Removal of Parabens from Aqueous Solution Using β-Cyclodextrin Cross-Linked Polymer
Abstract
:1. Introduction
2. Results and Discussion
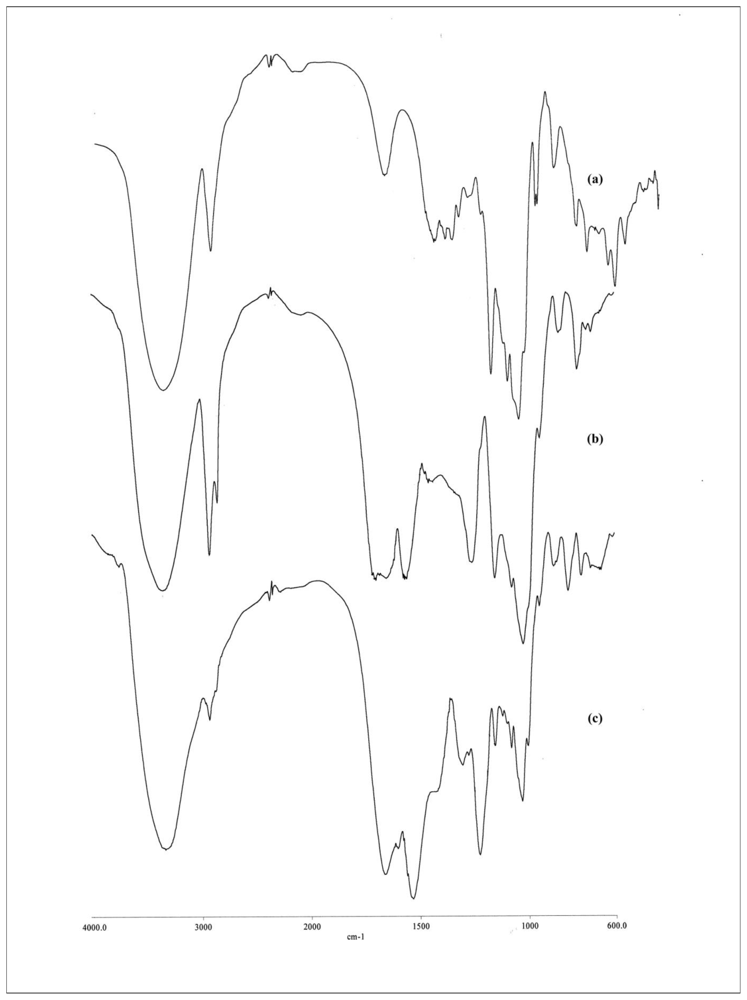
2.1. Characterization of β-CD-HMDI and β-CD-TDI Polymer
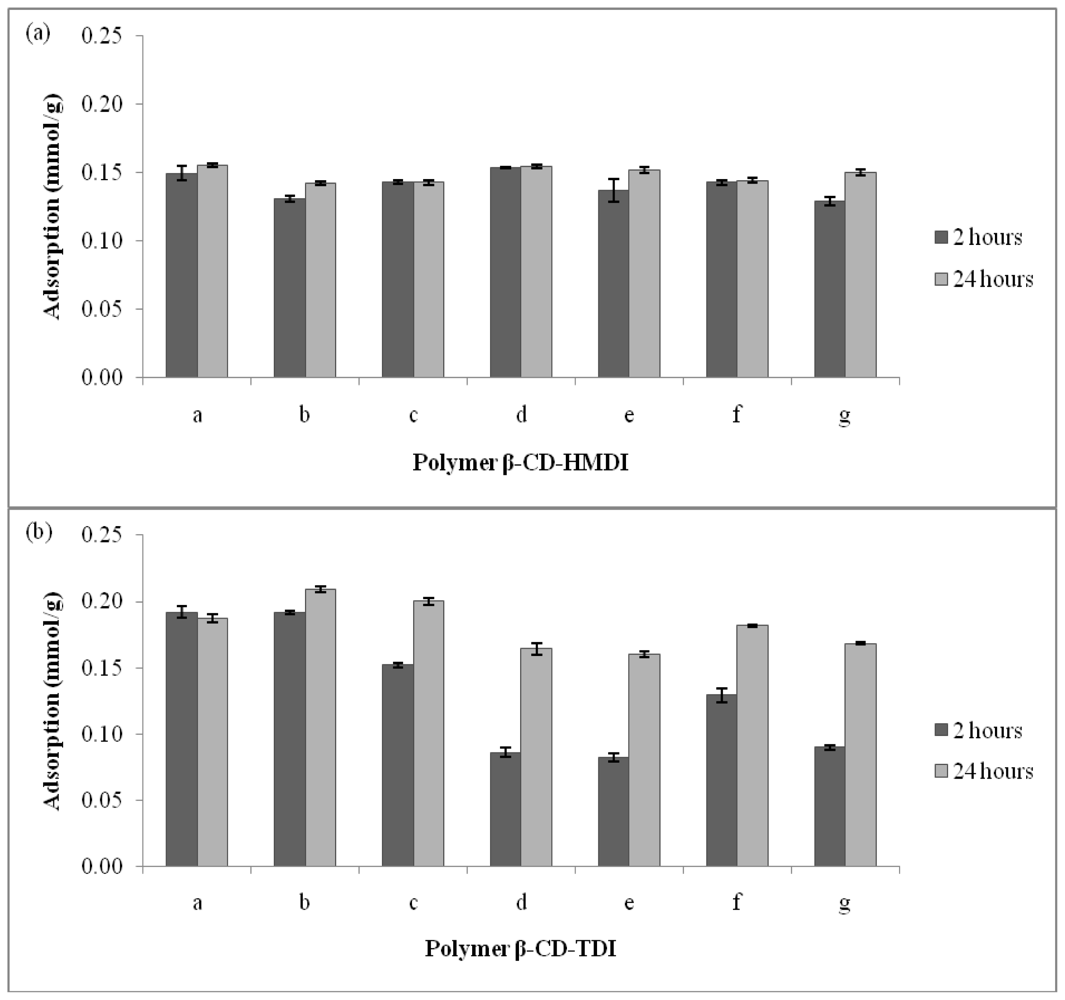
2.2. Effect of the Types and Amount of Cross-Linker
2.3. Adsorption Behavior of Polymers toward Parabens
2.4. Reusability
2.5. Application on Real Sample
3. Experimental Section
3.1. Reagents and Solutions
3.2. Instrumentation
3.3. Preparation of β-CD Polymer
3.4. Characterization of Polymer
3.5. Removal of Parabens from Water
3.6. Reusibility of β-CD Polymer as Adsorbent
3.7. Application of Polymer to Real Samples
4. Conclusions
Acknowledgements
References
- Darbre, PD. Environment oestrogens, cosmetics and breast cancer. Best Pract. Res. Cl. En 2006, 20, 121–143. [Google Scholar]
- Soni, MG; Carabin, IG; Burdock, GA. Safety assessment of esters of p-hydrobenzoic acid (parabens). Food Chem. Toxicol 2005, 43, 985–1015. [Google Scholar]
- Nagel, JE; Fuscaldo, JT; Fireman, P. Paraben allergy. JAMA 1977, 237, 1594–1595. [Google Scholar]
- Harvey, PW; Darbre, P. Endocrine disrupters and human health: Could oestrogenic chemicals in body care cosmetics adversely affect breast cancer incidence in woman? A review of evidence and call for further research. J. Appl. Toxicol 2004, 24, 167–176. [Google Scholar]
- Darbre, PD; Aljarrah, A; Miller, WR; Coldham, NG; Sauer, MJ; Pope, GS. Concentrations of parabens in human breast tumours. J. Appl. Toxicol 2004, 24, 5–13. [Google Scholar]
- Tavares, RS; Martins, FC; Oliveira, PJ; Ramalho-Santos, J; Peixoto, FP. Parabens in male- infertility- Is there a mitochondrial connection? Reprod. Toxicol 2009, 27, 1–7. [Google Scholar]
- Shen, H-Y; Jiang, H-L; Mao, H-L; Pan, G; Zhou, L; Cao, Y-F. Simultaneous determination of seven phthalates and four parabens in cosmetic products using HPLC-DAD and GC-MS methods. J. Sep. Sci 2007, 30, 48–54. [Google Scholar]
- Andersen, HR; Lundsbye, M; Wedel, HV; Eriksson, E; Ledin, A. Estrogenic personal care products in a greywater reuse system. Water Sci. Technol 2007, 56, 45–49. [Google Scholar]
- Benijts, T; Lambert, W; De Leenheer, A. Analysis of multiple endocrine disruptors in environmental waters via wide-spectrum solid-phase extraction and dual-polarity ionization LC-Ion Trap-MS/MS. Anal. Chem 2004, 76, 704–711. [Google Scholar]
- Blanco, E; Casais, MDC; Mejuto, MDC; Cela, R. Combination of off-line solid-pahse extraction and on-column sample stacking for sensitive determination of parabens and p-hydroxybenzoic acid in waters by non-aqueous capillary electrophoresis. Anal. Chim. Acta 2009, 647, 104–111. [Google Scholar]
- Lee, H-B; Peart, TE; Svoboda, ML. Determination of endocrine-disrupting phenols, acidic pharmaceuticals, and personal-care products in sewage by solid-phase extraction and gas chromatography- mass spectrometry. J. Chromatogr. A 2005, 1094, 122–129. [Google Scholar]
- Regueiro, J; Llompart, M; Psillakis, E; Garcia-Monteagudo, J; Garcia-Jares, C. Ultrasound-assisted emulsification-microextraction of phenolic preservatives in water. Talanta 2009, 79, 1387–1397. [Google Scholar]
- Trenholm, RA; Vanderford, BJ; Drewers, JE; Snyder, SA. Determination of household chemicals using gas chromatography with tandem mass spectrometry. J. Chromatogr. A 2008, 1190, 253–262. [Google Scholar]
- Grylik, D; Lach, M; Miller, JS. The aqueous photosentitized degradation of butylparaben. Photochem. Photobiol. Sci 2009, 8, 549–555. [Google Scholar]
- Tay, KS; Rahman, NA; Abas, MRB. Kinetic studies of the degradation of parabens in aqueous solution by ozone oxidation. Environ. Chem. Lett 2010, 1–7. [Google Scholar] [CrossRef]
- Metcalf; Eddy. Wastewater Engineering: Treatment and Reuse, 3rd ed; McGraw-Hill Professional: Boston, MA, USA, 2003. [Google Scholar]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci 2005, 30, 38–70. [Google Scholar]
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigm 2008, 77, 415–426. [Google Scholar]
- Ravi Kumar, MNV. A review of chitin and chitosan applications. React. Funct. Polym 2000, 46, 1–27. [Google Scholar]
- Synowiecki, J; Al-Khateeb, NA. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci 2003, 43, 145–171. [Google Scholar]
- Juang, R-S; Tseng, R-L; Wu, F-C; Lee, S-H. Adsorption behaviour of reactive dyes from aqueous solution on chitosan. J. Chem. Technol. Biotechnol 1997, 70, 391–399. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci 2006, 31, 603–632. [Google Scholar]
- Guo, L; Li, G; Liu, J; Yin, P; Li, Q. Adsorption of aniline on cross-linked starch from aqueous solution. Ind. Eng. Chem. Res 2009, 48, 10657–10663. [Google Scholar]
- Guo, L; Liu, J; Xing, G; Wen, Q. Adsorption and desorption of zinc(II) on water-insoluble starch phosphates. J. Appl. Polym. Sci 2009, 111, 1110–1114. [Google Scholar]
- Mostafa, KM; Samarkandy, AR; El-Sanabary, AA. Preparation of poly(MAA)-crosslinked pregelled starch graft copolymer and its application in waste water treatment. J. Appl. Polym. Sci 2009, 112, 2838–2846. [Google Scholar]
- Crini, G; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci 2002, 25, 789–813. [Google Scholar]
- Murai, S; Imajo, S; Takasu, Y; Takahashi, K; Hattori, K. Removal of phthalic acid esters from aqueous solution by inclusion and adsorption on β-cyclodextrin. Environ. Sci. Technol 1998, 32, 782–787. [Google Scholar]
- Yilmaz, E; Sezgin, M; Yilmaz, M. Immobilized copper-ion affinity adsorbent based on a cross-linked β-cyclodextrin polymer for adsorption of Candida rugosa lipase. Biocatal. Biotransform 2009, 27, 360–366. [Google Scholar]
- Stoddart, JF. A century of cyclodextrins. Carbohydr. Res 1989, 192, xii–xv. [Google Scholar]
- Prabaharan, M; Mano, JF. Chitosan derivatives bearing cyclodextrin cavities as novel adsorbent matrices. Carbohydr. Polym 2006, 63, 153–166. [Google Scholar]
- Lu, D; Yang, L; Zhou, T; Lei, Z. Synthesis, characterization and properties of biodegradable polylactic acid-β-cyclodextrin cross-linked copolymer microgels. Eur. Polym. J 2008, 44, 2140–2145. [Google Scholar]
- Murai, S; Imajo, S; Maki, Y; Takahashi, K; Hattori, K. Adsorption and recovery of nonionic surfactants by β-cyclodextrin polymer. J. Colloid Interface Sci 1996, 183, 118–123. [Google Scholar]
- Crini, G; Morin, N; Rouland, J-C; Janus, L; Morcellet, M; Bertini, S. Adsorption de béta-naphtol sur des gels de cyclodextrin-carboxyméthylcellulose reticules. Eur. Polym. J 2002, 38, 1095–1103. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev 1998, 98, 1743–1753. [Google Scholar]
- Del Valle, EMM. Cyclodextrins and their uses: A review. Process Biochem 2004, 39, 1033–1046. [Google Scholar]
- Mamba, BB; Krause, RW; Malefetse, TJ; Nxumalo, EN. Monofunctionalized cyclodextrin polymers for the removal of organic pollutants from water. Environ. Chem. Lett 2007, 5, 79–84. [Google Scholar]
- Yamasaki, H; Makihata, Y; Fukunaga, K. Efficient removal of wastewater from phenolic resin plants using crosslinked particles. J. Chem. Technol. Biotechnol 2006, 81, 1271–1276. [Google Scholar]
- Moon, J-Y; Jung, H-J; Moon, MH; Chung, BC; Choi, MH. Inclusion complex-based solid-phase extraction of steroidal compounds with entrapped β-cyclodextrin polymer. Steroids 2008, 73, 1090–1097. [Google Scholar]
- Bhaskar, M; Aruna, P; Jeevan, RG; Radhakrishnan, G. β-cyclodextrin-polyurethane polymer as solid-phase extraction material for the analysis of carcinogenic aromatic amines. Anal. Chim. Acta 2004, 509, 39–45. [Google Scholar]
- Wang, X; Zeng, H; Wei, Y; Lin, J-M. A reversible fluorescence sensor based on insoluble β-cyclodextrin polymer for direct determination of bisphenol A (BPA). Sens. Actuat. B 2006, 114, 565–572. [Google Scholar]
- Zhu, X; Wu, M; Gu, Y. β-cyclodextrin-cross-linked polymer as solid-phase extraction material coupled with inductively coupled plasma mass spectrometry for the analysis of trace Co(II). Talanta 2009, 78, 565–569. [Google Scholar]
- Chan, LW; Kurup, TRR; Muthanaiah, A; Thenmozhiyal, JC. Interaction of p-hydrobenzoic esters with beta-cyclodextrin. Int. J. Pharm 2000, 195, 71–79. [Google Scholar]
- de Vries, EJC; Caira, MR. A structural and thermal investigation of the inclusion of parabens in heptakis(2,6-di-O-methyl)cyclomaltoheptaose. Carbohydr. Res 2008, 343, 2433–2438. [Google Scholar]
- de Vries, EJC; Caira, MR; Bogdan, M; Farcas, SI; Bogdan, D. Inclusion of parabens in β-cyclodextrin: A solution NMR and X-ray structural investigation. Supramol. Chem 2009, 21, 358–366. [Google Scholar]
- García-Zubiri, IX; González-Gaitano, G; Isasi, JR. Sorption models in cyclodextrin polymers: Langmuir, Freundlich, and a dual-mode approach. J. Colloid Interface Sci 2009, 337, 11–18. [Google Scholar]


| Adsorbent | Paraben | Single solute solution | Mix solution | ||
|---|---|---|---|---|---|
| Adsorption capacity (mmol/g) | R.S.D.(%) n = 3 | Adsorption capacity (mmol/g) | R.S.D.(%) n = 3 | ||
| β-CD-HMDI(a) | MP | 0.0305 | 9.08 | 0.0239 | 7.51 |
| EP | 0.0376 | 9.36 | 0.0357 | 7.56 | |
| PP | 0.1854 | 5.67 | 0.0715 | 7.33 | |
| BP | 0.3026 | 8.10 | 0.2769 | 7.06 | |
| Total | 0.4080 | ||||
| β-CD-TDI(b) | MP | 0.1019 | 3.03 | 0.0324 | 5.42 |
| EP | 0.1286 | 3.22 | 0.0508 | 6.31 | |
| PP | 0.2551 | 3.04 | 0.1150 | 2.73 | |
| BP | 0.3699 | 3.44 | 0.3570 | 9.69 | |
| Total | 0.5552 | ||||
| Sample | Methyl paraben | Ethyl paraben | Propyl paraben | Benzyl paraben | ||||
|---|---|---|---|---|---|---|---|---|
| % removed | R.S.D. (%) | % removed | R.S.D. (%) | % removed | R.S.D. (%) | % removed | R.S.D. (%) | |
| Deionized water (Standard) | 15.38 | 4.54 | 23.21 | 5.41 | 48.44 | 13.46 | 100.00 | 0.00 |
| Tap water | 13.28 | 5.75 | 21.08 | 3.67 | 46.27 | 1.88 | 100.00 | 0.00 |
| Drinking water | 15.02 | 7.46 | 25.52 | 2.75 | 58.16 | 4.07 | 100.00 | 0.00 |
| River water | 14.05 | 7.87 | 24.13 | 3.66 | 56.70 | 1.64 | 100.00 | 0.00 |
| Swimming pool water | 13.46 | 6.68 | 22.07 | 8.22 | 49.59 | 8.15 | 100.00 | 0.00 |
| Waste water effluent | 12.80 | 6.45 | 20.83 | 4.94 | 46.85 | 3.39 | 100.00 | 0.00 |
| Soy sauce | 17.02 | 3.78 | 28.12 | 6.58 | 61.90 | 7.69 | 100.00 | 0.00 |
| Mouth rinse | 11.36 | 1.97 | 22.94 | 4.42 | 47.07 | 7.78 | 100.00 | 0.00 |
| Sample | Methyl paraben | Ethyl paraben | Propyl paraben | Benzyl paraben | ||||
|---|---|---|---|---|---|---|---|---|
| % removed | R.S.D. (%) | % removed | R.S.D. (%) | % removed | R.S.D. (%) | % removed | R.S.D. (%) | |
| Deionized water (Standard) | 33.56 | 1.88 | 51.55 | 2.62 | 95.51 | 1.31 | 100.00 | 0.00 |
| Tap water | 29.76 | 7.37 | 48.07 | 11.04 | 89.98 | 7.82 | 100.00 | 0.00 |
| Drinking water | 32.58 | 2.76 | 52.42 | 0.41 | 97.93 | 1.43 | 100.00 | 0.00 |
| River water | 28.56 | 5.92 | 46.28 | 5.30 | 88.29 | 4.31 | 100.00 | 0.00 |
| Swimming pool water | 27.09 | 0.27 | 47.35 | 3.59 | 93.34 | 2.89 | 100.00 | 0.00 |
| Waste water effluent | 24.24 | 2.41 | 38.77 | 1.61 | 73.63 | 2.21 | 100.00 | 0.00 |
| Soy sauce | 28.74 | 6.97 | 44.49 | 9.62 | 85.66 | 6.15 | 100.00 | 0.00 |
| Mouth rinse | 20.87 | 3.83 | 44.99 | 7.74 | 84.03 | 6.79 | 100.00 | 0.00 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chin, Y.P.; Mohamad, S.; Abas, M.R.B. Removal of Parabens from Aqueous Solution Using β-Cyclodextrin Cross-Linked Polymer. Int. J. Mol. Sci. 2010, 11, 3459-3471. https://doi.org/10.3390/ijms11092459
Chin YP, Mohamad S, Abas MRB. Removal of Parabens from Aqueous Solution Using β-Cyclodextrin Cross-Linked Polymer. International Journal of Molecular Sciences. 2010; 11(9):3459-3471. https://doi.org/10.3390/ijms11092459
Chicago/Turabian StyleChin, Yuk Ping, Sharifah Mohamad, and Mhd Radzi Bin Abas. 2010. "Removal of Parabens from Aqueous Solution Using β-Cyclodextrin Cross-Linked Polymer" International Journal of Molecular Sciences 11, no. 9: 3459-3471. https://doi.org/10.3390/ijms11092459




