Antiproliferative Effect of Aaptamine on Human Chronic Myeloid Leukemia K562 Cells
Abstract
:1. Introduction
2. Results and Discussion
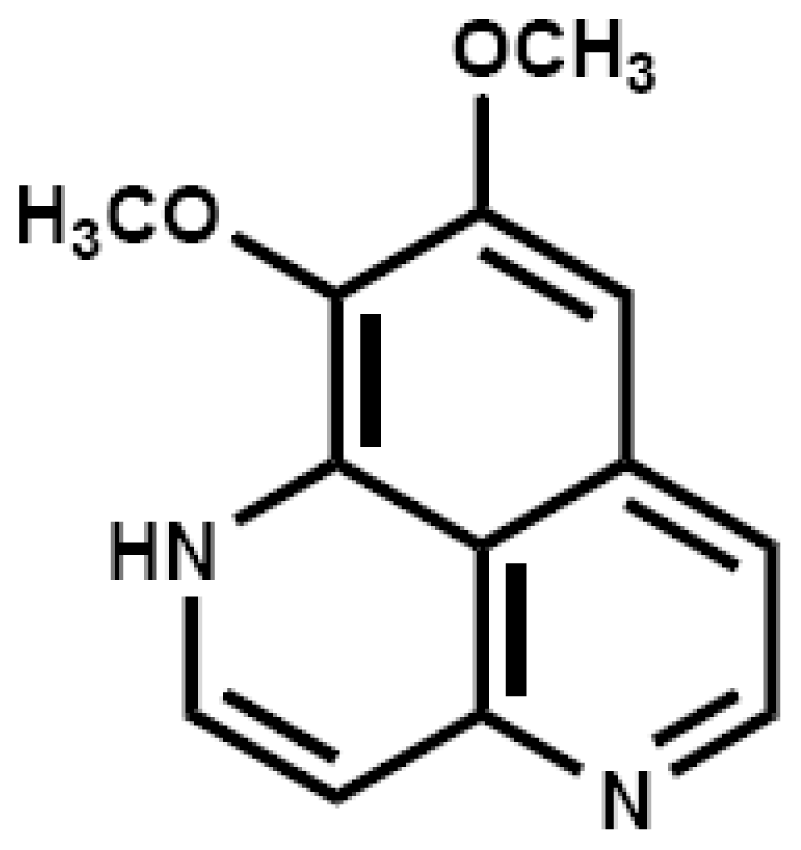
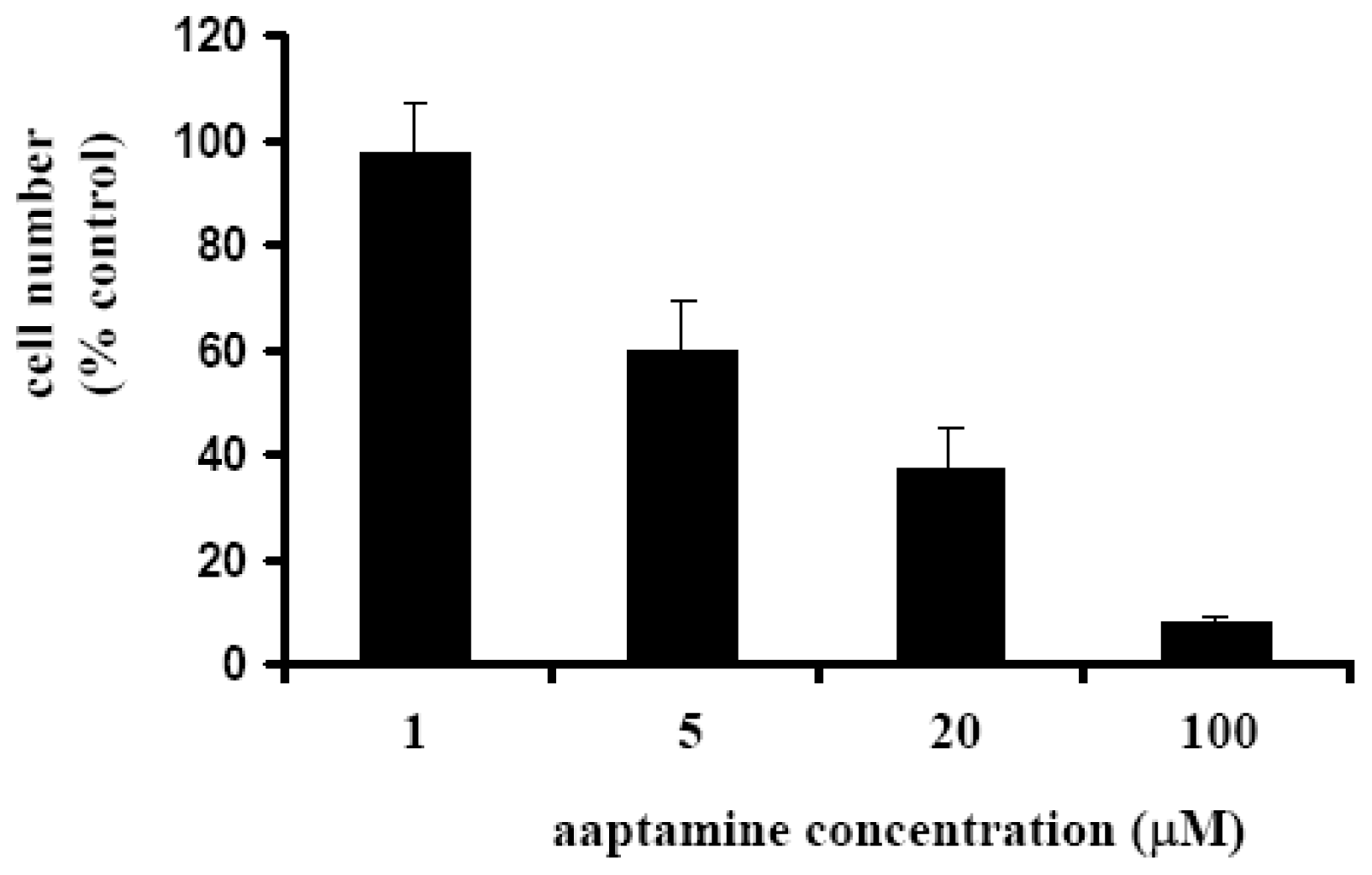
2.1. Aaptamine Inhibited the Proliferation of K562 Cells
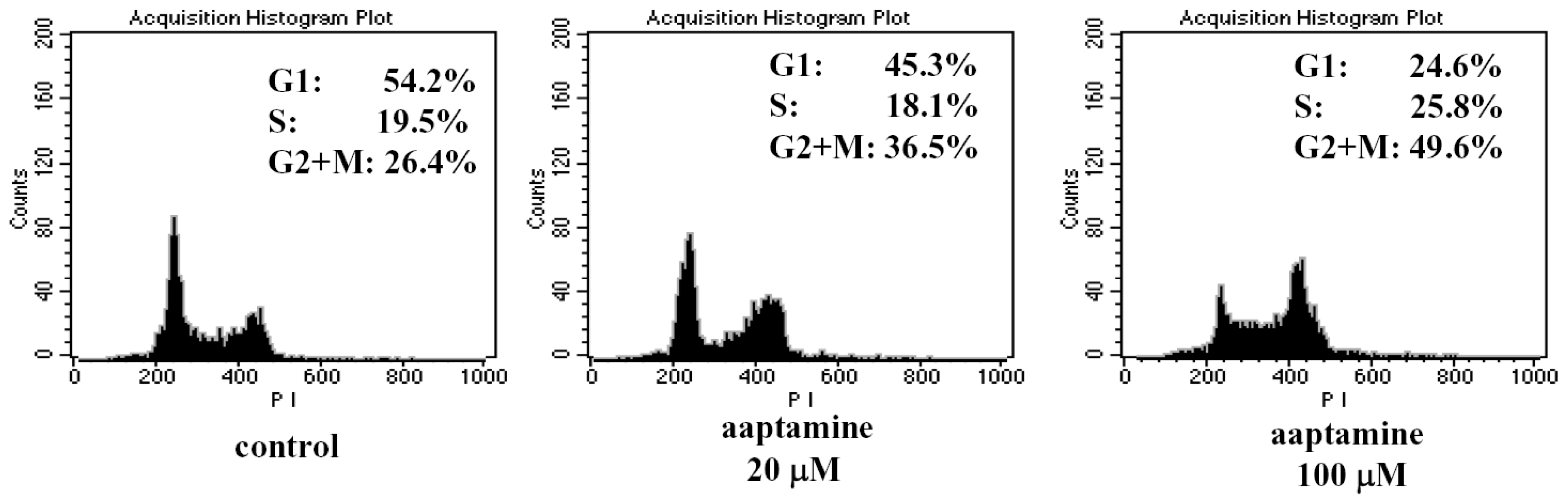
2.2. Aaptamine Arrested the Cell Cycle of K562 Cells at G2/M Phase
2.3. Aaptamine Induced p21 Expression in K562 Cells
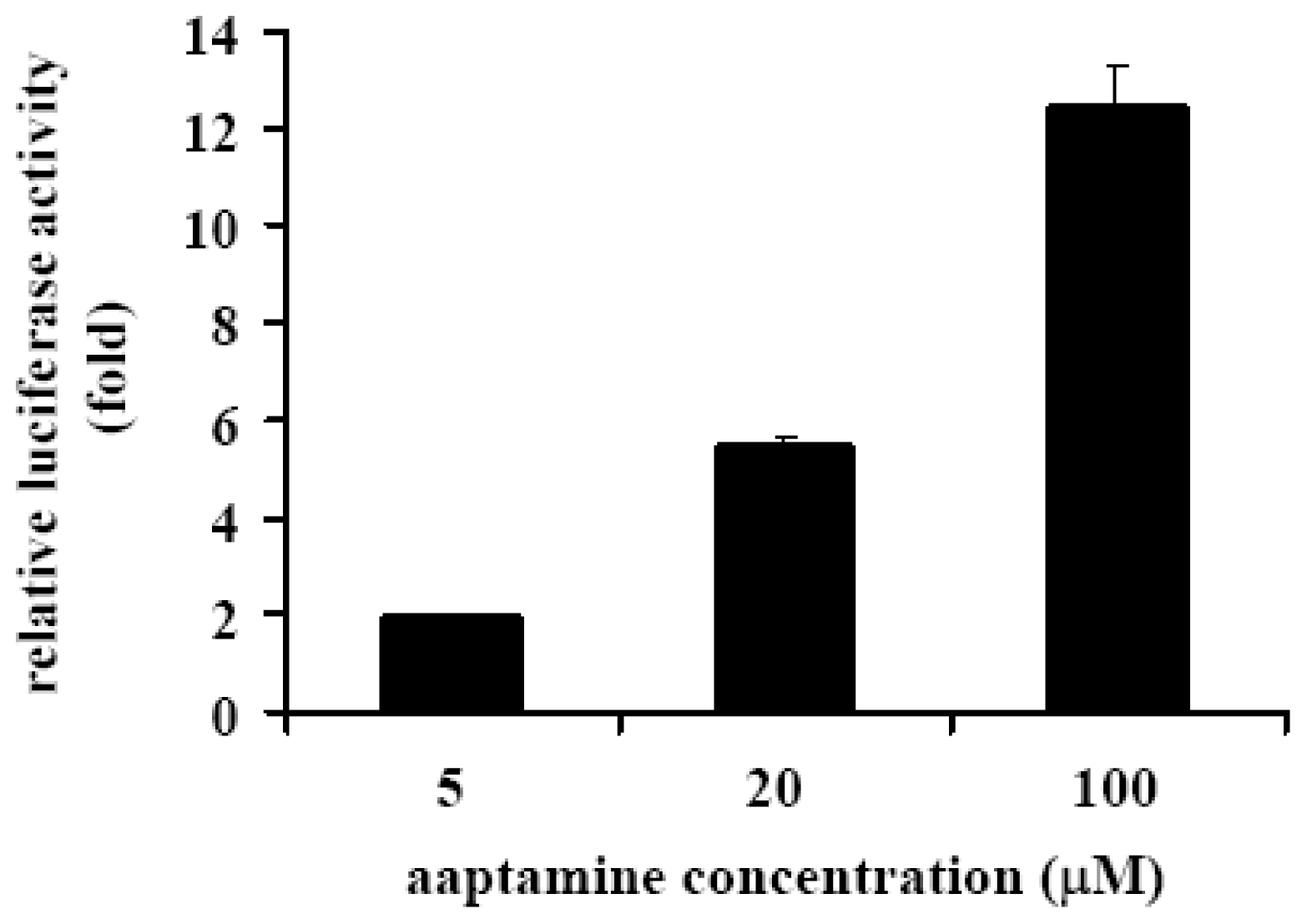
2.4. Aaptamine Activated p21 Promoter in K562 Cells
3. Experimental Section
3.1. Materials
3.2. Isolation and Identification of Aaptamine
3.3. Cell Culture
3.4. WST-8 Assay to Determine K562 Cell Growth
3.5. Flow Cytometric Analysis of Cell Cycle
3.6. Western Blot Analysis
3.7. Transfection of K562 Cells and Luciferase Assay
4. Conclusions
Acknowledgments
References
- De Klein, A.; van Kessel, A.G.; Grosveld, G.; Bartram, C.R.; Hagemeijer, A.; Bootsma, D.; Spurr, N.K.; Heisterkamp, N.; Groffen, J.; Stephenson, J.R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature 1982, 300, 765–767. [Google Scholar]
- Radich, J.P. Chronic myeloid leukemia 2010: Where are we now and where can we go? Hematology 2010, 2010, 122–128. [Google Scholar]
- Azam, M.; Latek, R.R.; Daley, G.Q. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell 2003, 112, 831–843. [Google Scholar]
- Melo, J.V.; Chuah, C. Resistance to imatinib mesylate in chronic myeloid leukaemia. Cancer Lett 2007, 249, 121–132. [Google Scholar]
- Nardi, V.; Azam, M.; Daley, G.Q. Mechanisms and implications of imatinib resistance mutations in BCR-ABL. Curr. Opin. Hematol 2004, 11, 35–43. [Google Scholar]
- Cuevas, C.; Francesch, A. Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat. Prod. Rep 2009, 26, 322–337. [Google Scholar]
- Aoki, S.; Kong, D.; Suna, H.; Sowa, Y.; Sakai, T.; Setiawan, A.; Kobayashi, M. Aaptamine, a spongean alkaloid, activates p21 promoter in a p53-independent manner. Biochem. Biophys. Res. Commun 2006, 342, 101–106. [Google Scholar]
- Aoki, S.; Kong, D.; Matsui, K.; Kobayashi, M. Smenospongine, a spongean sesquiterpene aminoquinone, induces erythroid differentiation in K562 cells. Anticancer Drugs 2004, 15, 363–369. [Google Scholar]
- Aoki, S.; Kong, D.; Matsui, K.; Rachmat, R.; Kobayashi, M. Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukemia, K562 cells. Chem. Pharm. Bull. Tokyo 2004, 52, 935–937. [Google Scholar]
- Kong, D.; Aoki, S.; Sowa, Y.; Sakai, T.; Kobayashi, M. Smenospongine, a sesquiterpene aminoquinone from a marine sponge, induces G1 arrest or apoptosis in different leukemia cells. Mar. Drugs 2008, 6, 480–488. [Google Scholar]
- Kong, D.; Yamori, T.; Kobayashi, M.; Duan, H. Antiproliferative and antiangiogenic activities of smenospongine, a marine sponge sesquiterpene aminoquinone. Mar. Drugs 2011, 9, 154–161. [Google Scholar]
- Aoki, S.; Kong, D.; Matsui, K.; Kobayashi, M. Erythroid differentiation in K562 chronic myelogenous cells induced by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer Res 2004, 24, 2325–2330. [Google Scholar]
- Kong, D.; Okamura, M.; Yoshimi, H.; Yamori, T. Antiangiogenic effect of ZSTK474, a novel phosphatidylinositol 3-kinase inhibitor. Eur. J. Cancer 2009, 45, 857–865. [Google Scholar]
- Hollstein, M.; Sidransky, D.; Vogelstein, B.; Harris, C.C. p53 mutations in human cancers. Science 1991, 253, 49–53. [Google Scholar]
- Vogelstein, B.; Kinzler, K.W. p53 function and dysfunction. Cell 1992, 70, 523–526. [Google Scholar]
- Tsukamoto, S.; Yamanokuchi, R.; Yoshitomi, M.; Sato, K.; Ikeda, T.; Rotinsulu, H.; Mangindaan, R.; de Voogd, N.J.; van Soest, R.W.; Yokosawa, H. Aaptamine, an alkaloid from the sponge Aaptos suberitoides, functions as a proteasome inhibitor. Bioorg. Med. Chem. Lett 2010, 20, 3341–3343. [Google Scholar]

© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jin, M.; Zhao, W.; Zhang, Y.; Kobayashi, M.; Duan, H.; Kong, D. Antiproliferative Effect of Aaptamine on Human Chronic Myeloid Leukemia K562 Cells. Int. J. Mol. Sci. 2011, 12, 7352-7359. https://doi.org/10.3390/ijms12117352
Jin M, Zhao W, Zhang Y, Kobayashi M, Duan H, Kong D. Antiproliferative Effect of Aaptamine on Human Chronic Myeloid Leukemia K562 Cells. International Journal of Molecular Sciences. 2011; 12(11):7352-7359. https://doi.org/10.3390/ijms12117352
Chicago/Turabian StyleJin, Meihua, Wennan Zhao, Yanwen Zhang, Motomasa Kobayashi, Hongquan Duan, and Dexin Kong. 2011. "Antiproliferative Effect of Aaptamine on Human Chronic Myeloid Leukemia K562 Cells" International Journal of Molecular Sciences 12, no. 11: 7352-7359. https://doi.org/10.3390/ijms12117352



