Distribution and Clinical Significance of Th17 Cells in the Tumor Microenvironment and Peripheral Blood of Pancreatic Cancer Patients
Abstract
:1. Introduction
2. Results and Discussion
2.1. Distribution of Th17 Cells in Pancreatic Tumor Tissues as Detected by Flow Cytometry Analysis (FACS)
2.2. Distribution of Th17 Cells in the Peripheral Blood of Pancreatic Cancer Patients as Detected by FACS
2.3. Expression of IL-17 in Pancreatic Tumors of Pancreatic in Situ as Detected by Immunohistochemisrty (IHC) and Its Correlation with Clinical Pathological Characteristics
2.4. Expression Levels of Serum IL-17 and IL-23 Detected by Enzyme Immunoassays (ELISA)
2.5. Correlation between the Distribution of Intratumoral Th17 Cells and Tumor Angiogenesis
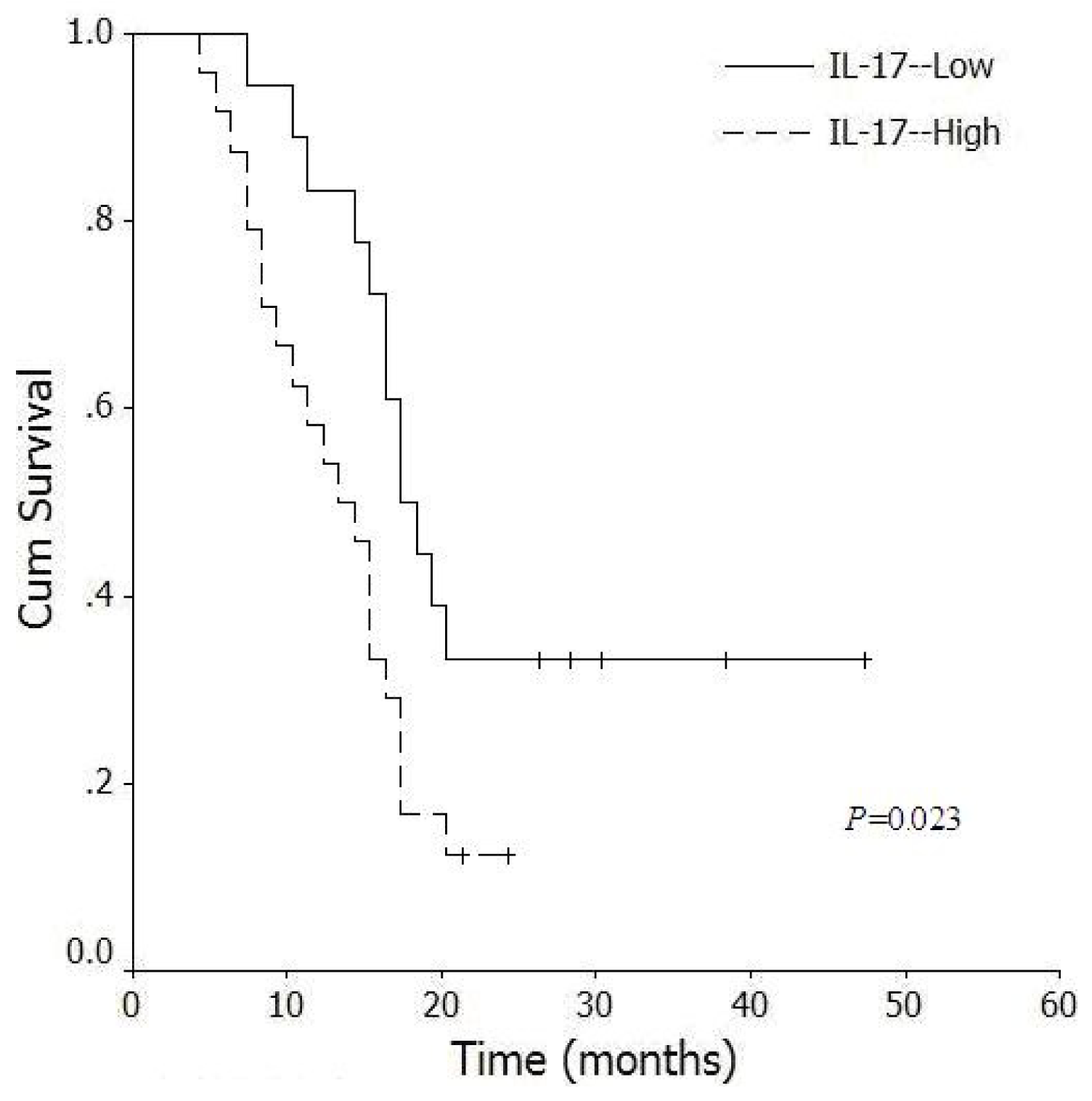
2.6. Correlation between Levels of Intratumoral IL-17+ Cells and Survival Time of Pancreatic Cancer Patients
2.7. Discussion
3. Materials and Methods
3.1. Patients and Specimens
3.2. Main Reagents
3.3. Flow Cytometry Analysis
3.4. Immunohistochemisrty
3.5. Enzyme Immunoassays
3.6. Statistical Analysis
4. Conclusions
Acknowledgement
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics. CA Cancer J. Clin 2010, 60, 277–300. [Google Scholar]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med 2010, 362, 1605–1617. [Google Scholar]
- Wray, C.J.; Ahmad, S.A.; Matthews, J.B.; Lowy, A.M. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology 2005, 128, 1626–1641. [Google Scholar]
- He, S.B.; Li, D.C. Mutated genes in pancreatic cancer. Chin. J. Cancer Res 2010, 22, 93–98. [Google Scholar]
- Dougan, M.; Dranoff, G. Immune therapy for cancer. Annu. Rev. Immunol 2009, 27, 83–117. [Google Scholar]
- Kleeff, J.; Beckhove, P.; Esposito, I.; Herzig, S.; Huber, P.E.; Lohr, J.M.; Friess, H. Pancreatic cancer microenvironment. Int. J. Cancer 2007, 121, 699–705. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar]
- Mueller, M.M.; Fusenig, N.E. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol 2005, 6, 1123–1132. [Google Scholar]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol 2005, 6, 1133–1141. [Google Scholar]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev 2008, 223, 87–113. [Google Scholar]
- Littman, D.R.; Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010, 140, 845–858. [Google Scholar]
- Hirota, K.; Martin, B.; Veldhoen, M. Development, regulation and functional capacities of Th17 cells. Semin. Immunopathol 2010, 32, 3–16. [Google Scholar]
- Kryczek, I.; Banerjee, M.; Cheng, P.; Vatan, L.; Szeliga, W.; Wei, S.; Huang, E.; Finlayson, E.; Simeone, D.; Welling, T.H.; et al. Phenotype, distribution, generation, functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009, 114, 1141–1149. [Google Scholar]
- Derhovanessian, E.; Adams, V.; Hahnel, K.; Groeger, A.; Pandha, H.; Ward, S.; Pawelec, G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int. J. Cancer 2009, 125, 1372–1379. [Google Scholar]
- Zhang, J.P.; Yan, J.; Xu, J.; Pang, X.H.; Chen, M.S.; Li, L.; Wu, C.; Li, S.P.; Zheng, L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J. Hepatol 2009, 50, 980–989. [Google Scholar]
- Su, X.; Ye, J.; Hsueh, E.C.; Zhang, Y.; Hoft, D.F.; Peng, G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J. Immunol 2010, 184, 1630–1641. [Google Scholar]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pages, F.; et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011, 71, 1263–1271. [Google Scholar]
- Maruyama, T.; Kono, K.; Mizukami, Y.; Kawaguchi, Y.; Mimura, K.; Watanabe, M.; Izawa, S.; Fujii, H. Distribution of Th17 cells and FoxP3 (+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci 2010, 101, 1947–1954. [Google Scholar]
- Kryczek, I.; Wei, S.; Zou, L.; Altuwaijri, S.; Szeliga, W.; Kolls, J.; Chang, A.; Zou, W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J. Immunol 2007, 178, 6730–6733. [Google Scholar]
- Atarashi, K.; Nishinura, J.; Shima, T.; Umesaki, Y.M.; Yamamoto, M.; Onoue, M.; Yagita, H.; Ishii, N.; Evans, R.; Honda, K.; et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 2008, 455, 808–812. [Google Scholar]
- Horlock, C.; Stott, B.; Dyson, P.J.; Morishita, M.; Coombes, R.C.; Savage, P.; Stebbing, J. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br. J. Cancer 2009, 100, 1061–1067. [Google Scholar]
- Sfanos, K.S.; Bruno, T.C.; Maris, C.H.; Xu, L.; Thoburn, C.J.; DeMarzo, A.M.; Meeker, A.K.; Isaacs, W.B.; Drake, C.G. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res 2008, 14, 3254–3261. [Google Scholar]
- Hirota, K.; Martin, B.; Veldhoen, M. Development, regulation and functional capacities of Th17 cells. Semin. Immunopathol 2010, 32, 3–16. [Google Scholar]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol 2007, 25, 821–852. [Google Scholar]
- Volpe, E.; Servant, N.; Zollinger, R.; Bogiatzi, S.I.; Hupe, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nature Immunol 2008, 9, 650–657. [Google Scholar]
- Benchetrit, F.; Ciree, A.; Vives, V.; Warnier, G.; Gey, A.; Sautes-Fridman, C.; Fossiez, F.; Haicheur, N.; Fridman, W.H.; Tartour, E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood 2002, 99, 2114–2121. [Google Scholar]
- Charles, K.A.; Kulbe, H.; Soper, R.; Escorcio-Correia, M.; Lawrence, T.; Schultheis, A.; Chakravarty, P.; Thompson, R.G.; Kollias, G.; Smyth, J.F.; et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Invest 2009, 119, 3011–3023. [Google Scholar]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med 2009, 206, 1457–1464. [Google Scholar]
- Gnerlich, J.L.; Mitchem, J.B.; Weir, J.S.; Sankpal, N.V.; Kashiwagi, H.; Belt, B.A.; Porembka, M.R.; Herndon, J.M.; Eberlein, T.J.; Goedegebuure, P.; et al. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J. Immunol 2010, 185, 4063–4071. [Google Scholar]
- Yamamoto, M.; Kamigaki, T.; Yamashita, K.; Hori, Y.; Hasegawa, H.; Kuroda, D.; Moriyama, H.; Nagata, M.; Ku, Y.; Kuroda, Y. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol. Rep 2009, 22, 337–343. [Google Scholar]
- Zou, W.; Restifo, N.P. T (H) 17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol 2010, 10, 248–256. [Google Scholar]
- Fang, J.; Shing, Y.; Wiederschain, D.; Yan, L.; Butterfield, C.; Jackson, G.; Harper, J.; Tamvakopoulos, G.; Moses, M.A. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc. Natl. Acad. Sci. USA 2000, 97, 3884–3889. [Google Scholar]
- Zhang, Y.L.; Li, J.; Mo, H.Y.; Qiu, F.; Zheng, L.M.; Qian, C.N.; Zeng, Y.X. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol. Cancer 2010, 9, 4:1–4:11. [Google Scholar]
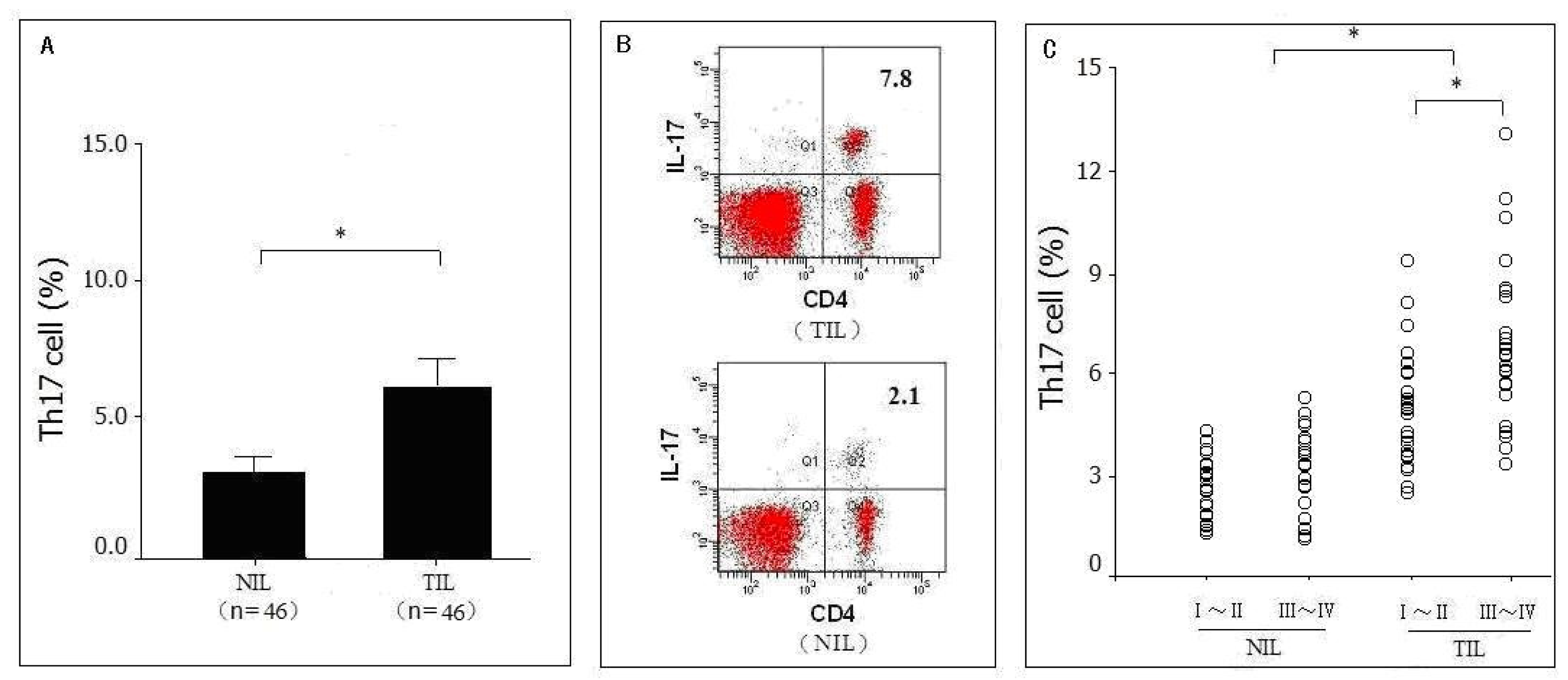
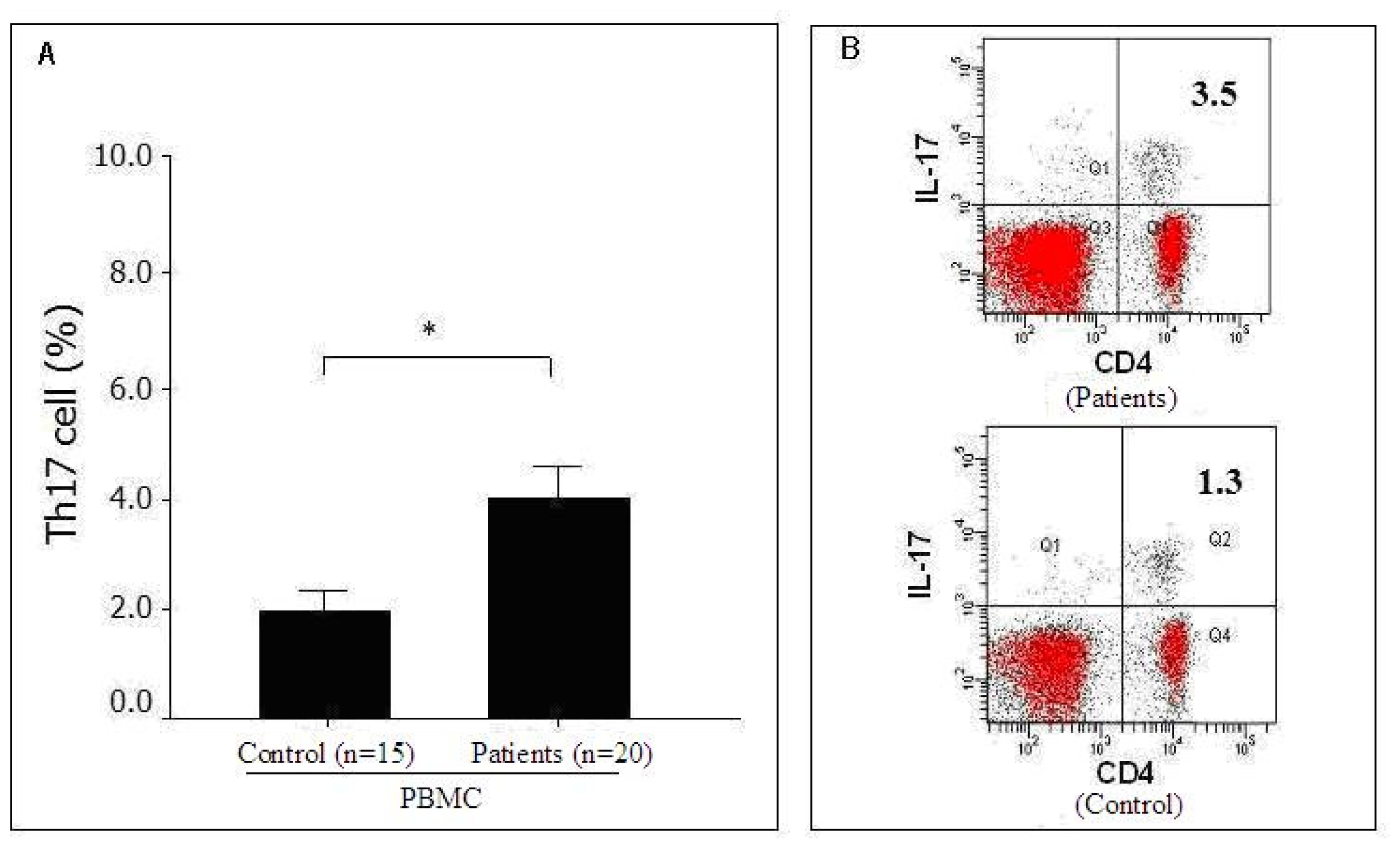
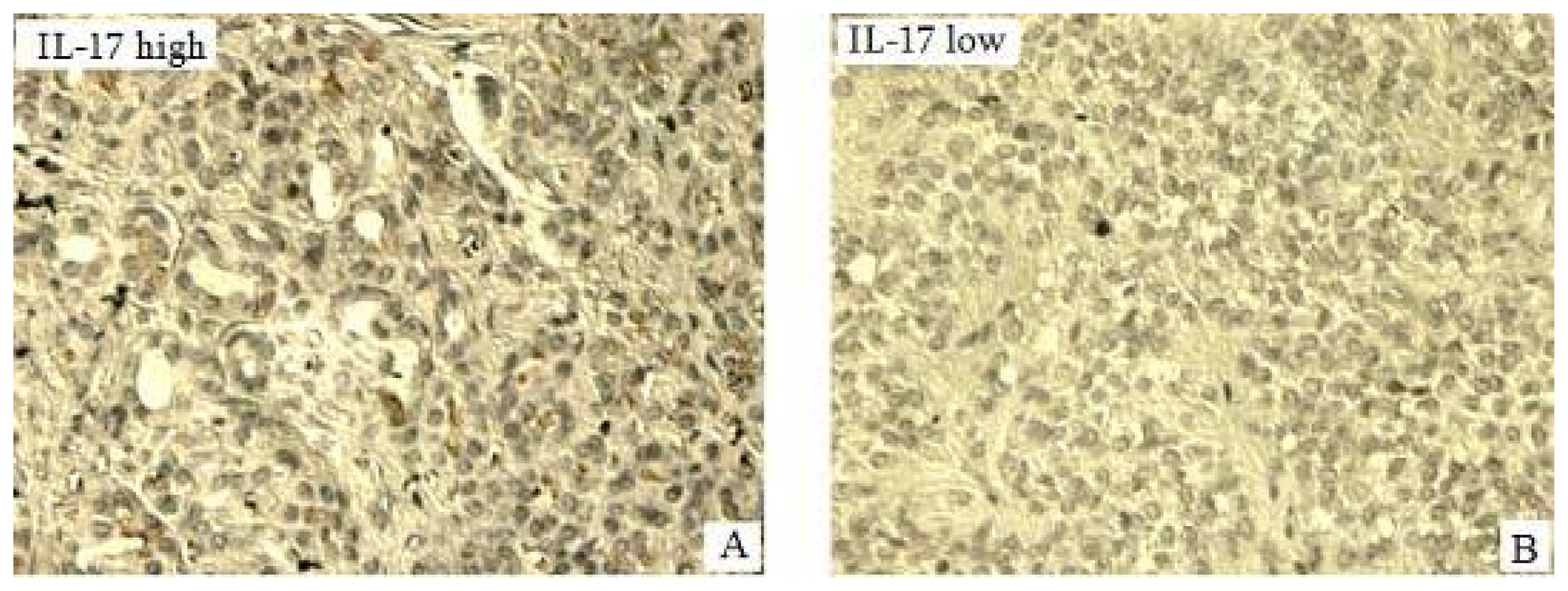

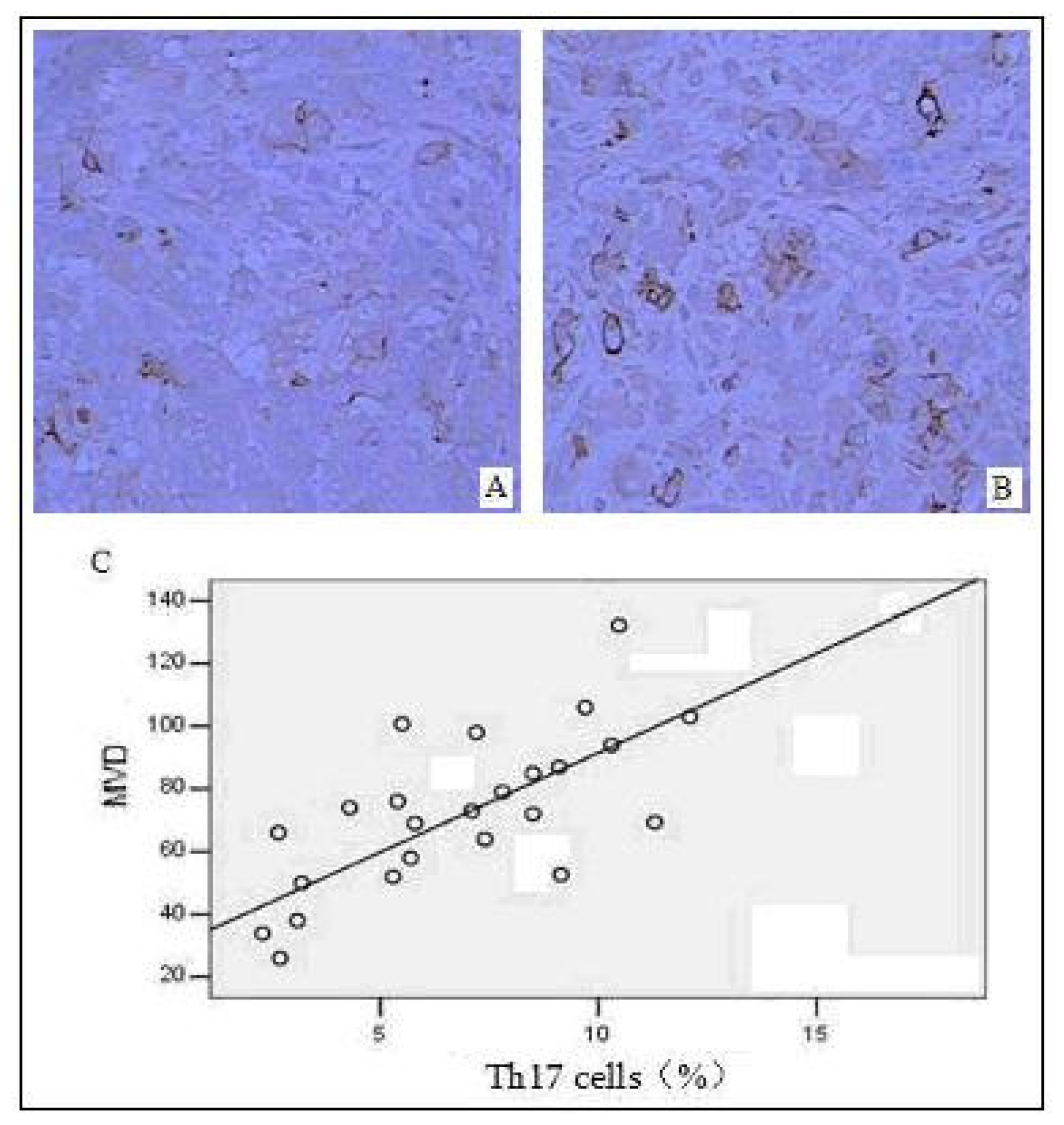
| Clinic pathological parameters | Cases (n) | Frequency of IL-17+ cells (%, mean ± SD) | P value |
|---|---|---|---|
| Sex | |||
| Male | 31 | 4.36 ± 0.85 | |
| Female | 15 | 4.12 ± 0.97 | 0.408 |
| Age (years) | |||
| ≤60 | 17 | 4.45 ± 0.72 | |
| >60 | 29 | 4.78 ± 0.91 | 0.184 |
| Tumor size (cm) | |||
| ≤2 | 32 | 4.33 ± 0.75 | |
| >2 | 14 | 4.91 ± 0.90 | 0.298 |
| Histological grade | |||
| Well differentiated | 12 | 4.35 ± 0.57 | |
| Moderate differentiated | 15 | 4.46 ± 0.71 | 0.315 |
| Poor differentiated | 19 | 4.70 ± 0.80 | |
| TNM stage | |||
| Stage I-II | 22 | 3.98 ± 0.45 | |
| Stage III-IV | 24 | 5.97 ± 1.25 | 0.012 |
| Lymph node metastasis | |||
| Positive | 35 | 6.18 ± 1.64 | |
| Negative | 11 | 3.74 ± 0.55 | 0.009 |
| Group | Cases (n) | IL-17 | IL-23 |
|---|---|---|---|
| Healthy volunteer | 15 | 14.5 ± 6.8 | 95.1 ± 37.2 |
| Pancreatic cancer patient | 20 | 69.2 ± 28.5 * | 266.5 ± 98.1 * |
| Stage I-II | 11 | 59.1 ± 25.9 * | 227.4 ± 87.6 * |
| Stage III-IV | 9 | 75.8 ± 29.0 * | 279.9 ± 102.1 * |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, S.; Fei, M.; Wu, Y.; Zheng, D.; Wan, D.; Wang, L.; Li, D. Distribution and Clinical Significance of Th17 Cells in the Tumor Microenvironment and Peripheral Blood of Pancreatic Cancer Patients. Int. J. Mol. Sci. 2011, 12, 7424-7437. https://doi.org/10.3390/ijms12117424
He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and Clinical Significance of Th17 Cells in the Tumor Microenvironment and Peripheral Blood of Pancreatic Cancer Patients. International Journal of Molecular Sciences. 2011; 12(11):7424-7437. https://doi.org/10.3390/ijms12117424
Chicago/Turabian StyleHe, Songbing, Min Fei, Yugang Wu, Dingcheng Zheng, Daiwei Wan, Liang Wang, and Dechun Li. 2011. "Distribution and Clinical Significance of Th17 Cells in the Tumor Microenvironment and Peripheral Blood of Pancreatic Cancer Patients" International Journal of Molecular Sciences 12, no. 11: 7424-7437. https://doi.org/10.3390/ijms12117424




