Separation and Purification of Sulforaphane from Broccoli by Solid Phase Extraction
Abstract
:1. Introduction
2. Results and Discussion
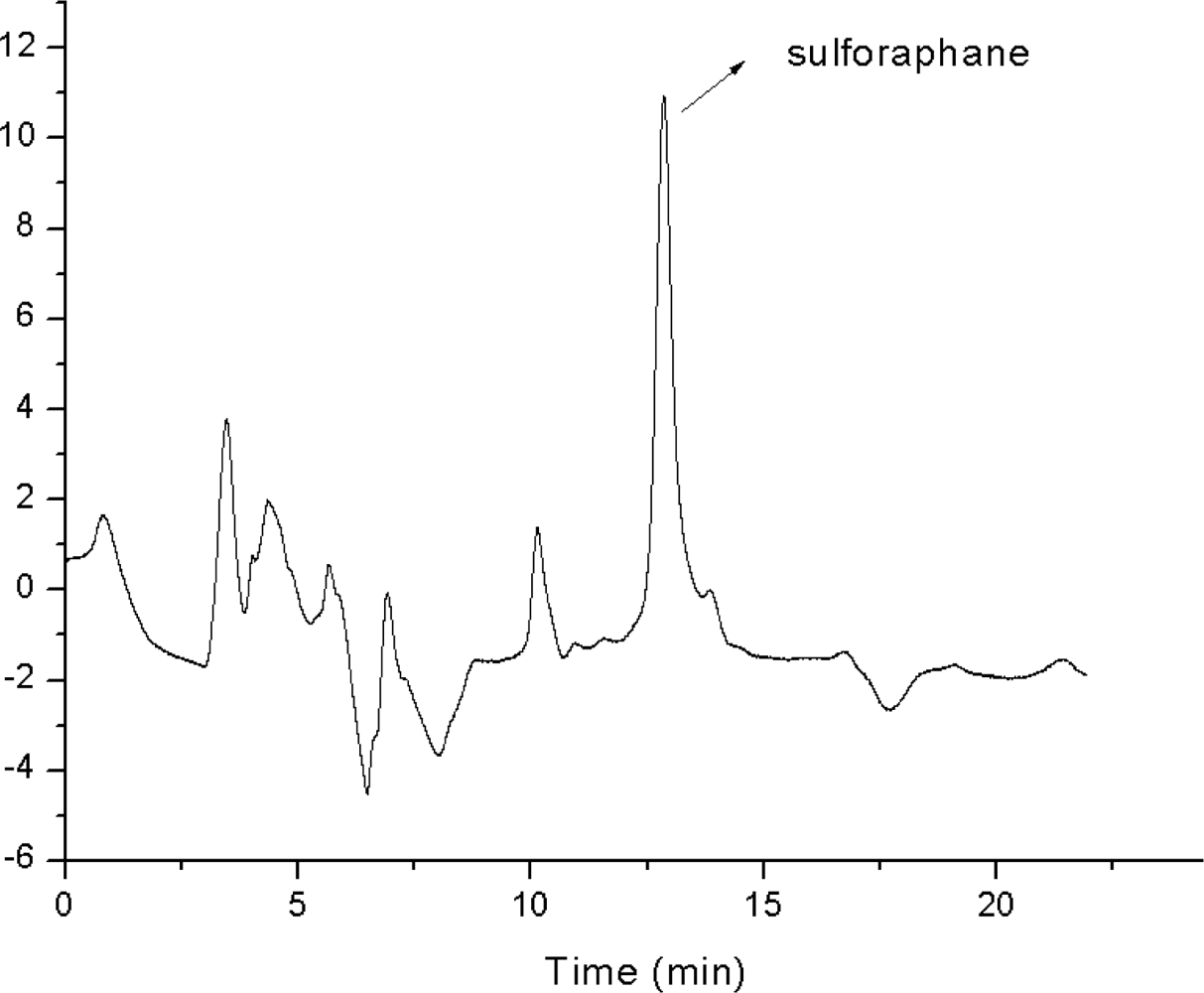
2.1. Optimization of Chromatographic Conditions
2.2. Optimization of the Hydrolyzation from Glucoraphanin to Sulforaphane
2.3. Optimization of SPE Conditions
2.3.1. Choice of Different SPE Cartridges
2.3.2. Influence of the Washing Solvent and Elution Solvent
2.3.3. Influence of Elution Solvent Volume
2.3.4. Validation of the Proposed Method
3. Experimental Section
3.1. Reagents and Materials
3.2. Chromatographic Conditions
3.3. Sample Clean-up and Preparation by SPE
4. Conclusions
Acknowledgments
References
- Vallejo, F; Tomás-Barberán, FA; Garcia-Viguera, C. Glucoraphanins and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur. Food Res. Tech 2002, 215, 310–316. [Google Scholar]
- Koh, E; Wimalasiri, KMS; Chassy, AW; Mitchell, AE. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Compos. Anal 2009, 22, 637–643. [Google Scholar]
- Chen, YJD; Conte, J; Lin, Y; Gemelli, A; Castro, A; Yarlagadda, S; Gonzalez, S; Philips, N. Sulforaphane, an extract from broccoli, prevents skin cancer. J. Plast. Dermatol 2008, 4, 165–166. [Google Scholar]
- Rose, P; Huang, Q; Ong, CN; Whiteman, M. Broccoli and watercress suppress matrix metalloproteinase-9 activity and invasiveness of human MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol 2005, 209, 105–113. [Google Scholar]
- Canene-Adams, K; Lindshield, BL; Wang, S; Jeffery, EH; Clinton, SK; Erdman, JW, Jr. Combinations of tomato and broccoli enhance antitumor activity in dunning R3327-H prostate adenocarcinomas. Cancer Res 2007, 67, 836–843. [Google Scholar]
- Sharma, R; Sharma, A; Chaudhary, P; Pearce, V; Vatsyayan, R; Singh, SV; Awasthi, S; Awasthi, YC. Role of lipid peroxidation in cellular responses to D,L-sulforaphane, a promising cancer chemopreventive agent. Biochemistry 2010, 49, 3191–3202. [Google Scholar]
- Hahm, ER; Singh, SV. Sulforaphane inhibits constitutive and interleukin-6-induced activation of signal transducer and activator of transcription 3 in prostate cancer cells. Cancer Prev. Res 2010, 3, 484–494. [Google Scholar]
- Rausch, V; Liu, L; Kallifatidis, G; Baumann, B; Mattern, J; Gladkich, J; Wirth, T; Schemmer, P; Büchler, MW; Zöller, M; Salnikov, AV; Herr, I. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res 2010, 70, 5004–5013. [Google Scholar]
- Dickinson, SE; Melton, TF; Olson, ER; Zhang, J; Saboda, K; Bowden, GT. Inhibition of activator protein-1 by sulforaphane involve interaction with cysteine in the cFos DNA-binding domain: Implications for chemoprevention of UVB-induced skin cancer. Cancer Res 2009, 69, 7103–7110. [Google Scholar]
- Rudolf, E; Andělová, H; Červinka, M. Activation of several concurrent proapoptic pathways by sulforaphane in human colon cancer cells SW620. Food Chem. Toxicol 2009, 47, 2366–2373. [Google Scholar]
- Agrawal, S; Winnik, B; Buckley, B; Mi, L; Chung, FL; Cook, TJ. Simultaneous determination of sulforaphane and its major metabolites from biological matrices with liquid chromatographytandem mass spectroscopy. J. Chrom. B 2006, 840, 99–107. [Google Scholar]
- Liang, H; Yuan, QP; Dong, HR; Liu, YM. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J. Food Compos. Anal 2006, 19, 473–476. [Google Scholar]
- Chiang, WCK; Pusateri, J; Leitz, REA. Gas chromatography/mass spectrometry method for the determination of sulforaphane and sulforaphane nitrile in broccoli. J. Agr. Food Chem 1998, 46, 1018–1021. [Google Scholar]
- Nakagawa, K; Umeda, T; Higuchi, O; Tsuzuki, T; Suzuki, T; Miyazawa, T. Evaporative light-scattering analysis of sulforaphane in broccoli samples: Quality of broccoli products regarding sulforaphane contents. J. Agr. Food Chem 2006, 54, 2479–2483. [Google Scholar]
- Matusheski, NV; Walling, MA; Juvic, JA; Klein, BP; Kushad, MM; Jeffery, EH. Preparative HPLC method for the purification of sulforaphane and sulforaphane nitrile from Brassica oleracea. J. Agr. Food Chem 2001, 49, 1867–1872. [Google Scholar]
- Liang, H; Li, C; Yuan, Q; Vriesekoop, F. Application of high-speed countercurrent chromatography for the isolation of sulforaphane from broccoli seed meal. J. Agr. Food Chem 2008, 56, 7746–7749. [Google Scholar]
- Campas-Baypoli, ON; Sánchez-Machado, DI; Bueno-Solano, C; Ramírez-Wong, B; López-Cervantes, J. HPLC method validation for measurement of sulforaphane level in broccoli by-products. Biomed. Chrom 2010, 24, 387–392. [Google Scholar]
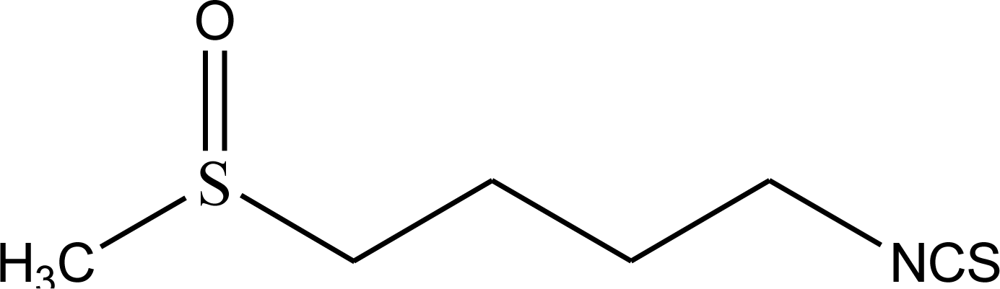

| pH | Amount of sulforaphane (mg/g) |
|---|---|
| 0 | 0.024 |
| 2.0 | 0.057 |
| 3.0 | 0.148 |
| 4.0 | 0.132 |
| 6.0 | 0.118 |
| Washing solvent | Amount of sulforaphane (mg/g) | ||
|---|---|---|---|
| Silica cartridge | C18 cartridge | Amino cartridge | |
| Water | 0.016 | 0.003 | 0.002 |
| Acetonitrile | 0.069 | 0.032 | 0.027 |
| Dichloromethane | 0.357 | - | 0.057 |
| 0.1M Acetic acid | - | 0.062 | - |
| Hexane | - | - | 0.006 |
| Ethyl acetate | 0 | 0 | - |
| Volume of dichloromethane (mL) | Amount of sulforaphane (mg/g) |
|---|---|
| 2 | 0.120 |
| 3 | 0.214 |
| 4 | 0.324 |
| 5 | 0.326 |
| 6 | 0.327 |
| Target compound | Linear range (μg/mL) | r2 | LOD (μg/mL) |
|---|---|---|---|
| Sulforaphane | 0.05–200 | 0.998 | 0.002 |
| Original (μg/mg) | Add amount (μg/mg) | Found amount (μg/mg) | Recovery (%) | RSD | |
|---|---|---|---|---|---|
| Sulforaphane | 0.513 | 10.0 | 9.59 | 90.8 | 3.54 |
| 0.513 | 50.0 | 47.10 | 93.2 | 3.17 | |
| 0.513 | 100.0 | 96.91 | 96.4 | 2.36 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Han, D.; Row, K.H. Separation and Purification of Sulforaphane from Broccoli by Solid Phase Extraction. Int. J. Mol. Sci. 2011, 12, 1854-1861. https://doi.org/10.3390/ijms12031854
Han D, Row KH. Separation and Purification of Sulforaphane from Broccoli by Solid Phase Extraction. International Journal of Molecular Sciences. 2011; 12(3):1854-1861. https://doi.org/10.3390/ijms12031854
Chicago/Turabian StyleHan, Dandan, and Kyung Ho Row. 2011. "Separation and Purification of Sulforaphane from Broccoli by Solid Phase Extraction" International Journal of Molecular Sciences 12, no. 3: 1854-1861. https://doi.org/10.3390/ijms12031854





