Functional Properties of Pea (Pisum sativum, L.) Protein Isolates Modified with Chymosin
Abstract
:1. Introduction
2. Results and Discussion
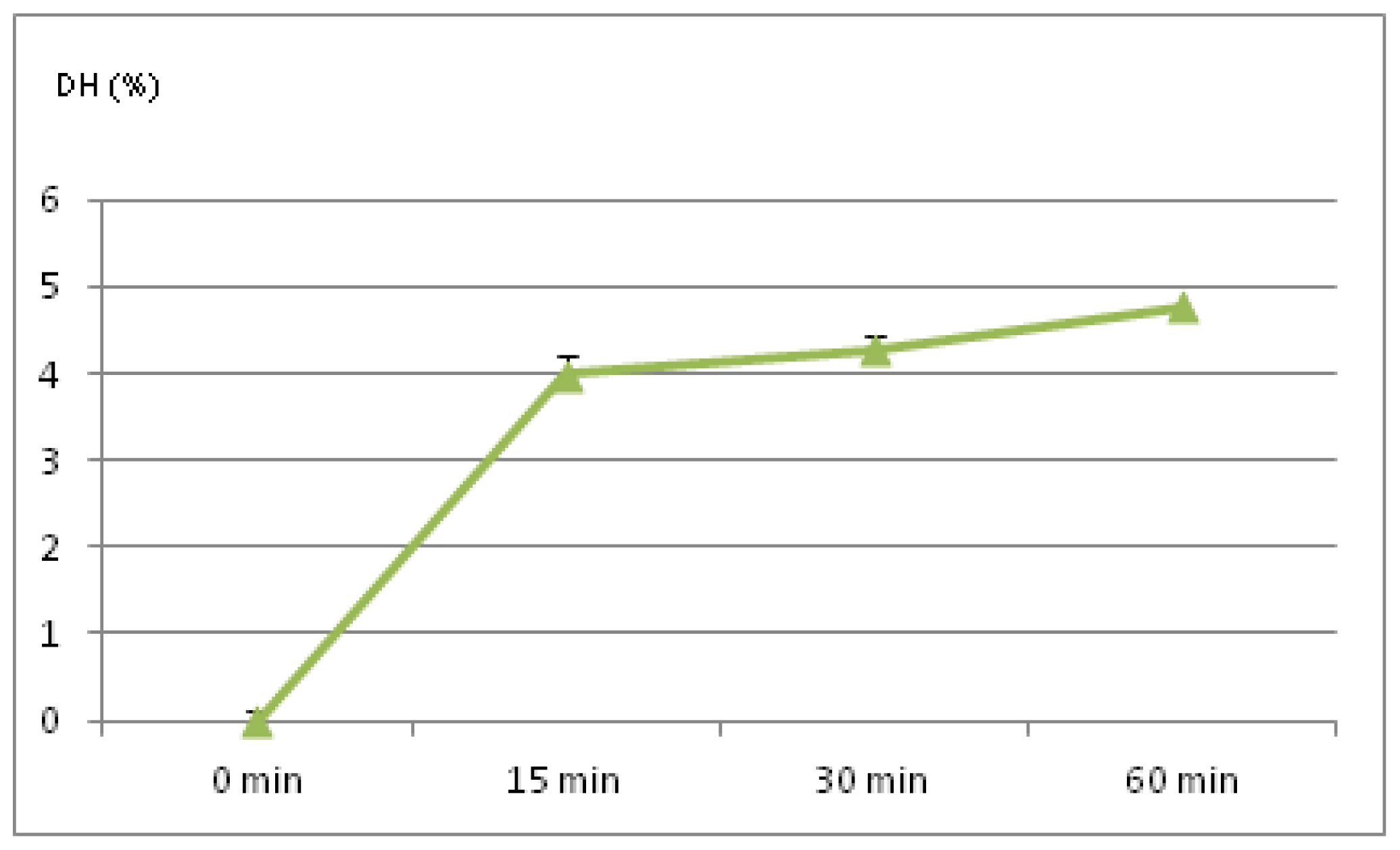
2.1. Degree of Hydrolysis
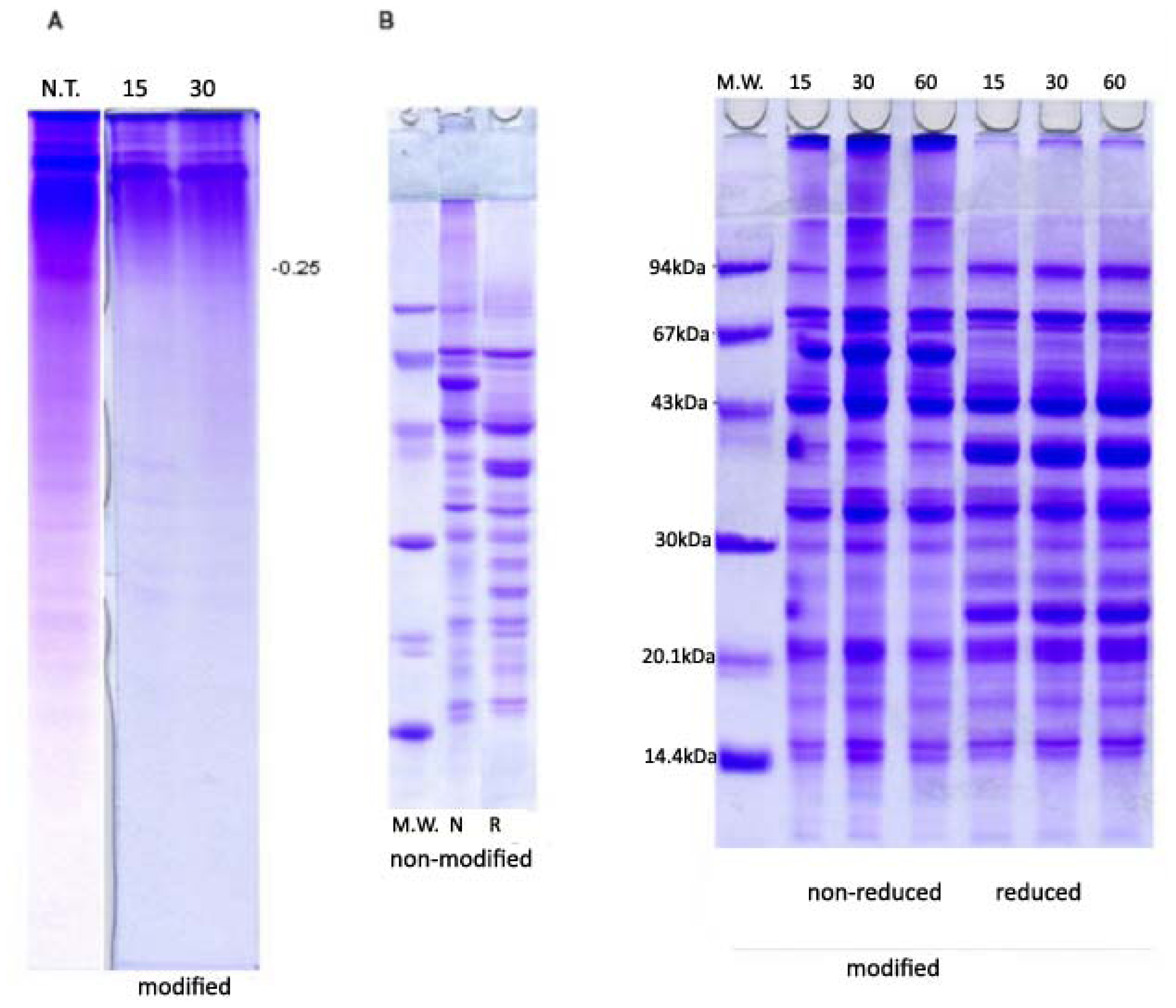
2.2. PAGE and SDS-PAGE
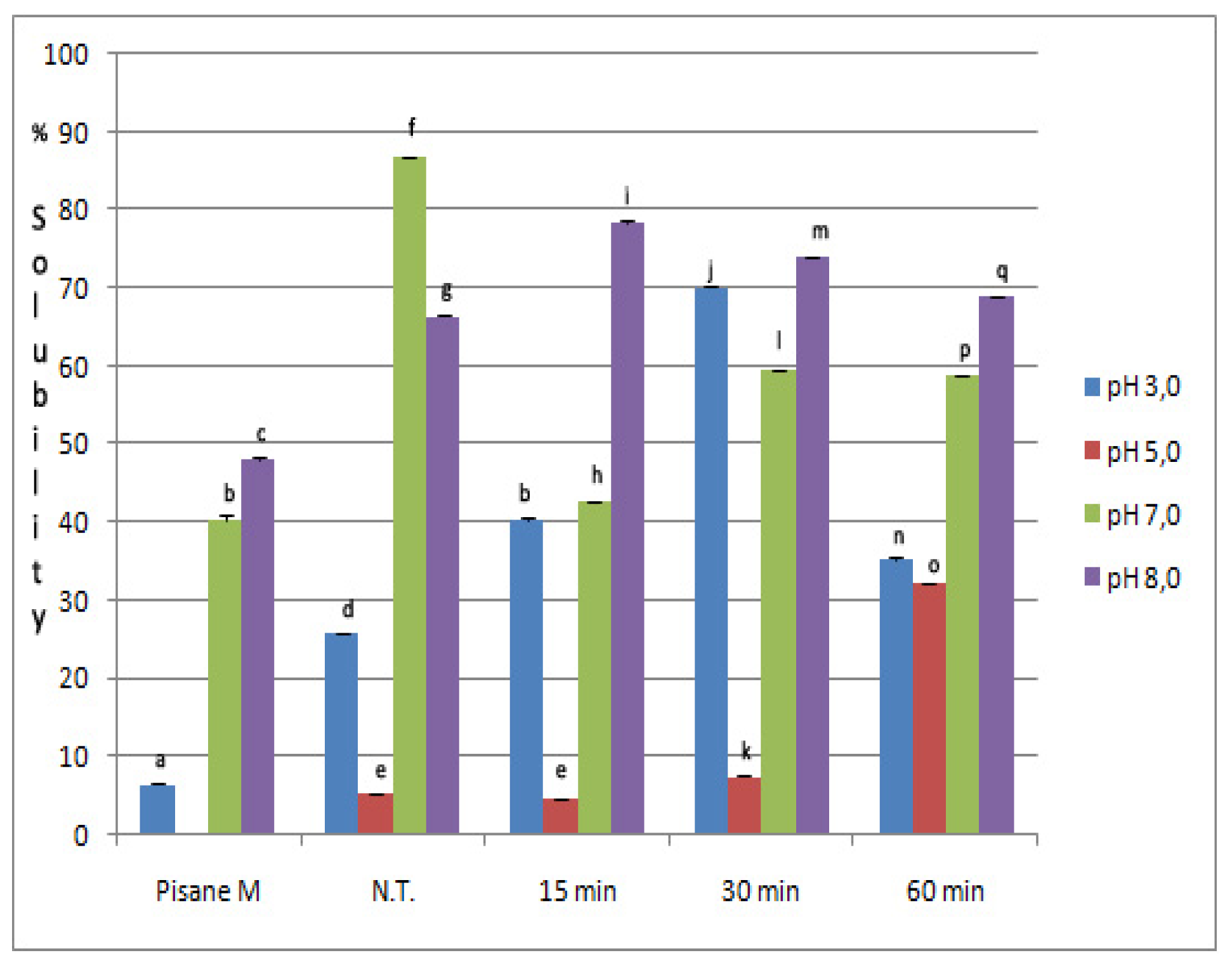
2.3. Solubility
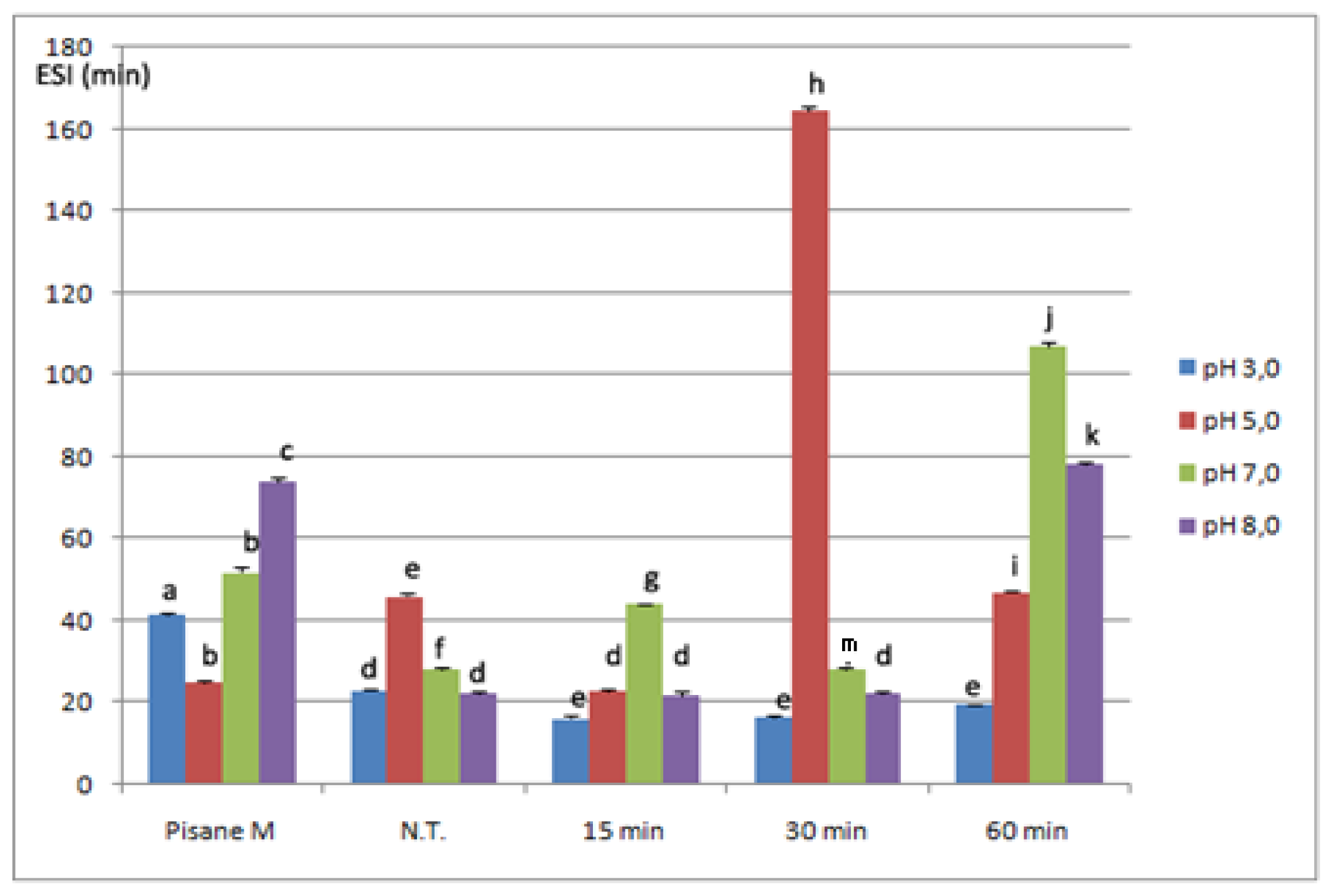
2.4. Emulsifying Properties
2.5. Foaming Properties
3. Material and Methods
3.1. Isolate Modification
3.2. Degree of Hydrolysis
3.3. Native-PAGE
3.4. SDS-PAGE
3.5. Densytometric Analysis
3.6. Protein Solubility
3.7. Emulsifying Properties
3.8. Foaming Properties
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Aluko, R.E. Determination of nutritional and bioactive properties of peptides in enzymatic pea, chickpea, and mung bean protein hydrolysates. J. AOAC Int 2008, 91, 947–956. [Google Scholar]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem 2010, 58, 4712–4718. [Google Scholar]
- Barbana, C.; Boye, J.I. Angiotensin I-converting enzyme inhibitory activity of chickpea and pea protein hydrolysates. Food Res. Int 2010, 43, 1642–1649. [Google Scholar]
- Barac, M.B.; Jovanovic, S.T.; Stanojevic, S.P.; Pesic, M.B. Effect of limited hydrolysis on traditional soy protein concentrate. Sensors 2006, 6, 1087–1101. [Google Scholar]
- Tsumura, K.; Saito, T.; Tsuge, K.; Ashida, H.; Kugimiya, W.; Inouye, K. Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT Food Sci. Technol 2005, 38, 255–261. [Google Scholar]
- Ribotta, P.D.; Rosell, C.M. Effects of enzymatic modification of soybean protein on the pasting and rheological profile of starch-protein systems. Starch Stärke 2010, 62, 373–383. [Google Scholar]
- Chen, L.; Chen, J.; Ren, J.; Zhao, M. Modifications of soy protein isolates using combined extrusion pre-treatment and controlled enzymatic hydrolysis for improved emulsifying properties. Food Hydrocoll 2011, 25, 887–897. [Google Scholar]
- Tsoukala, A.; Papalamprou, E.; Makri, E.; Doxastakis, G.; Braudo, E.E. Adsorption at the air-water interface and emulsification properties of grain legume protein derivatives from pea and broad bean. Coll. Surf. B 2006, 53, 203–208. [Google Scholar]
- Le Gall, M.; Gueguen, J.; Seve, B.; Quillien, L. Effects of grinding and thermal treatments on hydrolysis susceptibility of pea proteins (Pisum sativum L.). J. Agric. Food Chem 2005, 53, 3057–3064. [Google Scholar]
- Paraman, I.; Hettiarachchy, N.S.; Schaefer, C.; Beck, M.I. Hydrophobicity, solubility, and emulsifying properties of enzyme-modified rice endosperm protein. Cereal Chem 2007, 84, 343–349. [Google Scholar]
- Zhao, G.; Liu, Y.; Zhao, M.; Ren, J.; Yang, B. Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate. Food Chem 2011, 127, 1438–1443. [Google Scholar]
- Dong, X.; Zhao, M.; Shi, J.; Yang, B.; Li, J.; Luo, D.; Jiang, G.; Jiang, Y. Effects of combined high-pressure homogenization and enzymatic treatment on extraction yield, hydrolysis and function properties of peanut proteins. Innov. Food Sci. Emerg. Technol 2011, 12, 478–483. [Google Scholar]
- Horax, R.; Hettiarachchy, N.S.; Chen, P.; Jalaluddin, M. Preparation and characterization of protein isolate from cowpea (Vigna unguiculata L. Walp.). J. Food Sci 2004, 69, fct114–fct118. [Google Scholar]
- Guan, X.; Yao, H.; Chen, Z.; Shan, L.; Zhang, M. Some functional properties of oat bran protein concentrate modified by trypsin. Food Chem 2006, 101, 163–170. [Google Scholar]
- Yin, S.-W.; Tang, C.-H.; Cao, J.-S.; Hu, E.-K.; Wen, Q.-B.; Yang, X.-Q. Effects of limited enzymatic hydrolysis with trypsin on the functional properties of hemp (Cannabis sativa L.) protein isolate. Food Chem 2008, 106, 1004–1013. [Google Scholar]
- Bandyopadhyay, K.; Ghosh, S. Preparation and characterization of papain-modified sesame (Sesamum indicum L.) protein isolates. J. Agric. Food Chem 2002, 50, 6854–6857. [Google Scholar]
- Kamara, M.T.; Amadou, I.; Tarawalie, F.; Huiming, Z. Effect of enzymatic hydrolysis on the functional properties of foxtail millet (Setaria italica L.) proteins. Int. J. Food Sci. Technol 2010, 45, 1175–1183. [Google Scholar]
- Wu, W.; Hettiarachchy, N.; Qi, M. Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J. Am. Oil Chem. Soc 1998, 75, 845–850. [Google Scholar]
- Arcan, I.; Yemenicioglu, A. Effects of controlled pepsin hydrolysis on antioxidant potential and fractional changes of chickpea proteins. Food Res. Int 2010, 43, 140–147. [Google Scholar]
- Yoshie-Stark, Y.; Wada, Y.; Schott, M.; Wäsche, A. Functional and bioactive properties of rapeseed protein concentrates and sensory analysis of food application with rapeseed protein concentrates. LWT Food Sci. Technol 2006, 39, 503–512. [Google Scholar]
- Betancur-Ancona, D.; Martínez-Rosado, R.; Corona-Cruz, A.; Castellanos-Ruelas, A.; Jaramillo-Flores, M.E.; Chel-Guerrero, L. Functional properties of hydrolysates from Phaseolus lunatus seeds. Int. J. Food Sci. Technol 2009, 44, 128–137. [Google Scholar]
- Sijtsma, L.; Tezera, D.; Hustinx, J.; Vereijken, J.M. Improvement of pea protein quality by enzymatic modification. Nahrung Food 1998, 42, 215–216. [Google Scholar]
- Chabanon, G.; Chevalot, I.; Framboisier, X.; Chenu, S.; Marc, I. Hydrolysis of rapeseed protein isolates: Kinetics, characterization and functional properties of hydrolysates. Process Biochem 2007, 42, 1419–1428. [Google Scholar]
- Kim, S.Y.; Park, P.S.W.; Rhee, K.C. Functional properties of proteolytic enzyme modified soy protein isolate. J. Agric. Food Chem 1990, 38, 651–656. [Google Scholar]
- van der Ven, C.; Gruppen, H.; de Bont, D.B.A.; Voragen, A.G.J. Correlations between biochemical characteristics and foam-forming and -stabilizing ability of whey and casein hydrolysates. J. Agric. Food Chem 2002, 50, 2938–2946. [Google Scholar]
- Clemente, A.; Vioque, J.; Sanchez-Vioque, R.; Pedroche, J.; Millán, F. Production of extensive chickpea (Cicer arietinum L.) Protein hydrolysates with reduced antigenic activity. J. Agric. Food Chem 1999, 47, 3776–3781. [Google Scholar]
- Aluko, R.E.; Mofolasayo, O.A.; Watts, B.M. Emulsifying and foaming properties of commercial yellow pea (Pisum sativum L.) seed flours. J. Agric. Food Chem 2009, 57, 9793–9800. [Google Scholar]
- Maninder, K.; Sandhu, K.S.; Singh, N. Comparative study of the functional, thermal and pasting properties of flours from different field pea (Pisum sativum L.) and pigeon pea (Cajanus cajan L.) cultivars. Food Chem 2007, 104, 259–267. [Google Scholar]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int. J. Mol. Sci 2010, 11, 4973–4990. [Google Scholar]
- Gueguen, J.C.M.; Barbot, J.; Schaeffer, F. Dissociation and aggregation of pea legumin induced by pH and ionic strength. J. Sci. Food Agric. 1988, 53, 167–182. [Google Scholar]
- O’Kane, E. Molecular Characterisation and Heat-Induced Gellation of Pea Vicilin and Legumin. Ph.D. Dissertation, Wageningen Universitz, Wageningen, The Netherlands, 2004. [Google Scholar]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; van Boekel, M.A.J.S. Characterization of pea vicilin. 1. Denoting convicilin as the α-subunit of the pisum vicilin family. J. Agric. Food Chem 2004, 52, 3141–3148. [Google Scholar]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; van Boekel, M.A.J.S. Characterization of pea vicilin. 2. Consequences of compositional heterogeneity on heat-induced gelation behavior. J. Agric. Food Chem 2004, 52, 3149–3154. [Google Scholar]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; van Boekel, M.A.J.S. Heat-induced gelation of pea legumin: Comparison with soybean glycinin. J. Agric. Food Chem 2004, 52, 5071–5078. [Google Scholar]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int 2010, 43, 537–546. [Google Scholar]
- Sisson, T.; Thurston, A. Survival of dietary antigens in the digestive tract of calves intolerant to soyabean products. Res. Vet. Sci 1984, 37, 242–246. [Google Scholar]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Vucelic-Radovic, B.V. Assessment of soy genotype and processing method on quality of soybean tofu. J. Agric. Food Chem 2011, 59, 7368–7376. [Google Scholar]
- Karamac, M.; Amarowicz, R.; Kostura, H. Effect of temperature and enzyme/substrate ratio on the hydrolysis of pea protein isolates by trypsin. Czech J. Food Sci 2002, 20, 1–6. [Google Scholar]
- Szymanowska, U.; Jakubczyk, A.; Baraniak, B.; Kur, A. Characterisation of lipoxygenase from pea seeds (Pisum sativum var. Telephone L.). Food Chem 2009, 116, 906–910. [Google Scholar]
- Schwenke, K.D.; Henning, T.; Dudek, S.; Dautzenberg, H.; Danilenko, A.N.; Kozhevnikov, G.O.; Braudo, E.E. Limited tryptic hydrolysis of pea legumin: Molecular mass and conformational stability of legumin-T. Int. J. Biol. Macromol 2001, 28, 175–182. [Google Scholar]
- Gharsallaoui, A.; Saurel, R.; Chambin, O.; Voilley, A. Pea (Pisum sativum, L.) Protein isolate stabilized emulsions: A novel system for microencapsulation of lipophilic ingredients by spray drying. Food Bioprocess Technol 2011. [Google Scholar] [CrossRef]
- Ogunwolu, S.O.; Henshaw, F.O.; Mock, H.P.; Santros, A.; Awonorin, S.O. Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) nut. Food Chem 2009, 115, 852–858. [Google Scholar]
- Herceg, Z.; Režek, A.; Lelas, V.; Krešić, G.; Franetović, M. Effect of carbohydrates on the emulsifying, foaming and freezing properties of whey protein suspensions. J. Food Eng 2007, 79, 279–286. [Google Scholar]
- Helrich, K. Official methods of analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Davis, B.J. Disc electrophoresis—II method and application to human serum proteins. Ann. N. Y. Acad. Sci 1964, 121, 404–427. [Google Scholar]
- Fling, S.P.; Gregerson, D.S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal. Biochem 1986, 155, 83–88. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem 1978, 26, 716–723. [Google Scholar]

| Modified isolates | ||||
|---|---|---|---|---|
| Non-treated | 15 min | 30 min | 60 min | |
| Vicilin | 37.88 a | 33.52 b | 34.57 c | 34.3 c |
| Legumin | 32.96 a | 23.6 b | 26.15 c | 24.43 d |
| V/L ** | 1.15 a | 1.42 b | 1.32 c | 1.40 d |
| V+C/L *** | 1.46 a | 1.87 b | 1.74 c | 1.92 d |
| Foaming properties (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | Pisane M | N.T. | Modified isolates | |||||||
| 15 min | 30 min | 60 min | ||||||||
| FC | FS | FC | FS | FC | FS | FC | FS | FC | FS | |
| 3.0 | 324.2 ± 3.30 a | 89.6 ± 0.04 a | 187.9 ± 3.30 b | 27.4 ± 0.03 b | 293.4 ± 3.10 c | 17.56 ± 0.60 b | 203.0 ± 0.60 d | 10.45 c | 263.6 ± 1.70 e | 48.27 ± 0.50 d |
| 5.0 | 339.4 ± 4.40 a | 6.3 ± 0.03 a | 172.7 ± 8.40 b | 12.3 ± 0.04 b | 309.9 ± 4.10 c | 11.74 ± 1.16 b | 354.5 ± 6.50 d | 35.90 c | 278.8 ± 3.40 e | 72.82 ± 0.20 d |
| 7.0 | 270.8 ± 4.20 a | 0 a | 203.0 ± 4.20 b | 0 a | 339.4 ± 6.50 c | 0 a | 278.8 ± 8.10 a | 7.61b | 278.8 ± 3.00 a | 0 a |
| 8.0 | 203.0 ± 4.70 a | 0 a | 278.8 ± 6.40 b | 45.6 ± 0.03 b | 293.4 ± 4.20 c | 0 a | 263.6 ± 5.60 d | 0 a | 354.5 ± 4.20 e | 0 a |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barać, M.; Čabrilo, S.; Pešić, M.; Stanojević, S.; Pavlićević, M.; Maćej, O.; Ristić, N. Functional Properties of Pea (Pisum sativum, L.) Protein Isolates Modified with Chymosin. Int. J. Mol. Sci. 2011, 12, 8372-8387. https://doi.org/10.3390/ijms12128372
Barać M, Čabrilo S, Pešić M, Stanojević S, Pavlićević M, Maćej O, Ristić N. Functional Properties of Pea (Pisum sativum, L.) Protein Isolates Modified with Chymosin. International Journal of Molecular Sciences. 2011; 12(12):8372-8387. https://doi.org/10.3390/ijms12128372
Chicago/Turabian StyleBarać, Miroljub, Slavica Čabrilo, Mirjana Pešić, Slađana Stanojević, Milica Pavlićević, Ognjen Maćej, and Nikola Ristić. 2011. "Functional Properties of Pea (Pisum sativum, L.) Protein Isolates Modified with Chymosin" International Journal of Molecular Sciences 12, no. 12: 8372-8387. https://doi.org/10.3390/ijms12128372







