Effects of the Methanol Extract of Basella alba L (Basellaceae) on Steroid Production in Leydig Cells
Abstract
:1. Introduction
2. Results
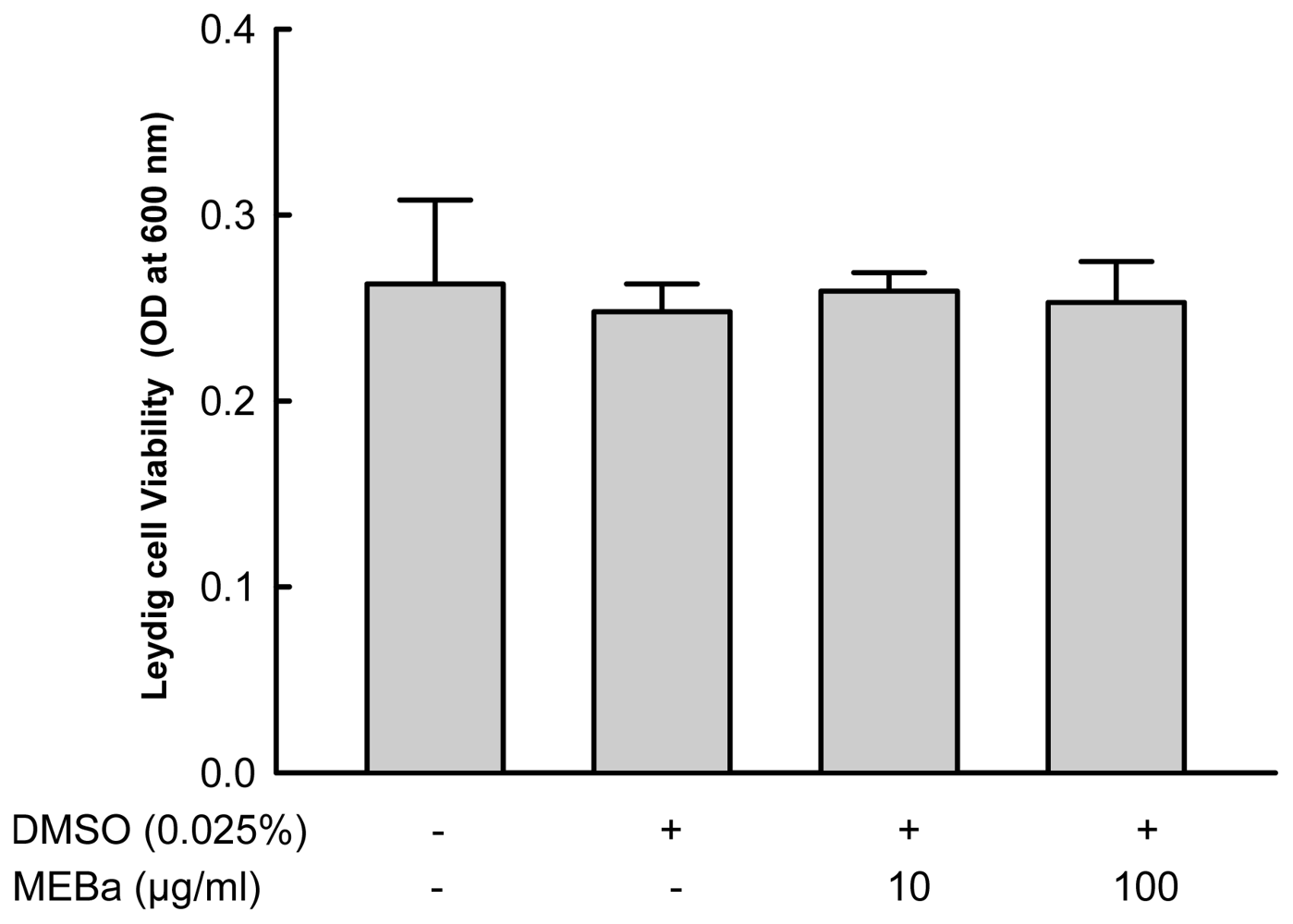
2.1. Effect of MEBA on Leydig Cell Viability
2.2. Testosterone and Estradiol Production by Leydig Cells
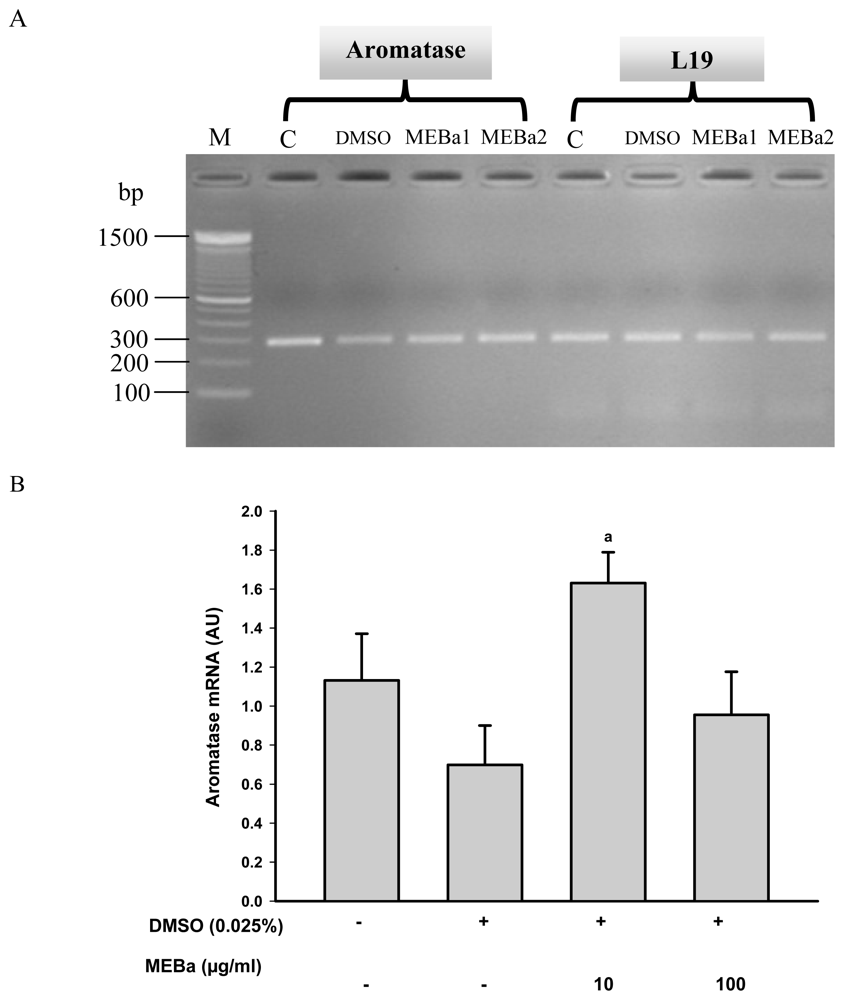
2.3. Aromatase mRNA Levels
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of the Methanol Extract of Basella alba
4.2. Chemicals
4.3. Animals
4.4. Purification of Leydig Cells
4.5. Study of the Effect of MEBA on Leydig Cell Viability
4.6. Study of the Effect of MEBA on Steroid Production and Aromatase mRNA Level
4.7. Statistical Analyses
5. Conclusions
Acknowledgements
References
- Nantia, EA; Moundipa, FP; Monsees, KT; Carreau, S. Medicinal plants as potential male antiinfertility agents: A review. Andrologie 2009, 19, 148–158. [Google Scholar]
- Swerdlow, J. Nature’s Medicine. In Plants That Heal; National Geographic Society: Washington, D.C., USA, 2000. [Google Scholar]
- Nantia, AE; Moundipa, FP; Beboy, ESN; Monsees, KT; Carreau, S. Study of the androgenic effect of the methanol extract of Basella alba L. (Basellaceae) on the male rat reproductive function. Andrologie 2007, 17, 129–133. [Google Scholar]
- Moundipa, FP; Beboy, ESN; Zelefack, F; Ngouela, S; Tsamo, E; Schill, WB; Monsees, TK. Effects of Basella alba and Hibiscus macranthus extracts on testosterone production by adult rat and bull Leydig cells. Asian J. Androl 2005, 7, 411–417. [Google Scholar]
- Moundipa, FP; Ngouela, S; Kamtchouing, P; Tsamo, E; Tchouanguep, MF; Carreau, S. Effects of extracts from Hibiscus macranthus and Basella alba mixture on testosterone production in vitro in adult rat testes slices. Asian J. Androlol 2006, 8, 111–114. [Google Scholar]
- Kandeel, RF; Koussa, TKV; Swerdloff, SR. Male sexual function and its disorders: Physiology, pathophysiology, clinical investigation, and treatment. Endocr. Rev 2001, 22, 342–388. [Google Scholar]
- Jones, EEM; Simpson, RE. Oestrogens in male reproduction. Baillière’s Clin. Endocrinol. Metab 2000, 14, 505–516. [Google Scholar]
- Walker, RV; Korach, SK. Estrogen receptor knockout mice as a model for endocrine research. ILAR J 2004, 45, 455–461. [Google Scholar]
- Carani, C; Granata, MRA; Rochira, V; Caffagni, G; Aranda, C; Antunez, P; Maffei, LE. Sex steroids and sexual desire in a man with a novel mutation of aromatase gene and hypogonadism. Psychoneuroendocrinology 2005, 30, 413–417. [Google Scholar]
- Rochira, V; Zirilli, L; Madeo, B; Aranda, C; Caffagni, G; Fabre, B; Montangero, VE; Roldan, EJ; Maffei, L; Carani, C. Case Report: Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: Evidences of a priming effect of estrogen for sex steroids action on bone. Bone 2007, 40, 1662–1668. [Google Scholar]
- Khosla, S; Melton, LJ; Atkinson, EJ; O’Fallon, WM; Klee, GG; Riggs, BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: A key role for bioavailable estrogen. J. Clin. Endocrinol. Metab 1998, 83, 2266–2274. [Google Scholar]
- Harman, MS; Metter, JE; Tobin, DJ; Pearson, J; Blackman, RM. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J. Clin. Endocrinol. Metab 2001, 86, 724–731. [Google Scholar]
- Papadopoulos, V; Carreau, S; Drosdowsky, MA. Effect of phorbol ester and phospholipase C on LH-stimulated steroidogenesis in purified rat Leydig cells. Febs. Lett 1985, 2, 312–316. [Google Scholar]
- Saotome, K; Morita, H; Umeda, M. Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicol in Vitro 1989, 3, 317–321. [Google Scholar]
- Travert, C; Carreau, S; Le Goff, D. Induction of apoptosis by 25-hydroxycholesterol in adult rat Leydig cells: Protective effect of 17β-estradiol. Reproduct. Toxicol 2006, 22, 564–570. [Google Scholar]
- Chomczynski, P; Sacchi, N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem 1987, 8, 155–161. [Google Scholar]
- Tena-Sempere, M; Barreiro, ML; González, LC; Gaytán, F; Zhang, FP; Caminos, EJ; Pinilla, L; Casanueva, FF; Diéguez, C; Aguilar, E. Novel expression and functional role of ghrelin in rat testis. Endocrinology 2002, 143, 717–725. [Google Scholar]
- Sharpe, RM; Donachie, K; Cooper, I. Re-evaluation of the intratesticular level of testosterone required for quantitative maintenance of spermatogenesis in the rat. J. Endocrinol 1988, 117, 19–26. [Google Scholar]
- Kung, WC. Androgen and bone mass in men. Asian J. Androl 2003, 5, 148–154. [Google Scholar]
- Sinha-Hikim, I; Taylor, EW; Gonzalez-Cadavid, FN; Zheng, W; Bhasin, S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: Up-regulation by androgen treatment. J. Clin. Endocrinol. Metab 2004, 89, 5245–5255. [Google Scholar]
- Motofei, IG; Rowland, DL. The physiological basis of human sexual arousal: Neuroendocrine sexual asymmetry. Int. J. Androl 2005, 28, 78–87. [Google Scholar]
- Bilińska, B; Wiszniewska, B; Kosiniak-Kamysz, K; Kotula-Balak, M; Gancarczyk, M; Hejmej, A; Sadowska, J; Marchlewicz, M; Kolasa, A; Wenda-Rózewicka, L. Hormonal status of male reproductive system: Androgens and estrogens in the testis and epididymis. In vivo and in vitro approaches. Reproduct. Biol 2006, 6, 43–58. [Google Scholar]
- Carreau, S; Silandre, D; Bourguiba, S; Hamden, K; Said, L; Lambard, S; Galeraud-Denis, I; Delalande, C. Estrogens and male reproduction: A new concept. Braz. J. Med. Biol. Res 2007, 40, 761–768. [Google Scholar]
- Winters, JS. Neuroendocrine control of testicular function. In Male Repoductive Function Pathophysiology and Treatment; Kandeel, RF, Swerdloff, SR, Pryor, LJ, Eds.; Informa, Healthcare: New York, NY, USA, 2007; pp. 21–34. [Google Scholar]
- Baines, H; Nwagwu, MO; Furneaux, EC; Stewart, J; Kerr, JB; Mayhew, TM; Ebling, FJ. Estrogenic induction of spermatogenesis in the hypogonadal (hpg) mouse: Role of androgens. Reproduction 2005, 130, 643–654. [Google Scholar]
- Li, Z-E; Li, X-D; Zhang, Q-S; Wang, Y-C; Zhang, M-X; Lu, Y-J; Duan, CM; Yang, XZ; Feng, LX. 17 beta-estradiol stimulates proliferation of spermatogonia in experimental cryptorchid mice. Asian J. Pharmacol 2007, 9, 659–667. [Google Scholar]
- Asah, P. Oligospermia due to partial maturation arrest responds to low dose estrogen-testosterone combination therapy resulting in live-birth: A case report. Asian J. Androl 2002, 4, 307–308. [Google Scholar]
| hCG | 0 UI/mL | 1 UI/mL | 10 UI/mL |
|---|---|---|---|
| Control | 0.33 ± 0.05 | ||
| 0.025% DMSO | 0.32 ± 0.01 | 1.63 ± 0.07b | 2.81 ± 0.10d |
| MEBa 10 μg/mL | 0.63 ± 0.04a | 1.99 ± 0.09c | 2.86 ± 0.17d |
| MEBa 100 μg/mL | 0.35± 0.02 | 1.61± 0.05b | 2.76 ± 0.08d |
| hCG | 0 UI/mL | 1 UI/mL | 10 UI/mL |
|---|---|---|---|
| Control | 62.14 ± 8.68 | ||
| 0.025% DMSO | 54.21 ± 11.50 | 201.37 ± 15.19b | 331.44 ± 39.84d |
| MEBa 10 μg/mL | 88.67 ± 6.05a | 246.42 ± 21.67c | 337.64 ± 35.31d |
| MEBa 100 μg/mL | 69.26 ± 8.34 | 204.89 ± 28.38b | 330.94 ± 42.17d |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nantia, E.A.; Travert, C.; Manfo, F.-P.T.; Carreau, S.; Monsees, T.K.; Fewou Moundipa, P. Effects of the Methanol Extract of Basella alba L (Basellaceae) on Steroid Production in Leydig Cells. Int. J. Mol. Sci. 2011, 12, 376-384. https://doi.org/10.3390/ijms12010376
Nantia EA, Travert C, Manfo F-PT, Carreau S, Monsees TK, Fewou Moundipa P. Effects of the Methanol Extract of Basella alba L (Basellaceae) on Steroid Production in Leydig Cells. International Journal of Molecular Sciences. 2011; 12(1):376-384. https://doi.org/10.3390/ijms12010376
Chicago/Turabian StyleNantia, Edouard Akono, Carine Travert, Faustin-Pascal T. Manfo, Serge Carreau, Thomas K. Monsees, and Paul Fewou Moundipa. 2011. "Effects of the Methanol Extract of Basella alba L (Basellaceae) on Steroid Production in Leydig Cells" International Journal of Molecular Sciences 12, no. 1: 376-384. https://doi.org/10.3390/ijms12010376




