Nanotechnology and Nanotoxicology in Retinopathy
Abstract
:1. Introduction
2. Retinopathy as the Target of NP-Based Medicine
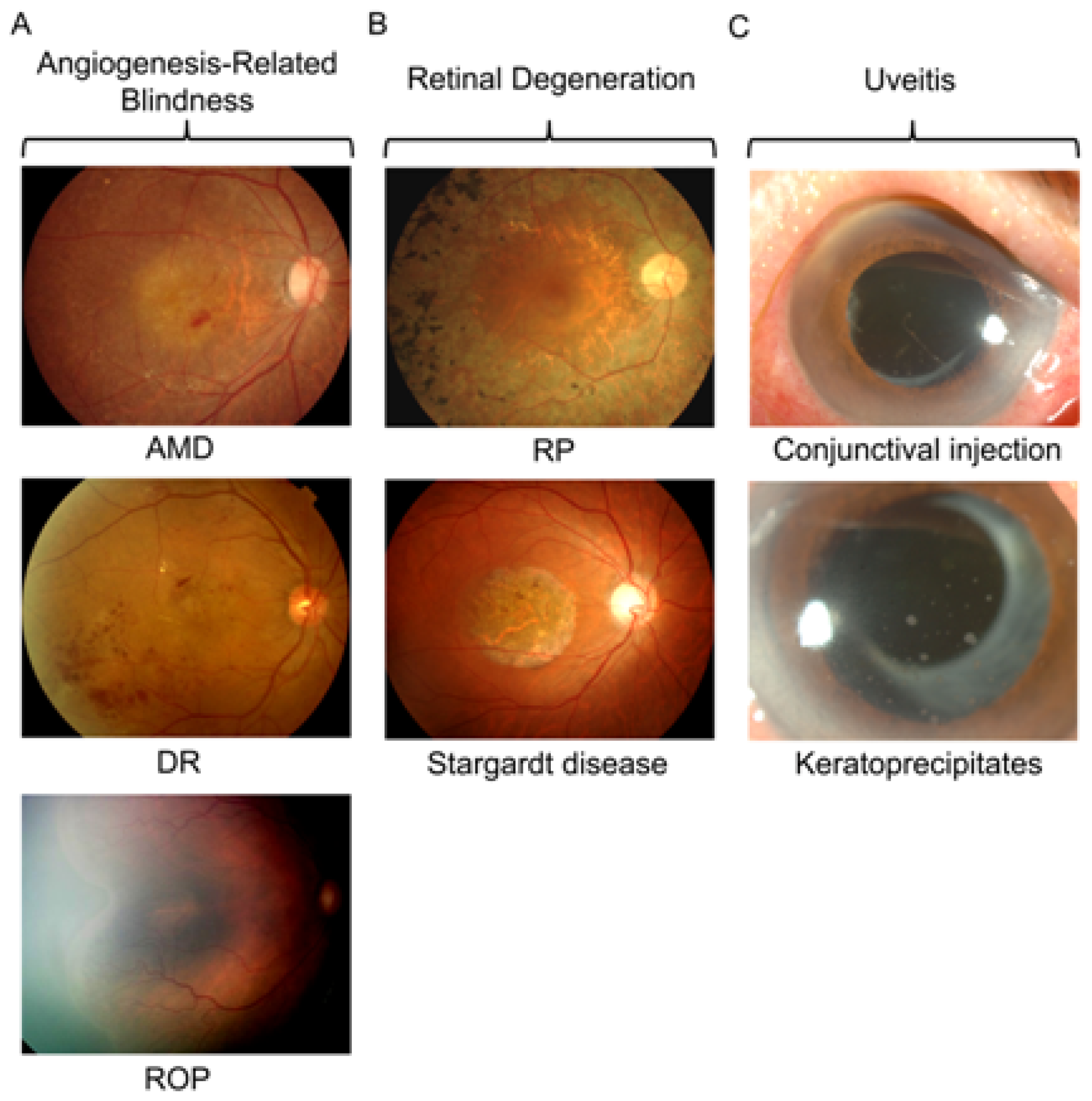
2.1. Angiogenesis-Related Blindness
2.2. Retinal Degeneration
2.3. Uveitis
3. Neuronal Toxicity of NPs in Retinopathy
3.1. Studies on Nanotoxicology in Retinopathy
3.2. Possible Mechanisms of Neuronal Toxicity Induced by NPs
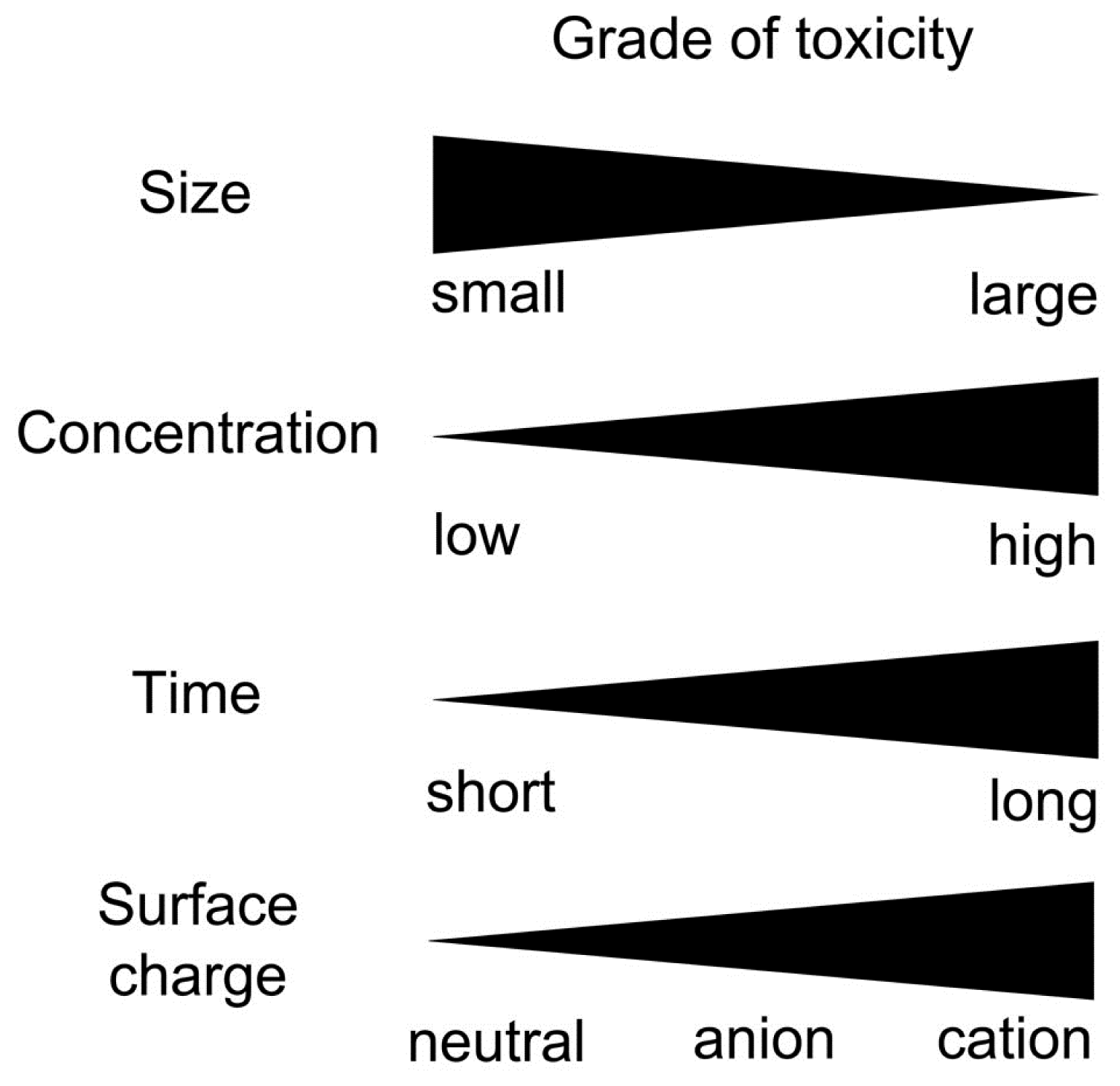
3.3. Factors Affecting Neuronal Toxicity of NPs
4. NPs on Blood-Retinal Barrier: Increasing the Bioavailability versus Affecting the BRB
5. Nanotechnology and Nanotoxicology in Retinopathy: Friends or Foes?
6. Future Directions
Acknowledgments
- Conflicts of InterestThe authors declare no conflict of interest.
References
- Farjo, K.M.; Ma, J.X. The potential of nanomedicine therapies to treat neovascular disease in the retina. J. Angiogenes. Res 2010, 2, 21. [Google Scholar]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res 2010, 29, 596–609. [Google Scholar]
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277. [Google Scholar]
- Jo, D.H.; Kim, J.H.; Kim, J.H. How to overcome retinal neuropathy: The fight against angiogenesis-related blindness. Arch. Pharm. Res 2010, 33, 1557–1565. [Google Scholar]
- Kim, J.H.; Kim, J.H.; Park, J.A.; Lee, S.W.; Kim, W.J.; Yu, Y.S.; Kim, K.W. Blood-neural barrier: Intercellular communication at glio-vascular interface. J. Biochem. Mol. Biol 2006, 39, 339–345. [Google Scholar]
- Cunha-Vaz, J.G. The blood-retinal barriers. Doc. Ophthalmol 1976, 41, 287–327. [Google Scholar]
- Kim, J.H.; Kim, J.H.; Kim, K.W.; Kim, M.H.; Yu, Y.S. Intravenously administered gold nanoparticles pass through the blood-retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology 2009, 20, 505101. [Google Scholar]
- Kim, J.H.; Kim, M.H.; Jo, D.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. The inhibition of retinal neovascularization by gold nanoparticles via suppression of vegfr-2 activation. Biomaterials 2011, 32, 1865–1871. [Google Scholar]
- Kalishwaralal, K.; Sheikpranbabu, S.; BarathManiKanth, S.; Haribalaganesh, R.; Ramkumarpandian, S.; Gurunathan, S. Gold nanoparticles inhibit vascular endothelial growth factor-induced angiogenesis and vascular permeability via src dependent pathway in retinal endothelial cells. Angiogenesis 2011, 14, 29–45. [Google Scholar]
- Zhou, X.; Wong, L.L.; Karakoti, A.S.; Seal, S.; McGinnis, J.F. Nanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the vldlr knockout mouse. PLoS One 2011, 6, e16733. [Google Scholar]
- Kim, H.; Csaky, K.G. Nanoparticle-integrin antagonist c16y peptide treatment of choroidal neovascularization in rats. J. Control. Release 2010, 142, 286–293. [Google Scholar]
- Singh, S.R.; Grossniklaus, H.E.; Kang, S.J.; Edelhauser, H.F.; Ambati, B.K.; Kompella, U.B. Intravenous transferrin, rgd peptide and dual-targeted nanoparticles enhance anti-vegf intraceptor gene delivery to laser-induced cnv. Gene Ther 2009, 16, 645–659. [Google Scholar]
- Jin, J.; Zhou, K.K.; Park, K.; Hu, Y.; Xu, X.; Zheng, Z.; Tyagi, P.; Kompella, U.B.; Ma, J.X. Anti-inflammatory and antiangiogenic effects of nanoparticle-mediated delivery of a natural angiogenic inhibitor. Invest. Ophthalmol. Vis. Sci 2011, 52, 6230–6237. [Google Scholar]
- Park, K.; Chen, Y.; Hu, Y.; Mayo, A.S.; Kompella, U.B.; Longeras, R.; Ma, J.X. Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes 2009, 58, 1902–1913. [Google Scholar]
- Jo, D.H.; Kim, J.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomedicine 2011. In press. [Google Scholar]
- Kalishwaralal, K.; Banumathi, E.; Ram Kumar Pandian, S.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver nanoparticles inhibit vegf induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B 2009, 73, 51–57. [Google Scholar]
- Kalishwaralal, K.; Barathmanikanth, S.; Pandian, S.R.; Deepak, V.; Gurunathan, S. Silver nano - a trove for retinal therapies. J. Control Release 2010, 145, 76–90. [Google Scholar]
- Sakai, T.; Kuno, N.; Takamatsu, F.; Kimura, E.; Kohno, H.; Okano, K.; Kitahara, K. Prolonged protective effect of basic fibroblast growth factor-impregnated nanoparticles in royal college of surgeons rats. Invest. Ophthalmol. Vis. Sci 2007, 48, 3381–3387. [Google Scholar]
- Strettoi, E.; Gargini, C.; Novelli, E.; Sala, G.; Piano, I.; Gasco, P.; Ghidoni, R. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2010, 107, 18706–18711. [Google Scholar]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar]
- Kong, L.; Cai, X.; Zhou, X.; Wong, L.L.; Karakoti, A.S.; Seal, S.; McGinnis, J.F. Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiol. Dis 2011, 42, 514–523. [Google Scholar]
- Read, S.P.; Cashman, S.M.; Kumar-Singh, R. Pod nanoparticles expressing gdnf provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol. Ther 2010, 18, 1917–1926. [Google Scholar]
- Cai, X.; Conley, S.M.; Nash, Z.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J 2010, 24, 1178–1191. [Google Scholar]
- Cai, X.; Nash, Z.; Conley, S.M.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS One 2009, 4, e5290. [Google Scholar]
- de Kozak, Y.; Andrieux, K.; Villarroya, H.; Klein, C.; Thillaye-Goldenberg, B.; Naud, M.C.; Garcia, E.; Couvreur, P. Intraocular injection of tamoxifen-loaded nanoparticles: A new treatment of experimental autoimmune uveoretinitis. Eur. J. Immunol 2004, 34, 3702–3712. [Google Scholar]
- Sakai, T.; Kohno, H.; Ishihara, T.; Higaki, M.; Saito, S.; Matsushima, M.; Mizushima, Y.; Kitahara, K. Treatment of experimental autoimmune uveoretinitis with poly(lactic acid) nanoparticles encapsulating betamethasone phosphate. Exp. Eye Res 2006, 82, 657–663. [Google Scholar]
- Saint-Geniez, M.; Maharaj, A.S.; Walshe, T.E.; Tucker, B.A.; Sekiyama, E.; Kurihara, T.; Darland, D.C.; Young, M.J.; D’Amore, P.A. Endogenous vegf is required for visual function: Evidence for a survival role on muller cells and photoreceptors. PLoS One 2008, 3, e3554. [Google Scholar]
- Lee, S.J.; Koh, H.J. Enlargement of the foveal avascular zone in diabetic retinopathy after adjunctive intravitreal bevacizumab (avastin) with pars plana vitrectomy. J. Ocul. Pharmacol. Ther 2009, 25, 173–174. [Google Scholar]
- Inan, U.U.; Avci, B.; Kusbeci, T.; Kaderli, B.; Avci, R.; Temel, S.G. Preclinical safety evaluation of intravitreal injection of full-length humanized vascular endothelial growth factor antibody in rabbit eyes. Invest. Ophthalmol. Vis. Sci 2007, 48, 1773–1781. [Google Scholar]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic properties of gold nanoparticles. Clin. Cancer Res 2005, 11, 3530–3534. [Google Scholar]
- Panyam, J.; Dali, M.M.; Sahoo, S.K.; Ma, W.; Chakravarthi, S.S.; Amidon, G.L.; Levy, R.J.; Labhasetwar, V. Polymer degradation and in vitro release of a model protein from poly(d,l-lactide-co-glycolide) nano- and microparticles. J. Control. Release 2003, 92, 173–187. [Google Scholar]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev 2003, 55, 329–347. [Google Scholar]
- Rivas, M.A.; Vecino, E. Animal models and different therapies for treatment of retinitis pigmentosa. Histol. Histopathol 2009, 24, 1295–1322. [Google Scholar]
- Fishman, G.A. Historical evolution in the understanding of stargardt macular dystrophy. Ophthalmic. Genet 2010, 31, 183–189. [Google Scholar]
- Liu, M.M.; Tuo, J.; Chan, C.C. Gene therapy for ocular diseases. Br. J. Ophthalmol 2011, 95, 604–612. [Google Scholar]
- Lee, F.F.; Foster, C.S. Pharmacotherapy of uveitis. Expert Opin. Pharmacother 2010, 11, 1135–1146. [Google Scholar]
- Okabe, J.; Kimura, H.; Kunou, N.; Okabe, K.; Kato, A.; Ogura, Y. Biodegradable intrascleral implant for sustained intraocular delivery of betamethasone phosphate. Invest. Ophthalmol. Vis. Sci 2003, 44, 740–744. [Google Scholar]
- Kempen, J.H.; Altaweel, M.M.; Holbrook, J.T.; Jabs, D.A.; Louis, T.A.; Sugar, E.A.; Thorne, J.E. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: The multicenter uveitis steroid treatment trial. Ophthalmology 2011, 118, 1916–1926. [Google Scholar]
- Tian, N. Visual experience and maturation of retinal synaptic pathways. Vision Res 2004, 44, 3307–3316. [Google Scholar]
- Bourges, J.L.; Gautier, S.E.; Delie, F.; Bejjani, R.A.; Jeanny, J.C.; Gurny, R.; BenEzra, D.; Behar-Cohen, F.F. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest. Ophthalmol. Vis. Sci 2003, 44, 3562–3569. [Google Scholar]
- Merodio, M.; Irache, J.M.; Valamanesh, F.; Mirshahi, M. Ocular disposition and tolerance of ganciclovir-loaded albumin nanoparticles after intravitreal injection in rats. Biomaterials 2002, 23, 1587–1594. [Google Scholar]
- Bakri, S.J.; Pulido, J.S.; Mukherjee, P.; Marler, R.J.; Mukhopadhyay, D. Absence of histologic retinal toxicity of intravitreal nanogold in a rabbit model. Retina 2008, 28, 147–149. [Google Scholar]
- Ding, X.Q.; Quiambao, A.B.; Fitzgerald, J.B.; Cooper, M.J.; Conley, S.M.; Naash, M.I. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS One 2009, 4, e7410. [Google Scholar]
- Hafeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P.; Bardenstein, D. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol. Pharm 2009, 6, 1417–1428. [Google Scholar]
- Prow, T.W.; Bhutto, I.; Kim, S.Y.; Grebe, R.; Merges, C.; McLeod, D.S.; Uno, K.; Mennon, M.; Rodriguez, L.; Leong, K.; et al. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomedicine 2008, 4, 340–349. [Google Scholar]
- Gramowski, A.; Flossdorf, J.; Bhattacharya, K.; Jonas, L.; Lantow, M.; Rahman, Q.; Schiffmann, D.; Weiss, D.G.; Dopp, E. Nanoparticles induce changes of the electrical activity of neuronal networks on microelectrode array neurochips. Environ. Health Perspect 2010, 118, 1363–1369. [Google Scholar]
- Iavicoli, I.; Leso, V.; Fontana, L.; Bergamaschi, A. Toxicological effects of titanium dioxide nanoparticles: A review of in vitro mammalian studies. Eur. Rev. Med. Pharmacol. Sci 2011, 15, 481–508. [Google Scholar]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett 2009, 187, 15–21. [Google Scholar]
- Wang, J.; Rahman, M.F.; Duhart, H.M.; Newport, G.D.; Patterson, T.A.; Murdock, R.C.; Hussain, S.M.; Schlager, J.J.; Ali, S.F. Expression changes of dopaminergic system-related genes in pc12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology 2009, 30, 926–933. [Google Scholar]
- Choi, J.; Zheng, Q.; Katz, H.E.; Guilarte, T.R. Silica-based nanoparticle uptake and cellular response by primary microglia. Environ. Health Perspect 2010, 118, 589–595. [Google Scholar]
- Hutter, E.; Boridy, S.; Labrecque, S.; Lalancette-Hebert, M.; Kriz, J.; Winnik, F.M.; Maysinger, D. Microglial response to gold nanoparticles. ACS Nano 2010, 4, 2595–2606. [Google Scholar]
- Ariano, P.; Zamburlin, P.; Gilardino, A.; Mortera, R.; Onida, B.; Tomatis, M.; Ghiazza, M.; Fubini, B.; Lovisolo, D. Interaction of spherical silica nanoparticles with neuronal cells: Size-dependent toxicity and perturbation of calcium homeostasis. Small 2011, 7, 766–774. [Google Scholar]
- Liu, Z.; Ren, G.; Zhang, T.; Yang, Z. Action potential changes associated with the inhibitory effects on voltage-gated sodium current of hippocampal ca1 neurons by silver nanoparticles. Toxicology 2009, 264, 179–184. [Google Scholar]
- Prabhu, B.M.; Ali, S.F.; Murdock, R.C.; Hussain, S.M.; Srivatsan, M. Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat. Nanotoxicology 2010, 4, 150–160. [Google Scholar]
- Krug, H.F.; Wick, P. Nanotoxicology: An interdisciplinary challenge. Angew. Chem. Int. Ed. Engl 2011, 50, 1260–1278. [Google Scholar]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.; Geertsma, R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar]
- Deng, X.; Luan, Q.; Chen, W.; Wang, Y.; Wu, M.; Zhang, H.; Jiao, Z. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 2009, 20, 115101. [Google Scholar]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.A.; Olmedo, I.; Clos, A.; Sadagopa Ramanujam, V.M.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun 2010, 393, 649–655. [Google Scholar]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled tio(2) nanoparticles. Toxicology 2008, 254, 82–90. [Google Scholar]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem 2004, 15, 897–900. [Google Scholar]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J. Drug Target 2004, 12, 635–641. [Google Scholar]
- Baber, O.; Jang, M.; Barber, D.; Powers, K. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro beas-2b cells. Inhal. Toxicol 2011, 23, 532–543. [Google Scholar]
| Material | Type | Size (nm) | Concentration | Animal model | Administration | Reference |
|---|---|---|---|---|---|---|
| Angiogenesis-related blindness | ||||||
| Gold | Nanoparticle | 20 | 1 μM | OIR | IVT | [8] |
| Gold | Nanoparticle | 50 | 500 nM | Cell work only | N/A | [9] |
| Nanoceria | Nanoparticle | 3–5 | 1 mM | VLDLR KO | IVT | [10] |
| PLA/PLA-PEO | Nanocapsule | 302 | 0.12 mg/μL | Laser CNV | IVT | [11] |
| PLGA | Nanoconjugate | 270–420 | N/A | Laser CNV | IV | [12] |
| PLGA-chitosan | Nanocapsule | 260 | N/A | Laser CNV | IVT | [13] |
| PLGA-chitosan | Nanocapsule | 260 | N/A | OIR | IVT | [14] |
| Silicate | Nanoparticle | 57 | 10 μg/mL | OIR | IVT | [15] |
| Silver | Nanoparticles | 50 | 500 nM | Cell work only | N/A | [16,17] |
| Retinal degeneration | ||||||
| Gelatin | Nanoconjugate | 585 | N/A | RCS rat | IVT | [18] |
| Lipid | Nanocapsule | 40–200 | N/A | rd10 mouse | Topical | [19] |
| Nanoceria | Nanoparticle | −5 | 0.1–1 μM | Light-induced RD | IVT | [20] |
| Nanoceria | Nanoparticle | N/A | 1 mM | tubby mouse | Intracardial | [21] |
| PEG | Nanoconjugate | 175.9 | N/A | Light-induced RD | Subretinal | [22] |
| PEG | Nanocapsule | −8 | 3.06 μg/μL | rds+/− mouse | Subretinal | [23,24] |
| Uveitis | ||||||
| PEG | Nanocapsule | 95–112 | N/A | EAU | IVT | [25] |
| PLA | Nanocapsule | 100–200 | N/A | EAU | IV | [26] |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jo, D.H.; Lee, T.G.; Kim, J.H. Nanotechnology and Nanotoxicology in Retinopathy. Int. J. Mol. Sci. 2011, 12, 8288-8301. https://doi.org/10.3390/ijms12118288
Jo DH, Lee TG, Kim JH. Nanotechnology and Nanotoxicology in Retinopathy. International Journal of Molecular Sciences. 2011; 12(11):8288-8301. https://doi.org/10.3390/ijms12118288
Chicago/Turabian StyleJo, Dong Hyun, Tae Geol Lee, and Jeong Hun Kim. 2011. "Nanotechnology and Nanotoxicology in Retinopathy" International Journal of Molecular Sciences 12, no. 11: 8288-8301. https://doi.org/10.3390/ijms12118288




