Correlation of the Rates of Solvolysis of i-Butyl Fluoroformate and a Consideration of Leaving-Group Effects
Abstract
:1. Introduction
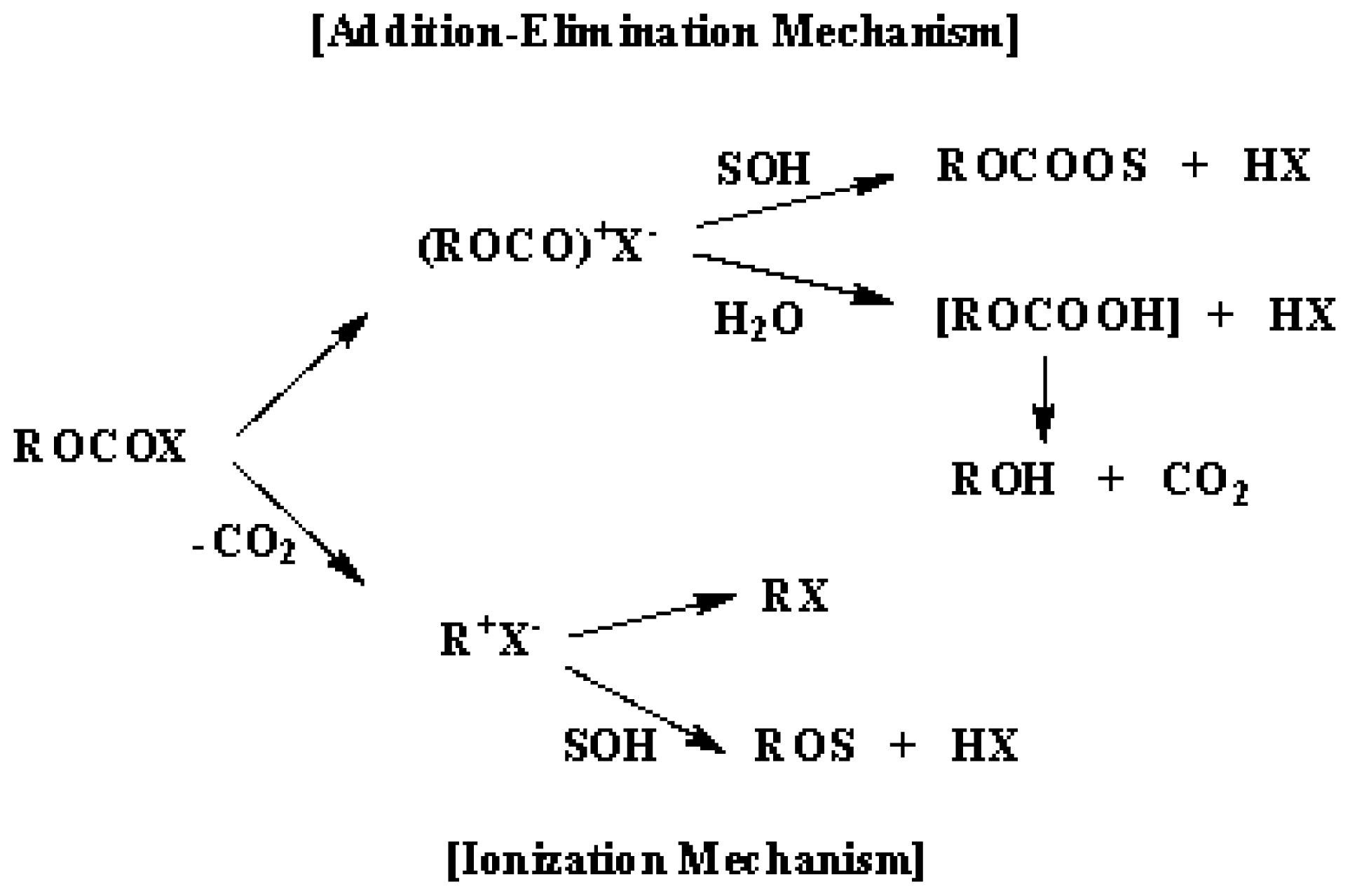
2. Results and Discussion
3. Experimental Section
4. Conclusions
References
- Grunwald, E.; Winstein, S. The correlation of solvolysis rates. J. Am. Chem. Soc 1948, 70, 846–854. [Google Scholar]
- Grunwald, E.; Winstein, S.; Jones, H.W. The correlation of solvolyses rates and the classification of solvolysis reactions into mechanistic categories. J. Am. Chem. Soc 1951, 73, 2700–2707. [Google Scholar]
- Kevill, D.N.; Anderson, S.W. An improved scale of solvent nucleophlicitiy based on the solvolysis of the S-methyldibenzothiophenium ion. J. Org. Chem 1991, 56, 1845–1850. [Google Scholar]
- Kevill, D.N. Development and Uses of Scales of Solvent Nucleophlicity. In In Advances in Quantitative Structure-Property Relationship; Charton, M., Ed.; JAI Press: Greenwich, CT, USA, 1996; Volume 1, pp. 81–115. [Google Scholar]
- Bentley, T.W.; Carter, G.E. The SN2-SN1 Spectrum. 4. The SN2 (intermediate) mechanism for solvolyses of tert-butyl chloride: A revised Y scale of solvent ionizing power based on solvolyses of 1-adamantyl chloride. J. Am. Chem. Soc 1982, 104, 5741–5747. [Google Scholar]
- Bentley, T.W.; Llewellyn, G. YX scales of solvent ionizing power. Prog. Phys. Org. Chem 1990, 17, 121–158. [Google Scholar]
- Kevill, D.N.; D’Souza, M.J. Additional YCl values and the correlation of the specific rates of solvolysis of tert-butyl chloride in terms of NT and YCl scales. J. Chem. Res. Synop 1993, 174–175. [Google Scholar]
- Von Schleyer, P.R.; Nicholas, R.D. The reactivity of bridgehead compounds of adamantane. J. Am. Chem. Soc 1961, 83, 2700–2707. [Google Scholar]
- Fainberg, A.H.; Winstein, S.J. Correlation of solvolysis rates. III. t-Butyl chloride in a wide range of solvent mixtures. J. Am. Chem. Soc 1956, 78, 2770–2777. [Google Scholar]
- Kevill, D.N.; Kim, J.C.; Kyong, J.B. Correlation of the rates of solvolysis of methyl chloroformate with solvent properties. J. Chem. Res. Synop 1999, 150–151. [Google Scholar]
- Kevill, D.N.; D’Souza, M.J. Concerning the two reaction channels for the solvolyses of ethyl chloroformate and ethyl chlorothioformate. J. Org. Chem 1998, 63, 2120–2124. [Google Scholar]
- Kyong, J.B.; Won, H.; Kevill, D.N. Application of the the extended grunwald-winstein equation to solvolyses of n-propyl chloroformate. Int. J. Mol. Sci 2005, 6, 87–96. [Google Scholar]
- D’Souza, M.J.; McAneny, M.J.; Kevill, D.N.; Kyong, J.B.; Choi, S.H. Kinetic evaluation of the solvolysis of isobutyl chloro- and chlorothioformate esters. Beilstein J. Org. Chem 2011, 7, 543–552. [Google Scholar]
- Kyong, J.B.; Kim, Y.G.; Kim, D.K.; Kevill, D.N. Dual pathways in the solvolyses of isopropyl chloroformate. Bull. Korean Chem. Soc 2000, 21, 662–664. [Google Scholar]
- Kevill, D.N.; Kyong, J.B.; Weitl, F.L. Solvolysis-decomposition of 1-adamantyl chloroformate: Evidence for ion pair return in 1-adamantyl chloride solvolysis. J. Org. Chem 1990, 55, 4304–4311. [Google Scholar]
- Seong, M.H.; Choi, S.H.; Lee, Y.W.; Kyong, J.B.; Kim, D.K.; Kevill, D.N. Correlation of the rates of solvolysis of methyl fluoroformate using the extended grunwald-winstein equation. Bull. Korean Chem. Soc 2009, 30, 2408–2412. [Google Scholar]
- Seong, M.H.; Kyong, J.B.; Lee, Y.H.; Kevill, D.N. Correlation of the specific rates of solvolysis of ethyl fluoroformate using the extended grunwald-winstein equation. Int. J. Mol. Sci 2009, 10, 929–941. [Google Scholar]
- Seong, M.H.; Kyong, J.B.; Kim, D.K.; Kevill, D.N. Application of the the extended grunwald-winstein equation to solvolyses of n-propyl fluoroformate and a consideration of leaving group effects. Bull. Korean Chem. Soc. 2008, 29, 1747–1751. [Google Scholar]
- Kevill, D.N.; D’Souza, M.J. Correlation of the rates of solvolysis of n-octyl fluoroformate and a comparison with n-octyl chloroformate solvolysis. J. Chem. Soc. Perkin Trans 2002, 2, 240–243. [Google Scholar]
- Lee, S.H.; Rhu, C.J.; Kyong, J.B.; Kim, D.K.; Kevill, D.N. Correlation of the rates of solvolysis of isopropyl fluoroformate using the extended grunwald-winstein equation. Bull. Korean Chem. Soc 2007, 28, 657–661. [Google Scholar]
- Lee, Y.W.; Seong, M.H.; Kyong, J.B.; Kevill, D.N. Correlation of the specific rates of solvolysis of t-butyl fluoroformate using the extended grunwald-winstein equation. Bull. Korean Chem. Soc 2010, 31, 3366–3370. [Google Scholar]
- Kevill, D.N.; Kyong, J.B. Multiple pathways in the solvolysis of 1-adamantyl fluoroformate. J. Org. Chem 1992, 57, 258–265. [Google Scholar]
- Kevill, D.N.; Lin, G.M.L. Solvent nucleophilicity. A scale based on triethyloxonium ion solvolysis. J. Am. Chem. Soc 1979, 101, 3916–3919. [Google Scholar]
- Kevill, D.N.; D’Souza, M.J. Use of the simple and extended grunwald-winstein equations in the correlation of the rates of solvolysis of highly hindered tertiary alkyl derivatives. Curr. Org. Chem 2010, 14, 1037–1049. [Google Scholar]
- Sykes, P. A Guidebook to Mechanism in Organic Chemistry, 6th ed; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Kevill, D.N. The Chemistry of the Functional Groups: The Chemistry of Acyl Halides; Patai, S., Ed.; John Wiley & Sons: New York, NY, USA, 1972. [Google Scholar]
- Swain, C.G.; Scott, C.B. Rates of solvolysis of some alkyl fluorides and chlorides. J. Am. Chem. Soc 1953, 75, 246–248. [Google Scholar]
- Queen, A.; Nour, T.A. Kinetics and mechanism of the acetate catalyzed hydrolysis of 4-methoxyphenyl chloroformate and 4-methoxyphenyl fluoroformate in aqueous dioxane. J. Chem. Soc. Perkin Trans 1976, 2, 935–939. [Google Scholar]
- Koo, I.S.; Yang, K.; Kang, D.H.; Park, H.J.; Kang, K.; Lee, I. Transition-state variation in the solvolyses of phenyl chlorothionoformate in alcohol-water mixtures. Bull. Korean Chem. Soc 1999, 20, 577–580. [Google Scholar]
- Ryu, Z.H.; Shin, S.H.; Lee, J.P.; Lim, G.T.; Bentley, T.W. Kinetics solvent isotope effects and correlations of rates of solvolyses for α-methylthio and other substitued acetyl chlorides. J. Chem. Soc. Perkin Trans 2002, 2, 1283–1287. [Google Scholar]
- Oh, Y.H.; Jang, G.G.; Lim, G.T.; Ryu, Z.H. Marked difference in solvation effects and mechanism between solvolyses of substituted acetyl chloride with alkyl groups and with aromatic rings in aqueous fluorinated alcohol and in 2,2,2-trifluoroethanol-ethanol solvent systems. Bull. Korean Chem. Soc 2002, 23, 1083–1096. [Google Scholar]
- Kyong, J.B.; Park, B.C.; Kim, C.B.; Kevill, D.N. Rate and product studies with benzyl and p-nitrobenzyl chloroformates under sovolytic conditions. J. Org. Chem 2000, 65, 8051–8058. [Google Scholar]
- Kyong, J.B.; Ryu, S.H.; Kevill, D.N. Rate and product studies of the solvolyses of benzyl fluoroformate. Int. J. Mol. Sci 2006, 7, 186–196. [Google Scholar]
- Queen, A. Kinetics of the hydrolysis of acyl chlorides in pure water. Can. J. Chem 1967, 45, 1619–1629. [Google Scholar]
- Park, K.H.; Kyong, J.B.; Kevill, D.N. Nucleophilic substution reactions of p-nitrobenzyl chloroformate. Bull. Korean Chem. Soc 2000, 21, 1267–1270. [Google Scholar]
- Kevill, D.N.; Miller, B. Application of the NT solvent nucleophilicity scale to attack at phosphorus: Solvolyses of N,N,N’,N’-tetramethyldiamidophosphorochloridate. J. Org. Chem 2002, 67, 7399–7406. [Google Scholar]
- Kevill, D.N.; D’Souza, M.J. Correlation of the rates of solvolysis of benzoyl fluoride and a consideration of leaving-group effects. J. Org. Chem 2004, 69, 7044–7050. [Google Scholar]
- Kyong, J.B.; Rhu, C.J.; Kim, Y.G.; Kevill, D.N. Rate and product studies with 2-adamantyl fluoroformate under solvolytic conditions. J. Phys. Org. Chem 2007, 20, 525–531. [Google Scholar]
- Anh, D.V.; Olofson, R.A.; Wolf, P.R.; Piteau, M.D.; Senet, J.P.G. A simple conversion of 1-chloroethyl carbonates to fluoroformates: Value in the preparation of tertiary alkyl fluoroformates. J. Org. Chem 1990, 55, 1847–1851. [Google Scholar]
- Cuomo, J.; Olofson, R.A. Efficient and convenient synthesis of fluoroformates and carbamoyl fluorides. J. Org. Chem 1979, 44, 1016–1017. [Google Scholar]

| Solvent b | 104 k (s−1) c | NTd | YCld |
|---|---|---|---|
| 100% MeOH e | 2.15 ± 0.02 g | 0.17 | −1.17 |
| 90% MeOH | 15.1 ± 0.2 | −0.01 | −0.18 |
| 80% MeOH | 42.5 ± 0.2 | −0.06 | 0.67 |
| 70% MeOH | 77.6 ± 0.2 | −0.40 | 1.46 |
| 100% EtOH | 0.378 ± 0.002 | 0.37 | −2.52 |
| 90% EtOH | 6.77 ± 0.04 | 0.16 | −0.94 |
| 80% EtOH | 14.4 ± 0.1 | 0.00 | 0.00 |
| 70% EtOH | 24.1 ± 0.1 | −0.20 | 0.78 |
| 60% EtOH | 35.3 ± 0.2 | −0.38 | 1.38 |
| 50% EtOH | 70.0 ± 0.2 | −0.58 | 2.02 |
| 90% Acetone | 0.0923 ± 0.0002 | −0.35 | −2.39 |
| 80% Acetone | 0.823 ± 0.001 | −0.37 | −0.80 |
| 70% Acetone | 2.60 ± 0.01 | −0.42 | 0.17 |
| 60% Acetone | 6.44 ± 0.03 | −0.52 | 1.00 |
| 97% TFE | 0.00678 ± 0.00005 | −3.30 | 2.83 |
| 90% TFE | 0.103 ± 0.003 | −2.55 | 2.85 |
| 70% TFE | 2.33 ± 0.04 | −1.98 | 2.96 |
| 50% TFE | 8.81 ± 0.04 | −1.73 | 3.16 |
| 80T-20E f | 0.0500 ± 0.0005 | −1.76 | 1.89 |
| 60T-40E f | 0.173 ± 0.002 | −0.94 | 0.63 |
| 40T-60E f | 0.440 ± 0.002 | −0.34 | −0.48 |
| 20T-80E f | 0.598 ± 0.004 | 0.08 | −1.42 |
| Solvent a | T, °C | 104 k (s−1) b | ΔH‡313c | ΔS‡313c |
|---|---|---|---|---|
| 100% MeOH | 40.0 | 2.15 ± 0.02 d | 9.5 ± 0.2 | −45.2 ± 0.7 |
| 45.0 | 2.80 ± 0.04 | |||
| 50.0 | 3.51 ± 0.02 | |||
| 55.0 | 4.55 ± 0.02 | |||
| 100% EtOH | 40.0 | 0.378 ± 0.002 d | 10.5 ± 0.2 | −45.3 ± 0.8 |
| 45.0 | 0.508 ± 0.002 | |||
| 50.0 | 0.671 ± 0.002 | |||
| 55.0 | 0.854 ± 0.003 | |||
| 80% EtOH | 40.0 | 14.4 ± 0.1 d | 9.8 ± 0.5 | −40.3 ± 1.5 |
| 45.0 | 19.5 ± 0.2 | |||
| 50.0 | 25.0 ± 0.2 | |||
| 55.0 | 31.0 ± 0.3 | |||
| 70% TFE | 40.0 | 2.33 ± 0.04 d | 11.1 ± 0.4 | −39.9 ± 1.4 |
| 45.0 | 3.08 ± 0.01 | |||
| 50.0 | 4.03 ± 0.04 | |||
| 55.0 | 5.54 ± 0.02 |
| Substrate | Mech.a | n b | l c | m c | c c | R d | l/m |
|---|---|---|---|---|---|---|---|
| 1 | A-E | 22 e | 1.78 ± 0.13 | 0.85 ± 0.10 | −0.07 ± 0.10 | 0.956 | 2.09 |
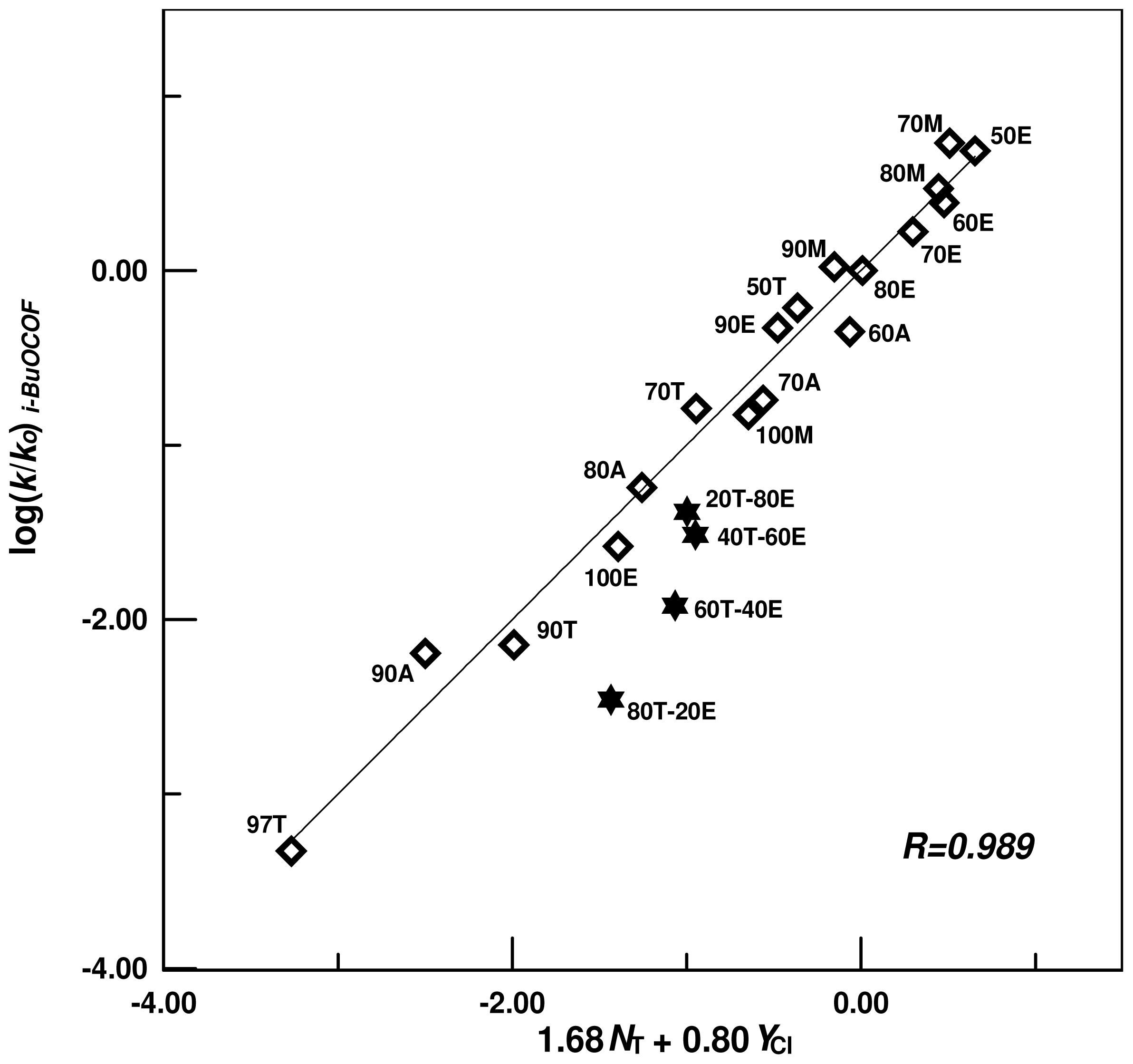
| A-E | 18 e,f | 1.68 ± 0.07 | 0.80 ± 0.04 | 0.01 ± 0.05 | 0.989 | 2.10 | |
| 2 | A-E | 18 g | 1.82 ± 0.15 | 0.53 ± 0.05 | 0.18 ± 0.07 | 0.957 | 3.43 |
| 3 | A-E | 14 h | 1.33 ± 0.09 | 0.73 ± 0.06 | −0.08 ± 0.08 | 0.972 | 1.82 |
| 4 | A-E | 17 i | 1.34 ± 0.14 | 0.77 ± 0.07 | −0.06 ± 0.10 | 0.942 | 1.74 |
| 5 | A-E | 16 j | 1.72 ± 0.12 | 0.91 ± 0.08 | 0.05 ± 0.08 | 0.970 | 1.89 |
| 6 | A-E | 19 k | 1.67 ± 0.07 | 0.76 ± 0.03 | −0.08 ± 0.18 | 0.988 | 2.20 |
| 7 | A-E | 20 l | 1.59 ± 0.16 | 0.80 ± 0.06 | 0.06 ± 0.08 | 0.957 | 1.99 |
| 8 | I | 17 m | 0.41 ± 0.05 | 0.65 ± 0.03 | 0.02 ± 0.04 | 0.989 | 0.63 |
| 9 | A-E | 10 n | 2.78 ± 0.21 | 1.01 ± 0.06 | 0.09 ± 0.16 | 0.987 | 2.78 |
| I | 16 n | ~0 | 0.70 ± 0.01 | −0.02 ± 0.05 | 0.999 | ~0 |
| Solvent c | methyl e | ethyl f | n-propyl g | i-butyl h | n-octyl i | i-propyl j | t-butyl k | 1-adamantyl l |
|---|---|---|---|---|---|---|---|---|
| 100% MeOH | 2.47 (5.81) | 0.836 (2.32) | - (2.19) | 0.952 (2.15) | 0.853 | 0.217 | 7.43 × 10−2 | 2.51 × 10−2 |
| 100% EtOH | 0.424 (1.09) | 0.135 (0.394) | - (0.437) | 0.155 (0.378) | 0.153 | 3.93 × 10−2 | 1.31 × 10−2 | 2.29 × 10−3 |
| 80% EtOH | 19.1 (43.6) | 6.52 (14.3) | - (14.0) | 6.31 (14.4) | 5.96 m | 1.71 | 0.616 | 0.150 |
| 70% TFE d | 4.75 (10.8) | 1.23 (3.61) | - (2.20) | 0.895 (2.33) | 0.430 m | 0.240 | 6.84 | - |
| kMeOH/kMeOD | 3.98 e | 3.10 f | 3.32 g | 3.40 h | - | 2.53 j | 1.26 k | - |
| Solvent a | methyl c | ethyl d | n-propyl e | i-butyl f | n-octyl g | i-propyl h | 1-adamantyl i |
|---|---|---|---|---|---|---|---|
| 100% EtOH | 0.83 | 0.57 | 0.57 | 0.45 | 0.62 | 0.18 | 1.20 × 10−5 |
| 80% EtOH | 8.28 | 8.74 | 5.62 | 5.43 | 8.09 | 2.11 | 3.17 × 10−5 |
| 60% EtOH | - | 14.0 | - | 8.43 | 15.1 | 1.79 | - |
| 100% MeOH | 1.12 | 0.93 | 0.75 | 0.66 | 0.95 | 0.39 | 1.56 × 10−5 |
| 90% MeOH | 5.11 | 4.82 | - | 2.42 | - | 1.76 | - |
| 80% Me2CO | 3.71 | 3.90 | 4.24 j | 2.60 | 2.86 | 0.53 | - |
| 70% TFE b | 27.2 | 19.3 | 7.72 | 8.86 | 10.2 k | 0.067 | - |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, Y.; Park, K.-H.; Seong, M.H.; Kyong, J.B.; Kevill, D.N. Correlation of the Rates of Solvolysis of i-Butyl Fluoroformate and a Consideration of Leaving-Group Effects. Int. J. Mol. Sci. 2011, 12, 7806-7817. https://doi.org/10.3390/ijms12117806
Lee Y, Park K-H, Seong MH, Kyong JB, Kevill DN. Correlation of the Rates of Solvolysis of i-Butyl Fluoroformate and a Consideration of Leaving-Group Effects. International Journal of Molecular Sciences. 2011; 12(11):7806-7817. https://doi.org/10.3390/ijms12117806
Chicago/Turabian StyleLee, Yelin, Kyoung-Ho Park, Mi Hye Seong, Jin Burm Kyong, and Dennis N. Kevill. 2011. "Correlation of the Rates of Solvolysis of i-Butyl Fluoroformate and a Consideration of Leaving-Group Effects" International Journal of Molecular Sciences 12, no. 11: 7806-7817. https://doi.org/10.3390/ijms12117806




