A 1HNMR-Based Metabonomics Study of Postmenopausal Osteoporosis and Intervention Effects of Er-Xian Decoction in Ovariectomized Rats
Abstract
:1 Introduction
2. Experimental
2.1. Materials
2.2. Animals
2.3. Establishment of the Osteoporotic Model and Drug Administration
2.4. Sample Collection
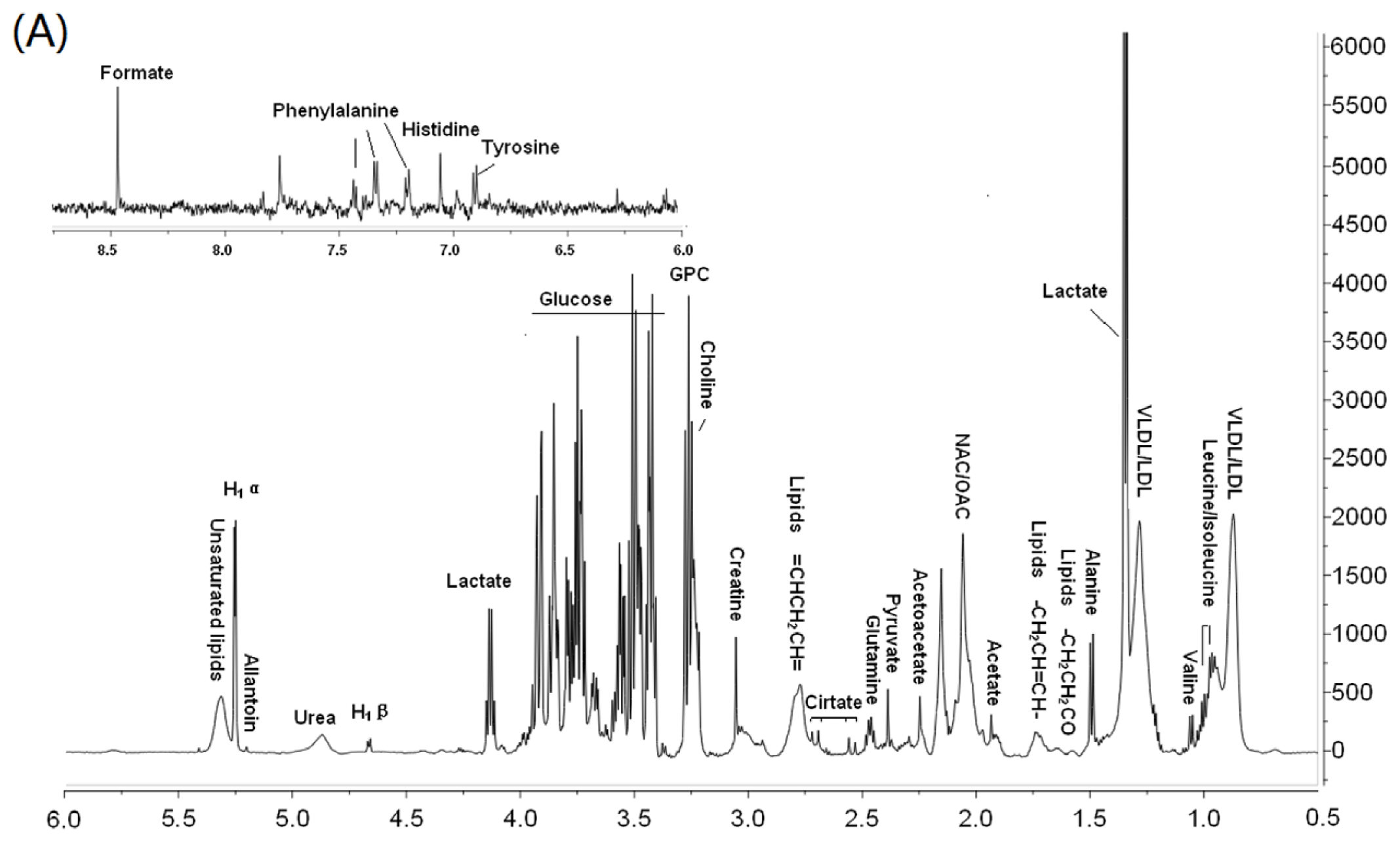
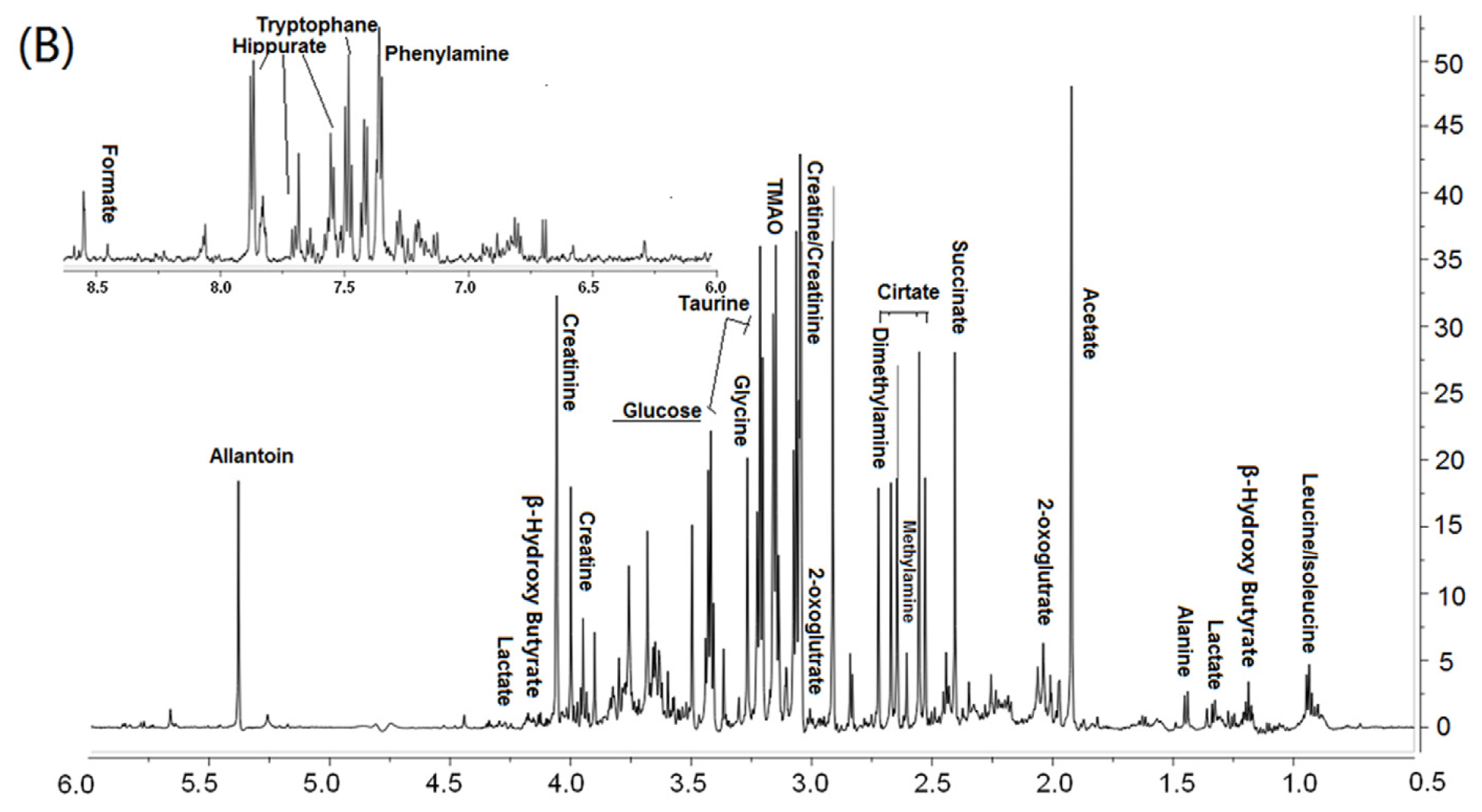
2.5. 1HNMR Spectroscopy
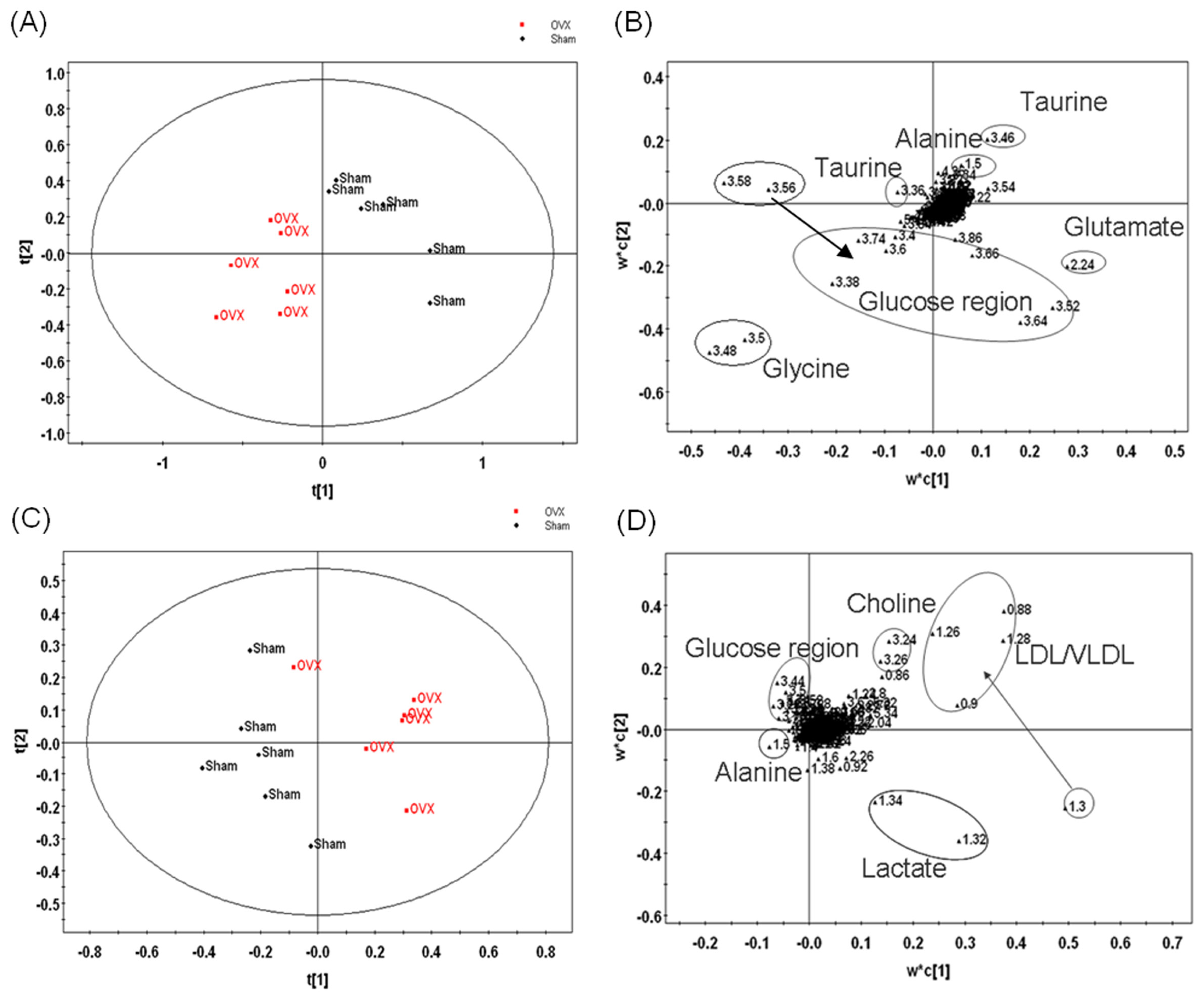
2.6. Multivariate Statistical Analysis
2.7. Statistics Analysis
3 Results and Discussion
3.1. Histopathology
3.2. Metabonomic Analysis of Osteoporosis Based on 1HNMR Metabolic Profiles in Rat Urine and Plasma
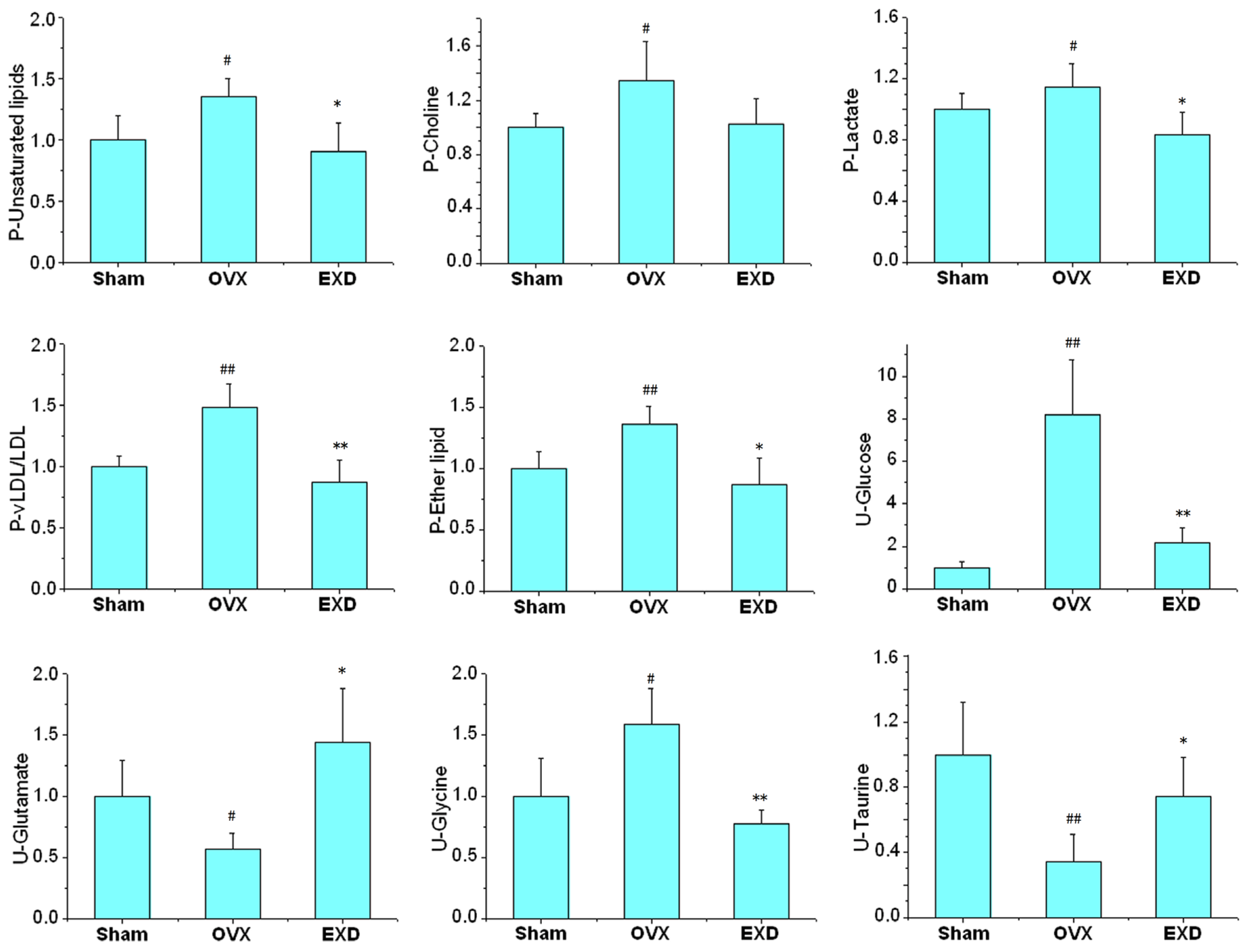
3.3. Biological Significance of the Potential Biomarkers in the Osteoporotic Rats
3.4. Metabonomic Analysis of EXD Treatment
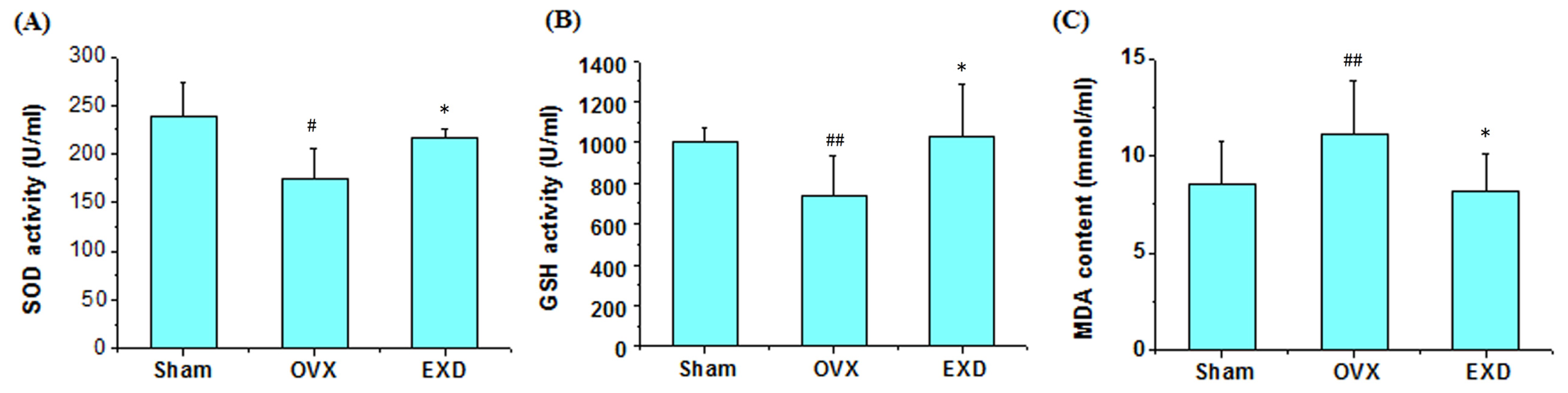
3.5. Determination of Superoxide Dismutase Activity, Glutathione Peroxidase Activity and Malondialdehyde Content
4. Conclusion
Acknowledgements
References
- Blazej, M.; Adam, C. Selective estrogen receptor modulators in treatment of postmenopausal osteoporosis. Ginekol. Pol 2009, 80, 213–217. [Google Scholar]
- Cole, Z.; Dennison, E.; Cooper, C. Update on the treatment of post-menopausal osteoporosis. Br. Med. Bull 2008, 86, 129–143. [Google Scholar]
- McNamara, L.M. Perspective on post-menopausal osteoporosis: Establishing an interdisciplinary understanding of the sequence of events from the molecular level to whole bone fractures. J. R. Soc. Interface 2010, 7, 353–372. [Google Scholar]
- Schairer, C.; Lubin, J.; Troisi, R.; Sturgeon, S.; Brinton, L.; Hoover, R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. J. Am. Med. Assoc 2000, 283, 485–491. [Google Scholar]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. J. Am. Med. Assoc 2002, 288, 321–333. [Google Scholar]
- Chen, H.Y.; Cho, W.C.; Sze, S.C.; Tong, Y. Treatment of menopausal symptoms with Er-xian decoction: A systematic review. Am. J. Chin. Med 2008, 36, 233–244. [Google Scholar]
- Lee, M.S.; Shin, B.C. Further consideration of combining clinical trials of er-xian decoction for menopausal symptoms. Am. J. Chin. Med 2008, 36, 1017–1028. [Google Scholar]
- Nian, H.; Qin, L.P.; Zhang, Q.Y.; Zheng, H.C.; Yu, Y.; Huang, B.K. Antiosteoporotic activity of Er-Xian Decoction, a traditional Chinese herbal formula, in ovariectomized rats. J. Ethnopharmacol 2006, 108, 96–102. [Google Scholar]
- Qin, L.P.; Han, T.; Zhang, Q.Y.; Cao, D.P.; Nian, H. Antiosteoporotic chemical constituents from Er-Xian Decoction, a traditional Chinese herbal formula. J. Ethnopharmacol 2008, 118, 271–279. [Google Scholar]
- Nicholson, J.K.; Holmes, E.; Lindon, J.C.; Brindle, J.T.; Grainger, D.J. Methods for analysis of spectral data and their applications osteoporosis. US Patent 20050037515, 2002. [Google Scholar]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. Metabonomics: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar]
- Long, W.F.; Li, L.; Chen, H.Q.; Tang, Y.; He, X.L.; Jing, R.Z. 1H-NMR-based metabonomics analysis of plasma from osteoporotic rats induced by ovariectomy. J. Sichuan Univ 2009, 40, 843–847. [Google Scholar]
- Ma, B.; Zhang, Q.; Wang, G. GC-TOF/MS-based metabonomic profiling of estrogen deficiency-induced obesity in ovariectomized rats. Acta Pharmacol. Sin 2011, 32, 270–278. [Google Scholar]
- Zhu, X.Y.; Liu, X.R.; He, P.; Cao, B.Q.; Lv, Y.H.; Zhang, W.D.; Ni, X. Metabonomics in serum of ovariectomised rats and those exposed to 17β-oestradiol and genistein. Gynecol. Endocrinol 2010, 26, 760–767. [Google Scholar]
- Granger, J.H.; Williams, R.; Lenz, E.M.; Plumb, R.S.; Stumpf, C.L.; Wilson, I.D. A metabonomic study of strain- and age-related differences in the Zucker rat. Rapid Commun. Mass Spectrom 2007, 21, 2039–2045. [Google Scholar]
- Atherton, H.J.; Bailey, N.J.; Zhang, W.; Taylor, J.; Major, H.; Shockcor, J.; Clarke, K.; Griffin, J.L. A combined 1H-NMR spectroscopy- and mass spectrometry-based metabonomic study of the PPAR-alpha null mutant mouse defines profound systemic changes in metabolism linked to the metabolic syndrome. Physiol. Genomics 2006, 27, 178–186. [Google Scholar]
- Fiehn, O.; Kristal, B.; van Ommen, B.; Sumner, L.W.; Sansone, S.A.; Taylor, C.; Hardy, N.; Kaddurah-Daouk, R. Establishing reporting standards for metabonomic and metabonomic studies: A call for participation. OMICS: J. Intergr. Biol 2006, 10, 158–163. [Google Scholar]
- Wishart, D.S.; Knox, C.; Guo, A.C.; et al. HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res 2009, 37, D603–D610. [Google Scholar]
- Psihogios, N.G.; Gazi, I.F.; Elisaf, M.S.; Seferiadis, K.I.; Bairaktari, E.T. Gender-related and age-related urinalysis of healthy subjects by NMR-based metabonomics. NMR Biomed 2008, 21, 195–207. [Google Scholar]
- Parhami, F.; Garfinkel, A.; Demer, L.L. Role of lipids in osteoporosis. Arterioscler. Thromb. Vasc. Biol 2000, 20, 2346–2348. [Google Scholar]
- Ozgocmen, S.; Kaya, H.; Fadillioglu, E.; Yilmaz, Z. Effects of calcitonin, risedronate, and raloxifene on erythrocyte antioxidant enzyme activity, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Arch. Med. Res 2007, 38, 196–205. [Google Scholar]
- Maritz, F.J.; Conradie, M.M.; Hulley, P.A.; Gopal, R.; Hough, S. Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler. Thromb. Vasc. Biol 2001, 21, 1636–1641. [Google Scholar]
- Schlemmer, C.; Coetzer, H.; Claassen, N.; Kruger, M. Oestrogen and essential fatty acid supplementation corrects bone loss due to ovariectomy in the female Sprague Dawley rat. Prostaglandins Leukot Essent. Fat. Acids 1999, 61, 381–390. [Google Scholar]
- Dixon, S.J.; Sims, S.M. P2 purinergic receptors on osteoblasts and osteoclasts: Potential targets for drug development. Drug Dev. Res 2000, 49, 187–200. [Google Scholar]
- Balint, E.; Szabo, P.; Marshall, C.F.; Sprague, S.M. Glucose-induced inhibition of in vitro bone mineralization. Bone 2001, 28, 21–28. [Google Scholar]
- Liu, M.L.; Xu, X.; Rang, W.Q.; Li, Y.J.; Song, H.P. Influence of ovariectomy and 17 [beta]-estradiol treatment on insulin sensitivity, lipid metabolism and post-ischemic cardiac function. Int. J. Cardiol 2004, 97, 485–493. [Google Scholar]
- Richard, D. Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol 1986, 250, 245–249. [Google Scholar]
- Hanaa, H.; Hamza, A.H. Potential role of arginine, glutamine and taurine in ameliorating osteoporotic biomarkers in ovariectomized rats. Rep. Opin 2009, 1, 24–35. [Google Scholar]
- Kum, K.Y.; Park, J.H.; Yoo, Y.J.; Choi, B.K.; Lee, H.J.; Lee, S.J. The inhibitory effect of alendronate and taurine on osteoclast differentiation mediated by Porphyromonas gingivalis sonicates in vitro. J. Endod 2003, 29, 28–30. [Google Scholar]
- Jeon, S.H.; Lee, M.Y.; Kim, S.J.; Joe, S.G.; Kim, G.B.; Kim, I.S.; Kim, N.S.; Hong, C.U.; Kim, S.Z.; Kim, J.S. Taurine increases cell proliferation and generates an increase in [Mg2+] i accompanied by ERK 1/2 activation in human osteoblast cells. FEBS Lett 2007, 581, 5929–5934. [Google Scholar]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 1999, 27, 29–34. [Google Scholar]
- Picard, F.; Deshaies, Y.; Lalonde, J.; Samson, P.; Labrie, C.; Belanger, A.; Labrie, F.; Richard, D. Effects of the estrogen antagonist EM-652. HCl on energy balance and lipid metabolism in ovariectomized rats. Int. J. Obes 2000, 24, 830–840. [Google Scholar]
- McFarlane, S.I.; Muniyappa, R.; Shin, J.J.; Bahtiyar, G.; Sowers, J.R. Osteoporosis and cardiovascular disease: Brittle bones and boned arteries, is there a link? Endocrine 2004, 23, 1–10. [Google Scholar]
- Ahmed-Sorour, H.; Bailey, C. Role of ovarian hormones in the long-term control of glucose homeostasis glycogen formation and gluconeogenesis. Ann. Nutr. Metab 1981, 25, 208–212. [Google Scholar]
- Tchernof, A.; Desmeules, A.; Richard, C.; Laberge, P.; Daris, M.; Mailloux, J.; Rhéaume, C.; Dupont, P. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J. Clin. Endocrinol. Metab 2004, 89, 3425–3430. [Google Scholar]
- Gupta, R.C.; Win, T.; Bittner, S. Taurine analogues; a new class of therapeutics: Retrospect and prospects. Curr. Med. Chem 2005, 12, 2021–2039. [Google Scholar]
- Muthusami, S.; Ramachandran, H.; Muthusamy, B.; Vasudevan, G.; Prabhu, V.; Subramaniam, V.; Jagadeesan, A.; Narasimhan, S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta 2005, 360, 81–86. [Google Scholar]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig 2003, 112, 915–923. [Google Scholar]
- Shen, X.H.; Fang, Z.Q.; Wu, D.X. Effect of er-xian decoction and its disassembled prescription on enzyme activities and their gene expression of antioxidant enzymes in aging rat. Zhongguo Zhong Xi Yi Jie He Za Zhi 1995, 15, 672–684. [Google Scholar]

| Group | Body weight(g) | Uterine index | E2 (pg/mL) | BMD (g/cm2) | TRAP (IU/L) | ALP (IU/L) | |
|---|---|---|---|---|---|---|---|
| Intial | Final | ||||||
| Sham | 251 ± 19 | 287 ± 10 | 4.0 ± 1.6 | 35.7 ± 2.6 | 0.26 ± 0.01 | 7.2 ± 1.9 | 80.6 ± 20.1 |
| OVX | 242 ± 6 | 346 ± 25 ## | 1.1 ± 0.7 ## | 16.7 ± 1.8 ## | 0.25 ± 0.01 # | 15.0 ± 2.6 ## | 109 ± 20.2 # |
| EXD | 253 ± 10 | 339 ± 32 | 1.8 ± 1.1 | 28.4 ± 2.2 ** | 0.26 ± 0.01 * | 3.3 ± 1.4 ** | 86.8 ± 18.2 * |
| Model | No.α | R2X | R2Y | Q2 |
|---|---|---|---|---|
| OVX-Sham | ||||
| Plasma | 3 | 0.768 | 0.78 | 0.612 |
| Urine | 3 | 0.756 | 0.88 | 0.626 |
| OVX-Sham-EXD | ||||
| Plasma | 3 | 0.792 | 0.602 | 0.625 |
| Urine | 3 | 0.504 | 0.876 | 0.685 |
| Compounds | Group | δH (ppm) | a OVX | b EXD | Pathway | Physiological action in bone metabolism | |
|---|---|---|---|---|---|---|---|
| Plasma | LDL/VLDL | −(CH2)n | 1.28–1.32 | ↑ | ↓ | PPAR signaling pathway | Lipid oxidation products (LPO) inhibit osteoblast differentiation [20,21]; |
| −CH3 | 0.86–0.9 | Elevated LDL are associated with low BMD [22] | |||||
| Choline | N(CH3)3 | 3.24 | ↑ | ↓ | Ether lipid metabolism | Elevated lipid inhibit bone remodeling [22,23] | |
| Lactate | βCH2 | 1.34 | ↑ | ↓ | Glycolysis/Gluconeogenesis | ATP improve osteoclast (OC) formation and inhibit osteoblast (OB) proliferation [24] | |
| CH2O | 4.14 | ||||||
| Lipids | CH2CH= | 5.3–5.34 | ↑ | ↓ | Peroxisome | Lipid peroxides altered bone oxidative system [21] | |
| Lipids | CH2C=CH | 2.78–2.82 | ↑ | ↓ | Glycerolipids metabolism | OVX increase hepatic lipid production [19] | |
| Alanine | αCH3 | 1.5 | ↓ | ↑ | Alanine, aspartate and glutamate metabolism | ||
| Acetoacetate | O=CCH3 | 2.26 | ↑ | ↓ | Valine, leucine and Isoleucine biosynthesis | ||
| Glucose | H4 | 3.44 | ↑ | ↓ | Carbohydrate metabolism | Glucose level related with OB proliferation [25], Ca uptake [26], bone formation [25,26] and lipid metabolism [23,27] | |
| H2 | 3.5 | ||||||
| H6 | 3.86, 3.92 | ||||||
| α-glucose | αCH | 5.26 | ↑ | ||||
| Isoleucine | γ CH3 | 0.92 | ↓ | ↑ | Valine, leucine and Isoleucine biosynthesis | ||
| Acetylglucoprotein | =OCNH | 2.02–2.06 | ↑ | ↓ | Glutamine and d-glutamate metabolism | Glu inhibit OC formation and increase BMD [28] | |
| Glycerophosphatide choline | ON(CH3)3 | 3.28 | ↑ | Ether lipid metabolism | |||
| Creatine | NH2C=O | 3.94 | ↑ | Arginine and Purine metabolism | |||
| Urine | |||||||
| Glycine | N−CH2 | 3.48–3.5 | ↑ | ↓ | Glycine, serine and threonine metabolism | ||
| Glutamate | βCH2 | 2.24 | ↓ | ↑ | Glutamate metabolism | [28] | |
| Glucose | H6′ | 3.74, 3.86 | ↓ | ↑ | Carbohydrate metabolism | ||
| H2 | 3.58–3.66 | ||||||
| Taurine | βCH2 | 3.46–3.44 | ↓ | ↑ | Taurine and hypotaurine metabolism | Taurine inhibit the formation of OC [29] and induce OB proliferation [30] | |
| αCH2 | 3.36–3.38 | ||||||
| Allantoin | CH | 5.4 | ↑ | ↓ | Purine metabolism | ||
| Alanine | αCH3 | 1.48–1.5 | ↓ | ↑ | Alanine, aspartate and glutamate metabolism | ||
| β-Hydroxy Butyrate | 4.28 | ↑ | ↓ | Butanoate metabolism | |||
| Hippurate | C6H ring | 7.86–7.9 | ↑ | ↓ | Phenylalanine metabolism | ||
| C2H ring | 7.58 | ||||||
| Lactate | CH2O | 4.14–4.1 | ↑ | ↓ | Glycolysis/Gluconeogenesis | ||
| βCH2 | 1.34 | ||||||
| Tryptophane | C4H, ring | 7.5–7.52 | ↑ | Glycine, serine and threonine metabolism | |||
| Citrate | CH2COO | 2.72 | ↑ | Citrate cycle | |||
| Creatine | NH2C=O | 4.0–4.04 | ↑ | Arginine and Purine metabolism | |||
| 2-oxo-glutamate | OOCCH2 | 2.98, 2.08 | ↑ | Citrate cycle | |||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xue, L.; Wang, Y.; Liu, L.; Zhao, L.; Han, T.; Zhang, Q.; Qin, L. A 1HNMR-Based Metabonomics Study of Postmenopausal Osteoporosis and Intervention Effects of Er-Xian Decoction in Ovariectomized Rats. Int. J. Mol. Sci. 2011, 12, 7635-7651. https://doi.org/10.3390/ijms12117635
Xue L, Wang Y, Liu L, Zhao L, Han T, Zhang Q, Qin L. A 1HNMR-Based Metabonomics Study of Postmenopausal Osteoporosis and Intervention Effects of Er-Xian Decoction in Ovariectomized Rats. International Journal of Molecular Sciences. 2011; 12(11):7635-7651. https://doi.org/10.3390/ijms12117635
Chicago/Turabian StyleXue, Liming, Yin Wang, Lei Liu, Lu Zhao, Ting Han, Qiaoyan Zhang, and Luping Qin. 2011. "A 1HNMR-Based Metabonomics Study of Postmenopausal Osteoporosis and Intervention Effects of Er-Xian Decoction in Ovariectomized Rats" International Journal of Molecular Sciences 12, no. 11: 7635-7651. https://doi.org/10.3390/ijms12117635




