Chemometric Analysis of the Amino Acid Requirements of Antioxidant Food Protein Hydrolysates
Abstract
:1. Introduction
2. Results and Discussions
3. Experimental Section
3.1. X-matrix
3.2. Y-matrix
3.3. PLS Modeling
4. Conclusions
Supplementary Information
ijms-12-03148-s001.pdfAcknowledgments
References
- Elias, RJ; Kellerby, SS; Decker, EA. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr 2008, 48, 430–441. [Google Scholar]
- Udenigwe, CC; Lu, YL; Han, CH; Hou, WC; Aluko, RE. Flaxseed protein-derived peptide fractions: Antioxidant properties and inhibition of lipopolysaccharide-induced nitric oxide production in murine macrophages. Food Chem 2009, 116, 277–284. [Google Scholar]
- Prior, RL; Wu, X; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem 2005, 53, 4290–4302. [Google Scholar]
- Huang, D; Ou, B; Prior, RL. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem 2005, 53, 1841–1856. [Google Scholar]
- Pownall, TL; Udenigwe, CC; Aluko, RE. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem 2010, 58, 4712–4718. [Google Scholar]
- Erdmann, K; Grosser, N; Schipporeit, K; Schroder, H. The ACE inhibitory dipeptide Met-Tyr diminishes free radical formation in human endothelial cells via induction of heme oxygenase-1 and ferritin. J. Nutr 2006, 136, 2148–2152. [Google Scholar]
- Hernández-Ledesma, B; Dávalos, A; Bartolomé, B; Amigo, L. Preparation of antioxidant enzymatic hydrolysates from -lactalbumin and -lactoglobulin. Identification of active peptides by HPLC-MS/MS. J. Agric. Food Chem 2005, 53, 588–593. [Google Scholar]
- Chen, HM; Muramoto, K; Yamauchi, F; Fujimoto, K; Nokihara, K. Antioxidant properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem 1998, 46, 49–53. [Google Scholar]
- Chen, HM; Muramoto, K; Yamauchi, F; Nokihara, K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem 1996, 44, 2619–2623. [Google Scholar]
- Saito, K; Jin, DH; Ogawa, T; Muramoto, K; Hatakeyama, E; Yasuhara, T; Nokihara, K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J. Agric. Food Chem 2003, 51, 3668–3674. [Google Scholar]
- Erdmann, K; Cheung, BWY; Schroder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem 2008, 19, 643–654. [Google Scholar]
- Kitts, DD; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des 2003, 9, 1309–1323. [Google Scholar]
- Wold, S; Sjöström, M; Eriksson, L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst 2001, 58, 109–130. [Google Scholar]
- Pripp, AH; Isaksson, T; Stepaniak, L; Sørhaug, T; Ardö, Y. Quantitative structure-activity relationship modelling of peptides and proteins as a tool in food science. Trends Food Sci. Technol 2005, 16, 484–494. [Google Scholar]
- Kim, HO; Li-Chan, ECY. Quantitative structure-activity relationship study of bitter peptides. J. Agric. Food Chem 2006, 54, 10102–10111. [Google Scholar]
- Wu, J; Aluko, RE. Quantitative structure-activity relationship study of bitter di- and tri-peptides with angiotensin I-converting enzyme inhibitory activity. J. Pept. Sci 2007, 13, 63–69. [Google Scholar]
- Wu, J; Aluko, RE; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: quantitative structure-activity relationship study of di- and tripeptides. J. Agric. Food Chem 2006, 54, 732–738. [Google Scholar]
- Wu, J; Aluko, RE; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: quantitative structure-activity relationship modelling of peptides containing 4–10 amino acid residues. QSAR Comb. Sci 2006, 25, 873–880. [Google Scholar]
- Yang, L; Shu, M; Ma, K; Mei, H; Jiang, Y; Li, Z. ST-scale as a novel amino acid descriptor and its application in QSAM of peptides and analogues. Amino Acids 2010, 38, 805–816. [Google Scholar]
- Udenigwe, CC; Aluko, RE. Quantitative structure–activity relationship modeling of renin-inhibiting dipeptides. Amino Acids 2011. [Google Scholar] [CrossRef]
- Siebert, KJ. Modeling protein functional properties from amino acid composition. J. Agric. Food Chem 2003, 51, 7792–7797. [Google Scholar]
- Khalebnikov, AI; Schepetkin, IA; Domina, NG; Kirpotina, LN; Quinn, MT. Improved quantitative structure-activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic, and cellular systems. Bioorg. Med. Chem 2007, 15, 1749–1770. [Google Scholar]
- Om, A; Kim, JH. A quantitative structure-activity relationship model for radical scavenging activity of flavonoids. J. Med. Food 2008, 11, 29–37. [Google Scholar]
- Roy, K; Mitra, I. Advances in quantitative structure-activity relationship models of antioxidants. Expert Opin. Drug Discov 2009, 4, 1157–1175. [Google Scholar]
- Van der Voet, H. Comparing the predictive accuracy of models using a simple randomization test. Chemom. Intell. Lab. Syst 1994, 25, 313–323. [Google Scholar]
- SIMCA-P 11 Software Analysis Advisor, version or edition; Umetrics AB: Umeå, Sweden, 2005.
- Sandberg, M; Eriksson, L; Jonsson, J; Sjöström, M; Wold, S. New chemical descriptors relevant for the design of biologically active peptides. A multivariate characterization of 87 amino acids. J. Med. Chem 1998, 41, 2181–2491. [Google Scholar]
- Davies, MJ. The oxidative environment and protein damage. Biochim. Biophys. Acta 2005, 1703, 93–109. [Google Scholar]
- Girgih, AT; Udenigwe, CC; Aluko, RE. In vitro antioxidant properties of hempseed (Cannabis sativa L.) protein hydrolysate fractions. J. Am. Oil Chem. Soc 2011, 88, 381–389. [Google Scholar]
- Pownall, TL; Udenigwe, CC; Aluko, RE. Effects of cationic property on the in vitro antioxidant properties of pea protein hydrolysate fractions. Food Res. Int 2011, 44, 1069–1074. [Google Scholar]
- Hellberg, S; Sjöström, M; Skagerberg, B; Wold, SJ. Peptide quantitative structure-activity relationships, a multivariate approach. J. Med. Chem 1987, 30, 1126–1135. [Google Scholar]
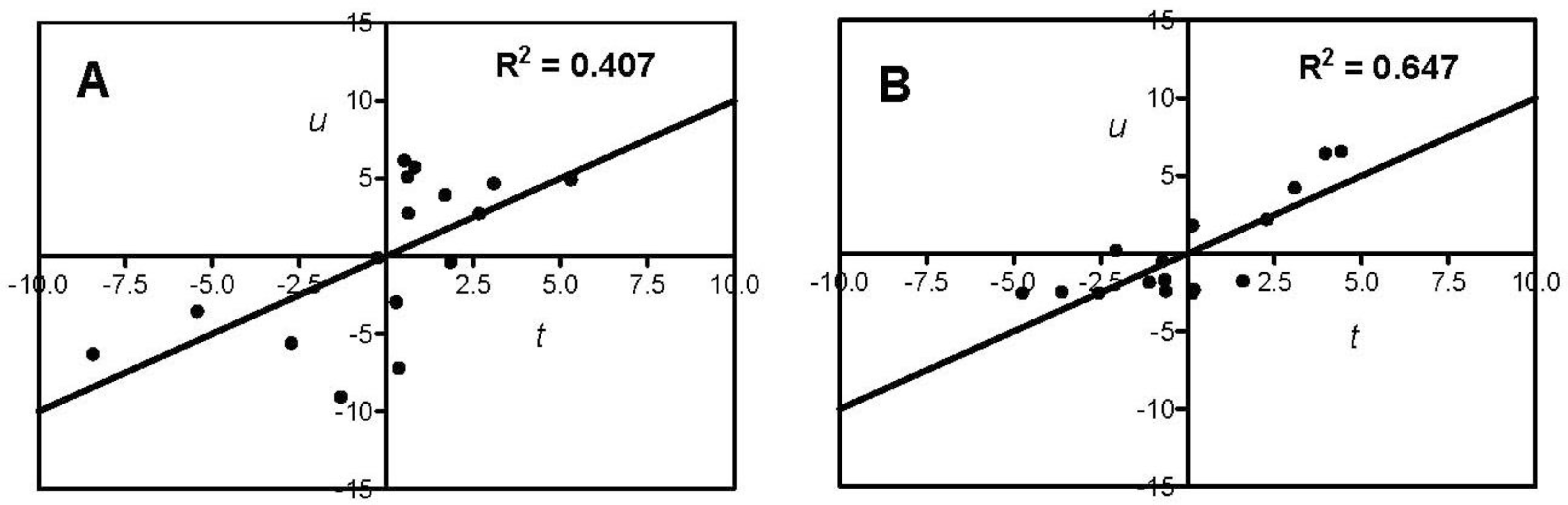
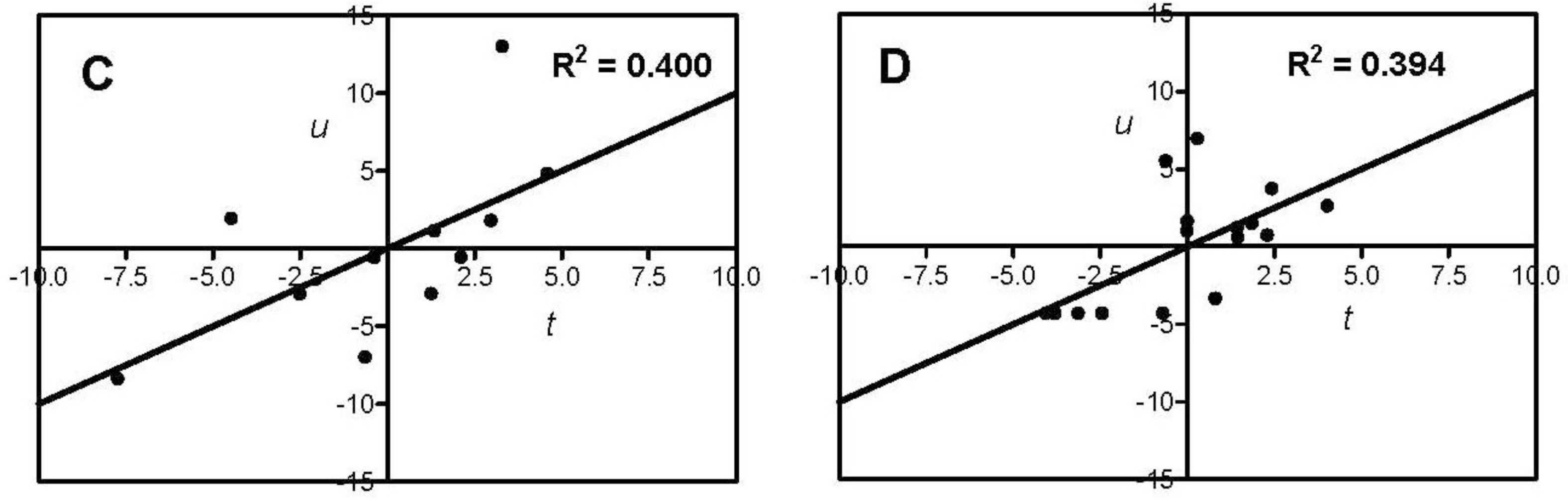


| Antioxidant Property | N a | X b | A c | R2 d | Q2cv e | Permutation Test f | |
|---|---|---|---|---|---|---|---|
| Int. R2cum | Int. Q2cv | ||||||
| DPPH-scavenging | 16 | AA + gAA+ Σzi | 1 | 0.407 | 0.327 | 0.241 | −0.096 |
| AA | 1 | 0.448 | 0.358 | 0.288 | −0.108 | ||
| gAA | 1 | 0.405 | 0.231 | 0.149 | −0.043 | ||
| Σzi | 1 | 0.304 | 0.288 | 0.006 | −0.127 | ||
| Ferric reducing | 16 | AA + gAA + Σzi | 1 | 0.647 | 0.604 | 0.166 | −0.208 |
| AA | 1 | 0.728 | 0.668 | 0.223 | −0.219 | ||
| gAA | 1 | 0.536 | 0.531 | 0.073 | −0.169 | ||
| H2O2-scavenging | 11 | AA + gAA+ Σzi | 1 | 0.400 | 0.137 | 0.261 | −0.122 |
| AA | 1 | 0.467 | 0.136 | 0.314 | −0.109 | ||
| Σzi | 1 | 0.340 | 0.232 | 0.088 | −0.008 | ||
| O2−-scavenging | 16 | AA + gAA + Σzi | 1 | 0.394 | 0.179 | 0.212 | −0.063 |
| gAA | 2 | 0.602 | 0.142 | 0.166 | −0.223 | ||
| Oxidative System | Positive Contributors | Negative Contributors |
|---|---|---|
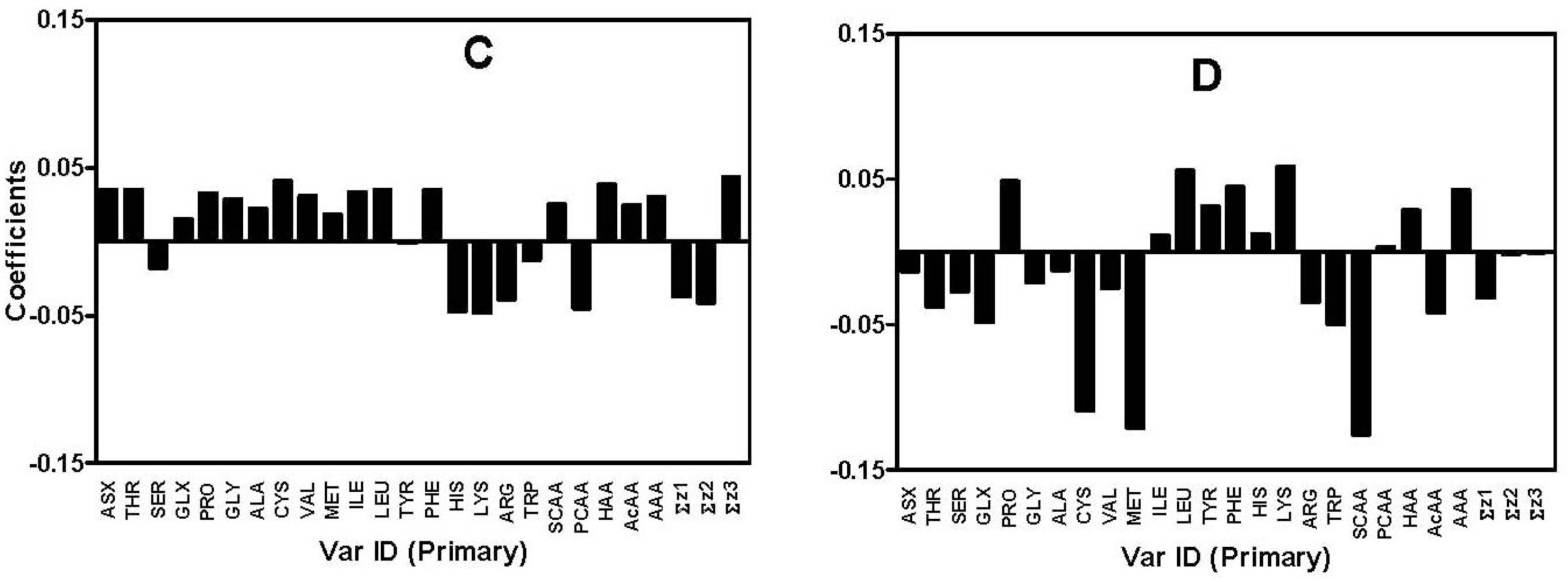
| DPPH radical scavenging | Asp + Asn (Asx), Thr, Val, Ile, HAA a, ↑Σz3 b, ↓Σz1 c, ↓Σz2d (Ala, Met, Leu, Phe, SCAA e, AcAA f, AAA g) | His, Lys, Arg, PCAA h |
| Ferric reducing | Cys, Met, Glu + Gln (Glx), SCAA, AcAA (Asp + Asp, Thr, Gly, ↑Σz3) | Lys (Ser, Leu, Tyr, Phe, His, PCAA, AAA) |
| H2O2-scavenging | Cys, Phe, Leu, Ile, Pro, Thr, Asx, HAA, ↑Σz3, ↓Σz1, ↓Σz2 (Gly, Ala, Val, Met, SCAA, AcAA, AAA) | His, Lys, Arg, PCAA (Ser) |
| Superoxide radical scavenging | Lys, Leu (Pro, Phe, Tyr, HAA, ↓Σz1) | SCAA, Met, Cys (Trp, Glx, AcAA, Thr, Arg, Ser) |
| Sample ID | X-variables (AA) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASX a | THR | SER | GLX b | PRO | GLY | ALA | CYS | VAL | MET | ILE | LEU | TYR | PHE | HIS | LYS | ARG | TRP | |
| 1 | 11.39 | 3.68 | 4.63 | 20.06 | 4.00 | 4.29 | 4.47 | 1.32 | 4.66 | 1.81 | 3.84 | 6.75 | 3.45 | 4.60 | 2.78 | 2.97 | 14.07 | 1.23 |
| 2 | 9.49 | 3.60 | 4.73 | 15.18 | 3.19 | 3.23 | 4.91 | 0.29 | 5.67 | 1.94 | 4.15 | 9.91 | 4.78 | 7.68 | 2.61 | 3.19 | 13.87 | 1.58 |
| 3 | 11.70 | 3.77 | 4.79 | 19.31 | 4.04 | 3.93 | 4.77 | 0.66 | 5.26 | 2.03 | 4.16 | 7.26 | 3.50 | 5.01 | 2.47 | 2.94 | 12.96 | 1.44 |
| 4 | 12.79 | 4.01 | 4.69 | 22.71 | 4.23 | 4.54 | 4.30 | 1.26 | 4.45 | 1.85 | 3.98 | 5.15 | 3.06 | 3.21 | 2.47 | 2.56 | 13.60 | 1.16 |
| 5 | 12.70 | 4.00 | 4.47 | 22.87 | 4.89 | 4.71 | 4.12 | 1.58 | 4.24 | 1.80 | 3.90 | 4.82 | 3.62 | 2.85 | 2.49 | 2.51 | 13.31 | 1.11 |
| 6 | 13.79 | 3.60 | 6.20 | 13.92 | 5.15 | 3.76 | 5.01 | 0.24 | 5.63 | 0.91 | 5.43 | 9.91 | 3.87 | 7.41 | 1.61 | 6.10 | 6.83 | 0.68 |
| 7 | 13.94 | 3.89 | 6.63 | 17.12 | 2.33 | 3.52 | 5.54 | 0.18 | 5.23 | 0.70 | 4.13 | 8.70 | 2.77 | 3.97 | 2.49 | 9.07 | 9.79 | 0.00 |
| 8 | 10.63 | 3.86 | 5.71 | 14.78 | 6.47 | 5.00 | 4.30 | 0.39 | 4.45 | 1.70 | 4.04 | 6.68 | 5.33 | 7.76 | 3.28 | 7.35 | 8.00 | 0.27 |
| 9 | 12.59 | 3.34 | 6.19 | 13.75 | 5.14 | 3.96 | 5.03 | 0.39 | 4.13 | 0.87 | 6.71 | 9.95 | 7.15 | 8.73 | 1.90 | 4.26 | 5.15 | 0.74 |
| 10 | 10.85 | 3.11 | 4.41 | 12.87 | 5.42 | 4.66 | 3.44 | 0.38 | 5.82 | 1.07 | 5.85 | 14.57 | 5.09 | 12.03 | 1.81 | 3.31 | 3.97 | 1.36 |
| 11 | 11.04 | 3.22 | 3.82 | 6.64 | 8.05 | 3.26 | 3.62 | 0.29 | 7.68 | 0.68 | 9.13 | 19.48 | 2.44 | 16.44 | 0.63 | 1.20 | 1.22 | 1.16 |
| 12 | 15.09 | 4.43 | 5.16 | 20.30 | 5.16 | 4.12 | 5.47 | 0.41 | 4.77 | 1.07 | 4.99 | 10.16 | 3.41 | 6.81 | 1.05 | 3.40 | 3.12 | 1.06 |
| 13 | 11.23 | 3.42 | 3.96 | 22.14 | 5.78 | 3.83 | 4.31 | 0.26 | 4.28 | 0.88 | 3.23 | 6.55 | 4.12 | 4.50 | 3.55 | 8.03 | 9.32 | 0.63 |
| 14 | 9.86 | 3.59 | 5.73 | 10.81 | 4.56 | 3.47 | 4.62 | 0.13 | 3.64 | 0.96 | 3.21 | 8.22 | 3.21 | 6.20 | 3.76 | 16.38 | 10.19 | 1.46 |
| 15 | 8.12 | 2.68 | 3.72 | 9.47 | 2.37 | 3.87 | 4.18 | 0.12 | 2.66 | 0.51 | 2.25 | 8.92 | 5.61 | 4.86 | 4.22 | 11.79 | 24.50 | 0.14 |
| 16 | 6.61 | 1.74 | 4.24 | 9.04 | 2.93 | 2.00 | 2.48 | 0.11 | 2.02 | 0.21 | 2.14 | 6.64 | 3.48 | 2.24 | 4.26 | 16.76 | 30.18 | 2.93 |
| Sample ID | X-Variables (gAA) | ||||
|---|---|---|---|---|---|
| SCAA a | PCAA b | HAA c | AcAA d | AAA e | |
| 1 | 3.13 | 19.82 | 36.13 | 31.45 | 9.28 |
| 2 | 2.22 | 19.67 | 44.10 | 24.67 | 14.05 |
| 3 | 2.70 | 18.37 | 38.14 | 31.00 | 9.95 |
| 4 | 3.11 | 18.62 | 32.64 | 35.50 | 7.42 |
| 5 | 3.39 | 18.31 | 32.95 | 35.56 | 7.58 |
| 6 | 1.15 | 14.54 | 44.24 | 27.71 | 11.96 |
| 7 | 0.88 | 21.35 | 33.55 | 31.06 | 6.74 |
| 8 | 2.09 | 18.63 | 41.39 | 25.41 | 13.36 |
| 9 | 1.26 | 11.31 | 48.84 | 26.34 | 16.62 |
| 10 | 1.45 | 9.09 | 55.03 | 23.72 | 18.48 |
| 11 | 0.97 | 3.05 | 68.97 | 17.68 | 20.04 |
| 12 | 1.48 | 7.58 | 43.32 | 35.39 | 11.28 |
| 13 | 1.14 | 20.90 | 34.53 | 33.37 | 9.24 |
| 14 | 1.09 | 30.33 | 36.21 | 20.67 | 10.88 |
| 15 | 0.63 | 40.51 | 31.63 | 17.59 | 10.62 |
| 16 | 0.33 | 51.19 | 25.18 | 15.65 | 8.65 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Udenigwe, C.C.; Aluko, R.E. Chemometric Analysis of the Amino Acid Requirements of Antioxidant Food Protein Hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148-3161. https://doi.org/10.3390/ijms12053148
Udenigwe CC, Aluko RE. Chemometric Analysis of the Amino Acid Requirements of Antioxidant Food Protein Hydrolysates. International Journal of Molecular Sciences. 2011; 12(5):3148-3161. https://doi.org/10.3390/ijms12053148
Chicago/Turabian StyleUdenigwe, Chibuike C., and Rotimi E. Aluko. 2011. "Chemometric Analysis of the Amino Acid Requirements of Antioxidant Food Protein Hydrolysates" International Journal of Molecular Sciences 12, no. 5: 3148-3161. https://doi.org/10.3390/ijms12053148




