectivity (%) |
|---|---|---|
| 1 | 1st run: 57 (58) | 99 |
| 2nd run: 56 (56) | 99 | |
| 3rd run: 42 (45) | 98 | |
| 2 | 1st run: 67 (72) | 99 |
| 2nd run:50 (60) | 97 | |
| 3rd run:45 (57) | 97 | |
| 3 | 1st run: 56 (58) | 99 |
| 2nd run: 56 (58) | 99 | |
| 3rd run: 49 (53) | 99 | |
| 4th run: 22 (30) | 99 | |
| 4 | 1st run: 74 (85) | 99 |
| 2nd run: 40 (54) | 95 | |
| 3rd run: 15 (23) | 95 |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thomas, P.A.; Marvey, B.B.; Ebenso, E.E. Metathesis of Fatty Acid Ester Derivatives in 1,1-Dialkyl and 1,2,3-Trialkyl Imidazolium Type Ionic Liquids. Int. J. Mol. Sci. 2011, 12, 3989-3997. https://doi.org/10.3390/ijms12063989
Thomas PA, Marvey BB, Ebenso EE. Metathesis of Fatty Acid Ester Derivatives in 1,1-Dialkyl and 1,2,3-Trialkyl Imidazolium Type Ionic Liquids. International Journal of Molecular Sciences. 2011; 12(6):3989-3997. https://doi.org/10.3390/ijms12063989
Chicago/Turabian StyleThomas, Priya A., Bassie B. Marvey, and Eno E. Ebenso. 2011. "Metathesis of Fatty Acid Ester Derivatives in 1,1-Dialkyl and 1,2,3-Trialkyl Imidazolium Type Ionic Liquids" International Journal of Molecular Sciences 12, no. 6: 3989-3997. https://doi.org/10.3390/ijms12063989




