Dynamics of Protein Phosphatase Gene Expression in Corbicula fluminea Exposed to Microcystin-LR and to Toxic Microcystis aeruginosa Cells
Abstract
:1. Introduction
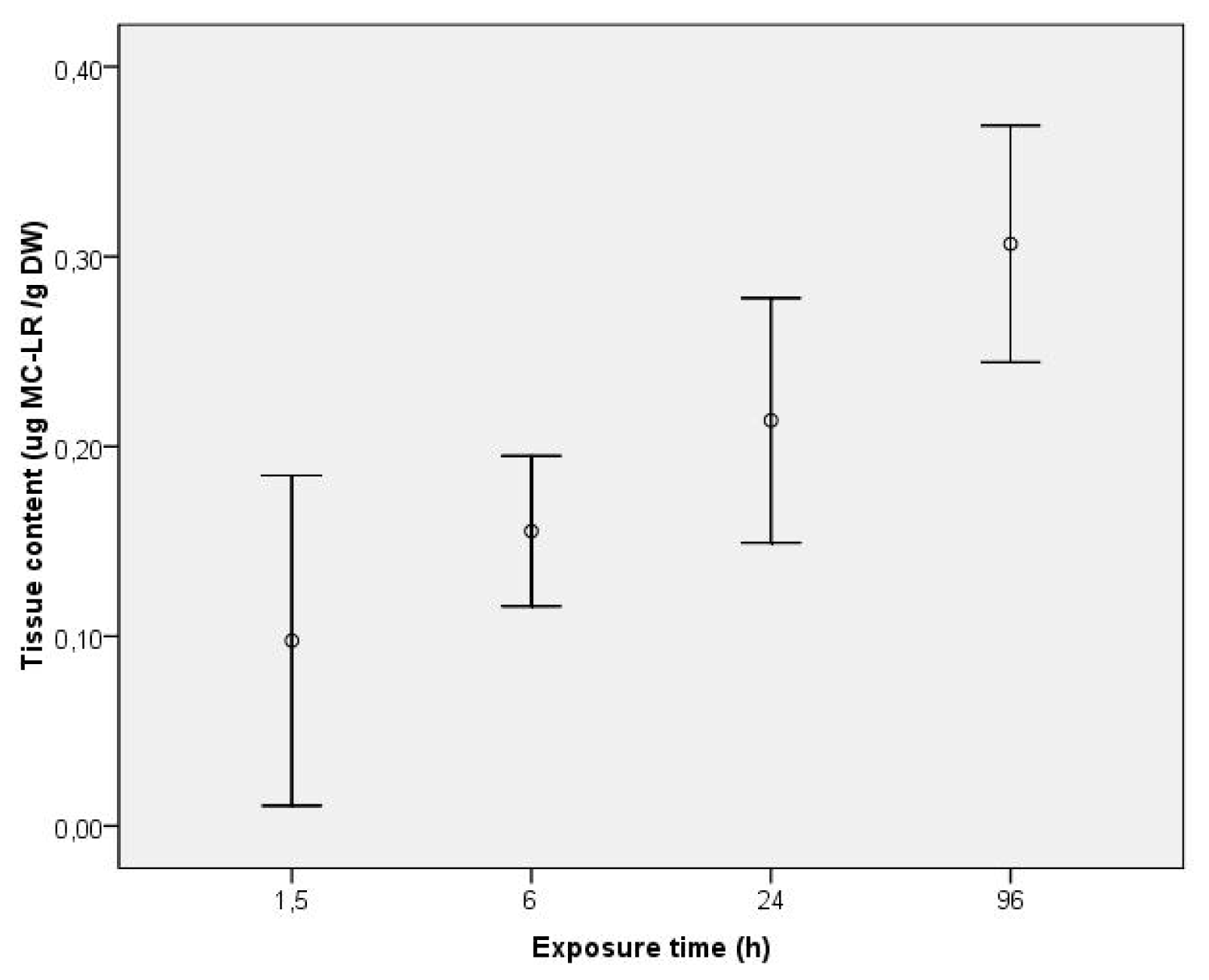
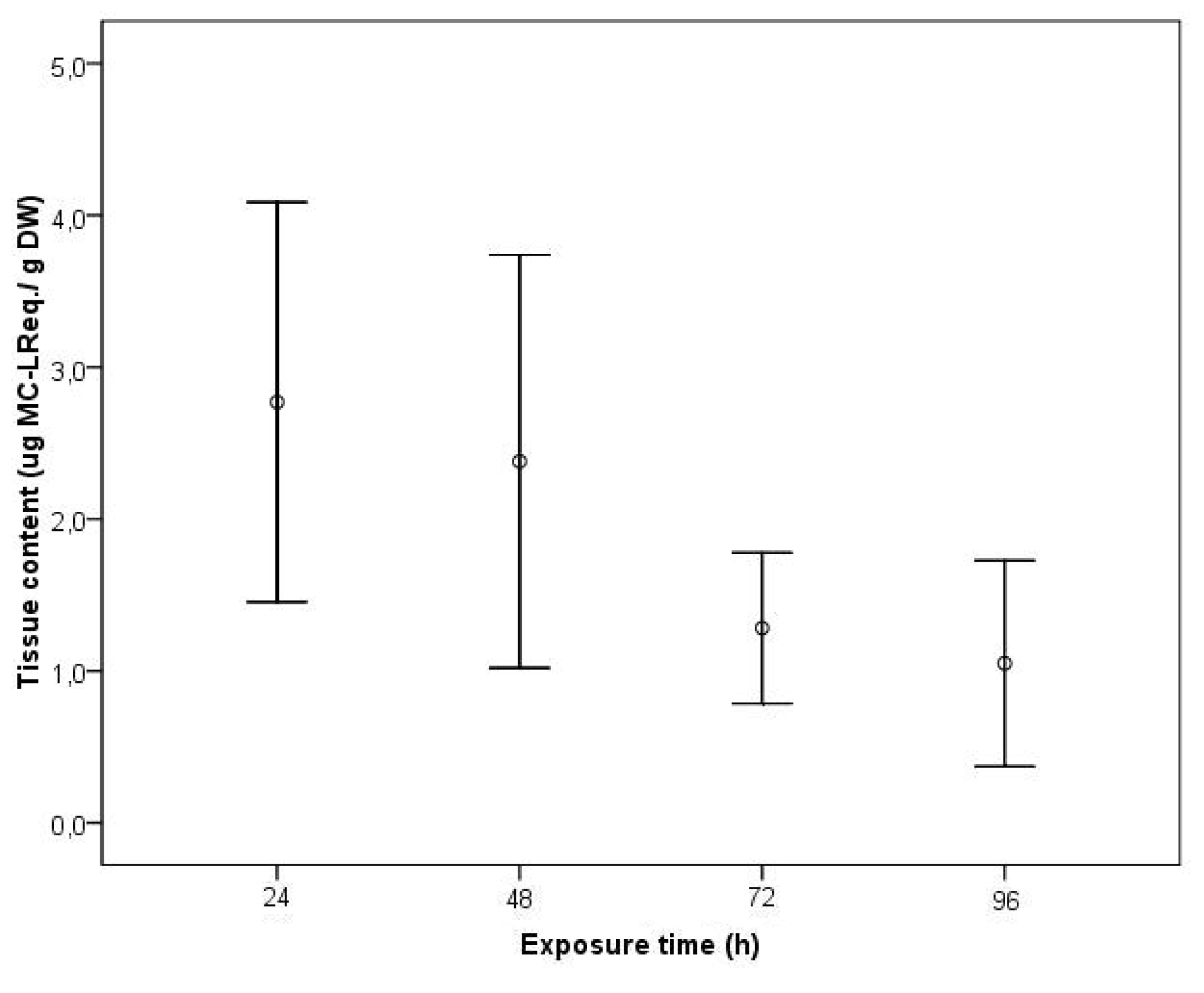
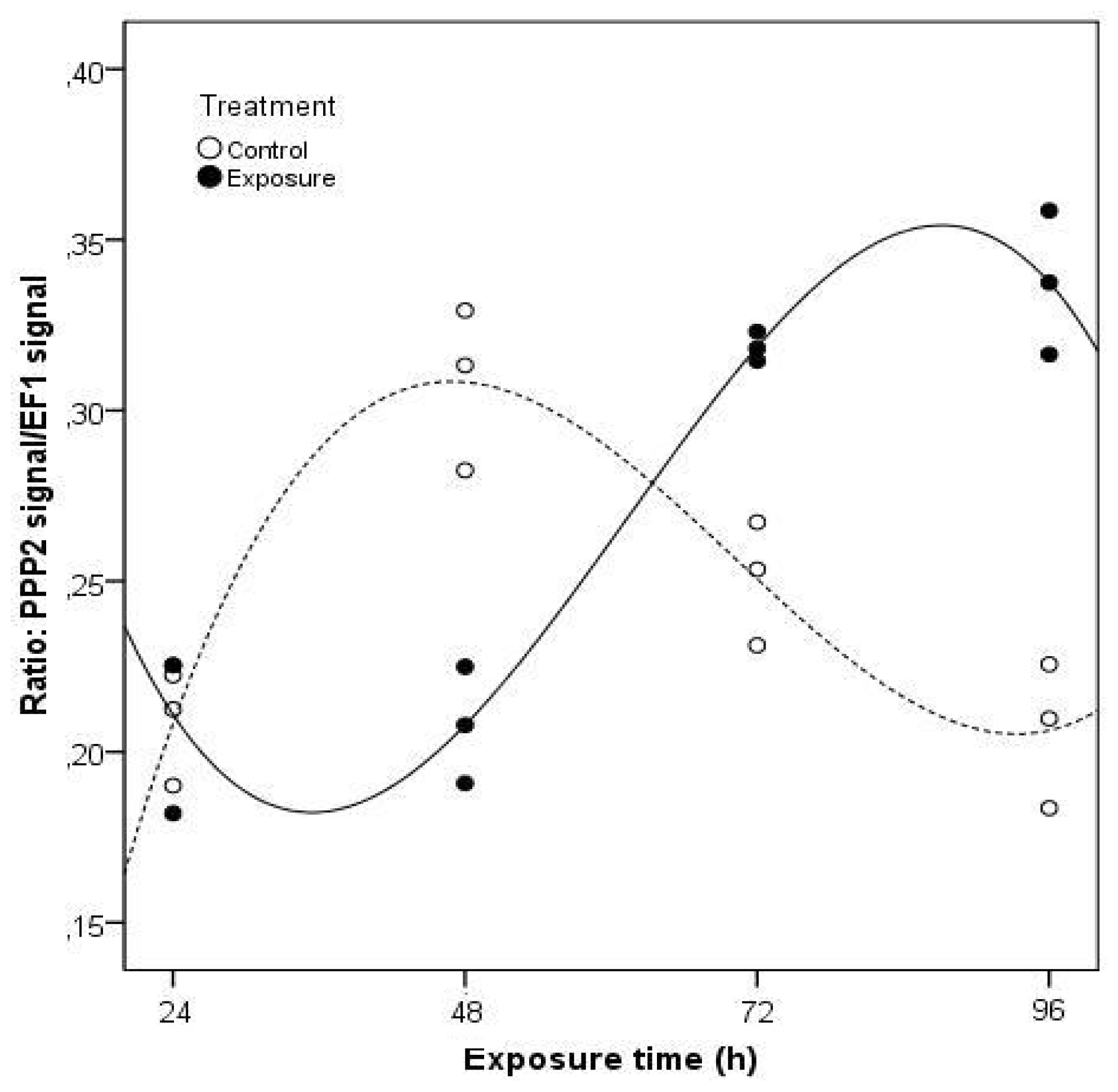
2. Results and Discussion
3. Experimental Section
3.1. Test Species and Cultures
3.2. MC Purification
3.2.1. MC-LR Extraction for Analytical HPLC
3.2.2. MC-LR Extraction for Semi-Preparative HPLC
3.2.3. Solid Phase Extraction
3.2.4. Quantification of MC-LR
3.3. Exposure Experiments
3.3.1. With Purified MC-LR
3.3.2. With M. aeruginosa Toxic Strain Cells
3.4. Toxin Analysis in the Clams
3.5. Gene Expression
3.5.1. RNA Extraction and cDNA Synthesis
3.5.2. Primers Design
3.5.3. Quantitative RT-PCR
3.6. Enzyme Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Chorus, I.; Falconer, I.R.; Salas, H.J.; Bartram, J. Health risks caused by freshwater cyanobacteria in recreational waters. J. Toxicol. Environ. Health B 2000, 3, 323–347. [Google Scholar]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol. Appl. Pharmacol 2005, 203, 273–289. [Google Scholar]
- Martins, J.C.; Vasconcelos, V.M. Microcystin dynamics in aquatic organisms. J. Toxicol. Environ. Health B 2009, 12, 1–18. [Google Scholar]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett 1990, 264, 187–192. [Google Scholar]
- Huang, X.; Honkanen, R.E. Molecular cloning, expression, and characterization of a novel human serine/threonine protein phosphatase, PP7, that is homologous to Drosophila retinal degeneration C gene product (rdgC). J. Biol. Chem 1998, 273, 1462–1468. [Google Scholar]
- Falconer, I.R. Tumour promotion and liver injury caused by oral consumption of cyanobacteria. Environ. Toxicol. Water Qual 1991, 6, 177–184. [Google Scholar]
- Nishiwaki-Matsushima, R.; Ohta, T.; Nishiwaki, S.; Suganuma, M.; Kohyama, K.; Ishikawa, T.; Carmichael, W.W.; Fujiki, H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin 1992, 118, 420–424. [Google Scholar]
- Humpage, A.R.; Hardy, S.J.; Moore, E.J.; Froscio, S.M.; Falconer, I.R. Microcystins (cyanobacterial toxins) in drinking water enhance the growth of aberrant crypt foci in the mouse colon. J. Toxicol. Environ. Health A 2000, 61, 155–165. [Google Scholar]
- Prieto, A.I.; Jos, Á.; Pichardo, S.; Moreno, I.; Cameán, A.M. Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat. Toxicol 2006, 77, 314–321. [Google Scholar]
- Zhao, Y.; Xie, P.; Zhang, X. Oxidative stress response after prolonged exposure of domestic rabbit to a lower dosage of extracted microcystins. Environ. Toxicol. Pharmacol 2009, 27, 195–199. [Google Scholar]
- Fujiki, H.; Suganuma, M. Carcinogenic aspects of protein phosphatase 1 and 2A inhibitors. In Marine Toxins as Research Tools: Progress in Molecular and Subcellular; Spring: Berlin, Germany, 2009; Marine Molecular Biotechnology 46; pp. 221–254. [Google Scholar]
- Johnson, L.N. The regulation of protein phosphorylation. Biochem. Soc. Trans 2009, 37, 627–641. [Google Scholar]
- Pereira, S.R.; Vasconcelos, V.M.; Antunes, A. The phosphoprotein phosphatase family of Ser/Thr phosphatases as principal targets of naturally occuring toxins. Crit. Rev. Toxicol 2011, 41, 83–110. [Google Scholar]
- Prickett, T.D.; Brautigan, D.L. The α4 regulatory subunit exerts opposing allosteric effects on protein phosphatases PP6 and PP2A. J. Biol. Chem 2006, 281, 30503–30511. [Google Scholar]
- Goldberg, J.; Huang, H.B.; Kwon, Y.G.; Greengard, P.; Nairn, A.C.; Kuriyan, J. Three dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 1995, 376, 745–753. [Google Scholar]
- Craig, M.; Luu, H.A.; McCready, T.L.; Williams, D.; Andersen, R.J.; Holmes, C.F. Molecular mechanisms underlying he interaction of motuporin and microcystins with type-1 and type-2A protein phosphatases. Biochem. Cell Biol 1996, 74, 569–578. [Google Scholar]
- Xing, Y.; Xu, Y.; Chen, Y.; Jeffrey, P.D.; Chao, Y.; Lin, Z.; Li, Z.; Strack, S.; Stock, J.B.; Shi, Y. Structure of protein phosphatase 2A core enzyme bound to tumor-inducing toxins. Cell 2006, 127, 341–353. [Google Scholar]
- Gehringer, M.M.; Shephard, E.G.; Downing, T.G.; Wiegand, C.; Neilan, B.A. An investigation into the detoxification of microcystin-LR by the glutathione pathway in Balb/c mice. Int. J. Biochem. Cell Biol 2004, 36, 931–941. [Google Scholar]
- Malbrouck, C.; Kestemont, P. Effects of microcystin on fish. Environ. Toxicol. Chem 2005, 25, 72–86. [Google Scholar]
- Pinho, G.L.L.; Rosa, C.M.; Maciel, F.E.; Bianchini, A.; Yunes, J.S.; Proença, L.A.O.; Monserrat, J.M. Antioxidant responses and oxidative stress after microcystin exposure in the hepatopancreas of an estuarine crab species. Ecotoxicol. Environ. Saf 2005, 61, 353–360. [Google Scholar]
- Gérard, C.; Poullain, V. Variation in the response of the invasive species Potamopyrgus antipodarum (Smith) to natural (cyanobacterial toxin) and anthropogenic (herbicide atrazine) stressors. Environ. Pollut 2005, 138, 28–33. [Google Scholar]
- Gérard, C.; Brient, L.; Le Rouzic, B. Variation in the response of juvenile and adult gastropods (Lymnaea stagnalis) to cyanobacterial toxin (microcystin-LR). Environ. Toxicol 2005, 20, 592–596. [Google Scholar]
- Lance, E.; Paty, C.; Bormans, M.; Brient, L.; Gérard, C. Interactions between cyanobacteria and gastropods II. Impact of toxic Planktothrix agardhii on the life-history traits of Lymnaea stagnalis. Aquat. Toxicol 2007, 81, 389–396. [Google Scholar]
- Zurawell, R.W.; Goldberg, J.I.; Holmes, C.F.B.; Prepas, E.E. Tissue distribution and oral dose effects of microcystin in the freshwater pulmonate snail Lymnaea stagnalis jugularis (Say). J. Toxicol. Environ. Health A 2007, 70, 620–626. [Google Scholar]
- Falconer, I.R. Cyanobacterial toxins present in Microcystis aeruginosa extracts—More than microcystins! Toxicon 2007, 50, 585–588. [Google Scholar]
- Amorim, A.; Vasconcelos, V. Dynamics of microcystins in the mussel Mytilus galloprovincialis. Toxicon 1999, 37, 1041–1052. [Google Scholar]
- Buetler, T.M.; Gallagher, E.P.; Wang, C.; Stahl, D.L.; Hayes, J.D.; Eaton, D.L. Induction of phase I and phase II drug-metabolizing enzyme mRNA, protein, and activity by BHA, ethoxyquin and oltipraz. Toxicol. Appl. Pharmacol 1995, 135, 45–57. [Google Scholar]
- Hemmings, B.A.; Adams-Pearson, C.; Maurer, F.; Müller, P.; Goris, J.; Merlevede, W.; Hofsteenge, J.; Stone, S.R. Alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry 1990, 29, 3166–3173. [Google Scholar]
- Stark, M.J. Yeast protein serine/threonine phosphatases: Multiple roles and diverse regulation. Yeast 1996, 12, 1647–1675. [Google Scholar]
- Gkelis, S.; Lanaras, T.; Sivonen, K. The presence of microcystins and other cyanobacterial bioactive peptides in aquatic fauna collected from Greek freshwaters. Aquat. Toxicol 2006, 78, 32–41. [Google Scholar]
- Wood, S.A.; Briggs, L.R.; Sprosen, J.; Ruck, J.G.; Wear, R.G.; Holland, P.T.; Bloxham, M. Changes in concentrations of microcystins in rainbow trout, freshwater mussels, and cyanobacteria in Lakes Rotoiti and Rotoehu. Environ. Toxicol 2006, 21, 205–222. [Google Scholar]
- Apeldoorn, M.E.; Egmond, H.P.; Speijers, G.J.A.; Bakker, G.J.I. Toxins of cyanobacteria. Mol. Nutr. Food Res 2007, 51, 7–60. [Google Scholar]
- Metcalf, J.S.; Beattie, K.A.; Pflugmacher, S.; Codd, G.A. Immuno-crossreactivity and toxicity assessment of conjugation products of the cyanobacterial toxin, microcystin-LR. FEMS Microbiol. Lett 2000, 189, 155–158. [Google Scholar]
- Ott, J.L.; Carmichael, W.W. LC/ESI/MS method development for the analysis of hepatotoxic cyclic peptide microcystins in animal tissues. Toxicon 2006, 47, 734–741. [Google Scholar]
- Vasconcelos, V.M. Uptake and depuration of the heptapeptide toxin microcystin-LR in Mytilus galloprovincialis. Aquat. Toxicol 1995, 32, 227–237. [Google Scholar]
- Prepas, E.E.; Kotak, B.G.; Campbell, L.M.; Evans, J.C.; Hrudey, S.E.; Holmes, C.F.B. Accumulation and elimination of cyanobacterial hepatotoxins by the freshwater clam Anodonta grandis simpsoniana. Can. J. Fish. Aquat. Sci 1997, 54, 41–46. [Google Scholar]
- Lance, E.; Brient, L.; Bormans, M.; Gérard, C. Interactions between cyanobacteria and Gastropods I. Ingestion of toxic Planktothrix agardhii by Lymnaea stagnalis and the kinetics of microcystin bioaccumulation and detoxification. Aquat. Toxicol 2006, 79, 140–148. [Google Scholar]
- Pires, L.M.D.; Karlsson, K.M.; Meriluoto, J.A.O.; Kardinaal, E.; Visser, P.M.; Siewertsen, K.; Donk, E.V.; Ibelings, B.W. Assimilation and depuration of microcystin-LR by the zebra mussel Dreissena polymorpha. Aquat. Toxicol 2004, 69, 385–396. [Google Scholar]
- Liu, Y.; Xie, P.; Wu, X. Grazing on toxic and non-toxic Microcystis aeruginosa PCC7820 by Unio douglasiae and Corbicula fluminea. Limnology 2009, 10, 1–5. [Google Scholar]
- Lance, E.; Neffling, M.; Gérard, C.; Meriluoto, J.; Bormans, M. Accumulation of free and covalently bound microcystins in tissues of Lymnaea stagnalis (Gastropoda) following toxic cyanobacteria or dissolved microcystin-LR exposure. Environ. Pollut 2010, 158, 674–680. [Google Scholar]
- Huang, P.; Zheng, Y.; Xu, L. Oral administration of cyanobacterial bloom extract induced the altered expression of the PP2A, Bax, and Bcl-2 in mice. Environ. Toxicol 2008, 23, 688–693. [Google Scholar]
- Malbrouck, C.; Trausch, G.; Devos, P.; Kestemont, P. Effect of microcystin-LR on protein phosphatase activity in fed and fasted juvenile goldfish Carassius auratus L. Toxicon 2004, 43, 295–301. [Google Scholar]
- Martins, J.C.; Leão, P.N.; Vasconcelos, V. Differential protein expression in Corbicula fluminea upon exposure to a Microcystis aeruginosa toxic strain. Toxicon 2009, 53, 409–416. [Google Scholar]
- Kotai, J. Instruction for Preparation of Modified Nutrient Solution Z8 for Algae, Publication B-11/69; Norwegian Institute for Water Research: Oslo, Norway, 1972; p. 5. [Google Scholar]
- Ramanan, S.; Tang, J.; Velayudhan, A. Isolation and preparative purification of microcystin variants. J. Chromatogr. A 2000, 883, 103–112. [Google Scholar]
- Lemaire-Gony, S.; Boudou, A. Mantle and gill fine structure in the freshwater Asiatic clam, Corbicula fluminea (Müller). Ann. Limnol. Int. J. Limnol 1997, 33, 163–178. [Google Scholar]
- Fernandes, S. Biodisponibilidade de cianotoxinas em bivalves. PhD Thesis, University of Porto, Porto, Portugal, 2008. [Google Scholar]
- Contardo-Jara, V.; Pflugmacher, S.; Wiegand, C. Multi-xenobiotic-resistance a possible explanation for the insensitivity of bivalves towards cyanobacterial toxins. Toxicon 2008, 52, 936–943. [Google Scholar]
- Morga, B.; Arzul, I.; Faury, N.; Renault, T. Identification of genes from flat oyster Ostrea edulis as suitable housekeeping genes for quantitative real time PCR. Fish Shellfish Immunol 2010, 29, 937–945. [Google Scholar]
- Whelan, J.A.; Russell, N.B.; Whelan, M.A. A method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods 2003, 278, 261–269. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenter: An Introduction to Design, Data Analysis and Model Building; John Wiley and Sons: New York, NY, USA, 1978; p. 653. [Google Scholar]
- Tomassone, R.; Lesquoy, E.; Millier, C. La Regression-Nouveaux Regards Sur Une Ancienne Method Statistique; Masson: Paris, France, 1983; p. 180. [Google Scholar]


| Sequence of Foward primer (5′–3′) | Sequence of Reverse primer (5′–3′) | Amplified fragment size (bp) | |
|---|---|---|---|
| PPP1 | AATGTGCCAGCATCAACAGA | ATCTGTTGGCCGCATAATTC | 206 |
| PPP2 | ACGGCAATGCTAATGTTTGG | GACCCTCATGTGGAACCTCT | 171 |
| PPP4 | ACGAGGGAATCATGAAAGCCGTC | TCGCGGACTCACTCCCCATCC | 301 |
| EF1-α | CGTTGGTGTCAACAAGATGG | TACAGCCCAACCCTTGTACC | 202 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martins, J.C.; Machado, J.; Martins, A.; Azevedo, J.; OlivaTeles, L.; Vasconcelos, V. Dynamics of Protein Phosphatase Gene Expression in Corbicula fluminea Exposed to Microcystin-LR and to Toxic Microcystis aeruginosa Cells. Int. J. Mol. Sci. 2011, 12, 9172-9188. https://doi.org/10.3390/ijms12129172
Martins JC, Machado J, Martins A, Azevedo J, OlivaTeles L, Vasconcelos V. Dynamics of Protein Phosphatase Gene Expression in Corbicula fluminea Exposed to Microcystin-LR and to Toxic Microcystis aeruginosa Cells. International Journal of Molecular Sciences. 2011; 12(12):9172-9188. https://doi.org/10.3390/ijms12129172
Chicago/Turabian StyleMartins, José Carlos, João Machado, António Martins, Joana Azevedo, Luís OlivaTeles, and Vitor Vasconcelos. 2011. "Dynamics of Protein Phosphatase Gene Expression in Corbicula fluminea Exposed to Microcystin-LR and to Toxic Microcystis aeruginosa Cells" International Journal of Molecular Sciences 12, no. 12: 9172-9188. https://doi.org/10.3390/ijms12129172





