Synthesis and Characterization of a Heteroleptic Ru(II) Complex of Phenanthroline Containing Oligo-Anthracenyl Carboxylic Acid Moieties
Abstract
:1. Introduction
2. Results and Discussion
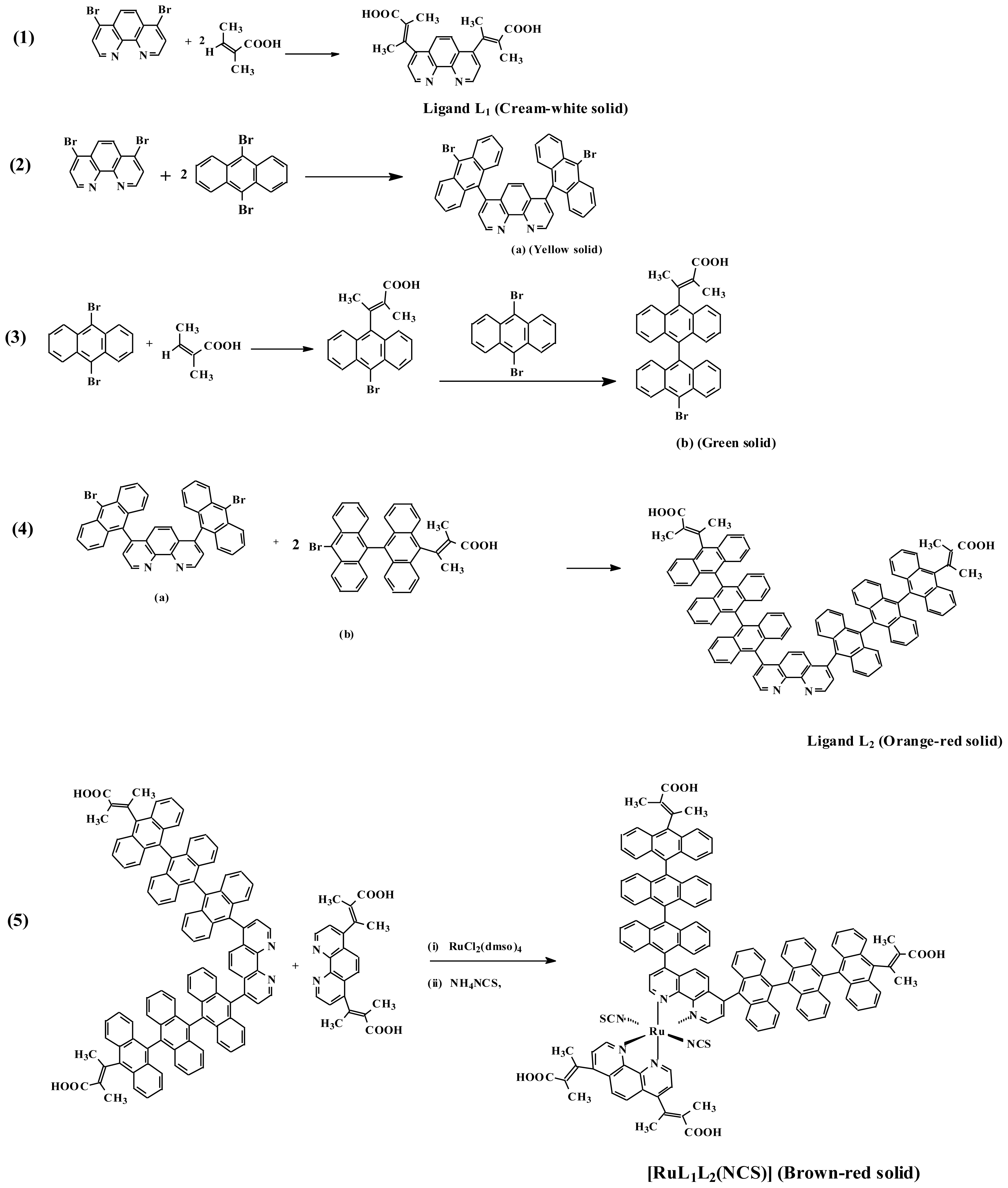
2.1. Syntheses
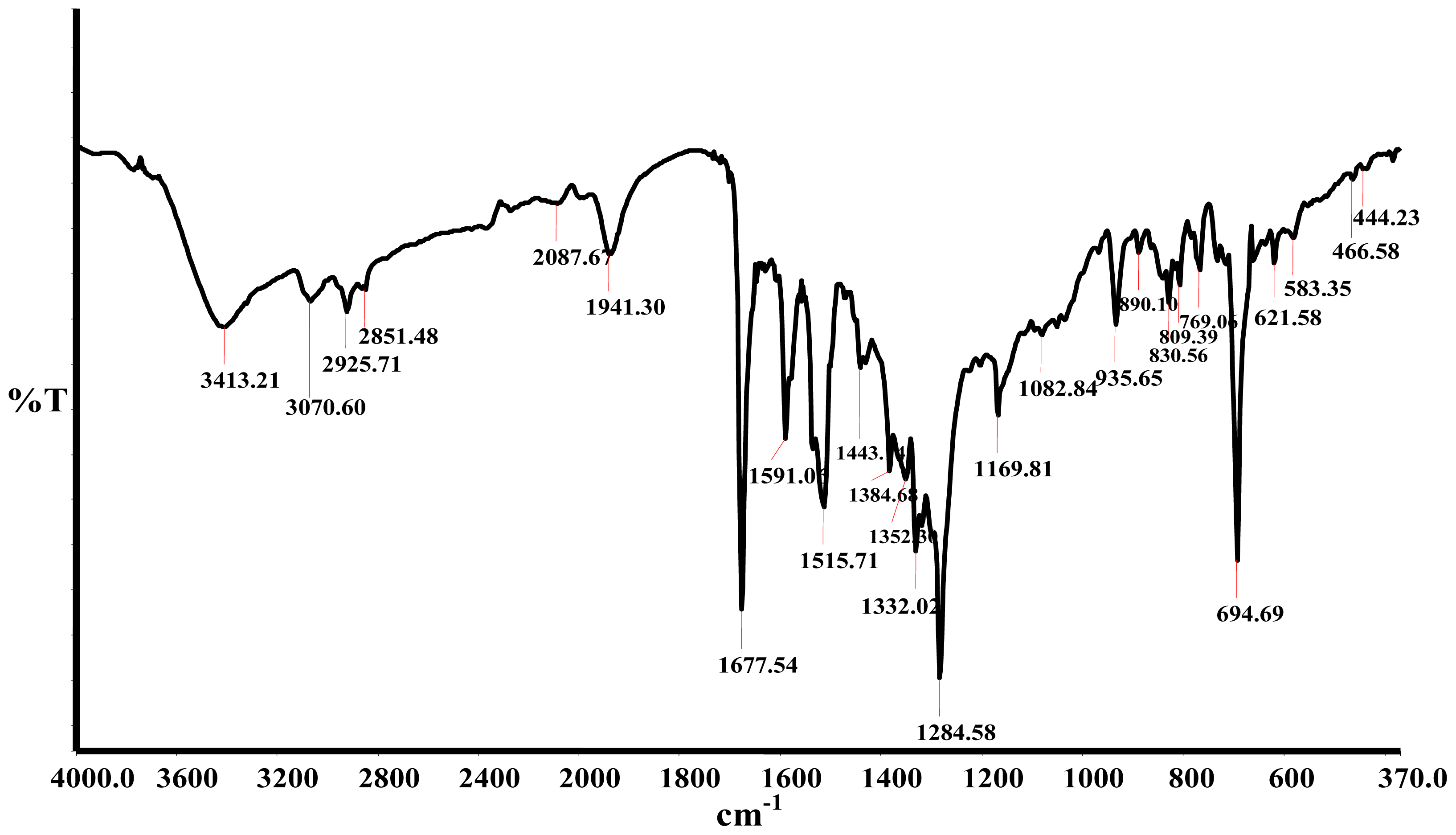
2.2. Infrared Spectra
2.3. NMR
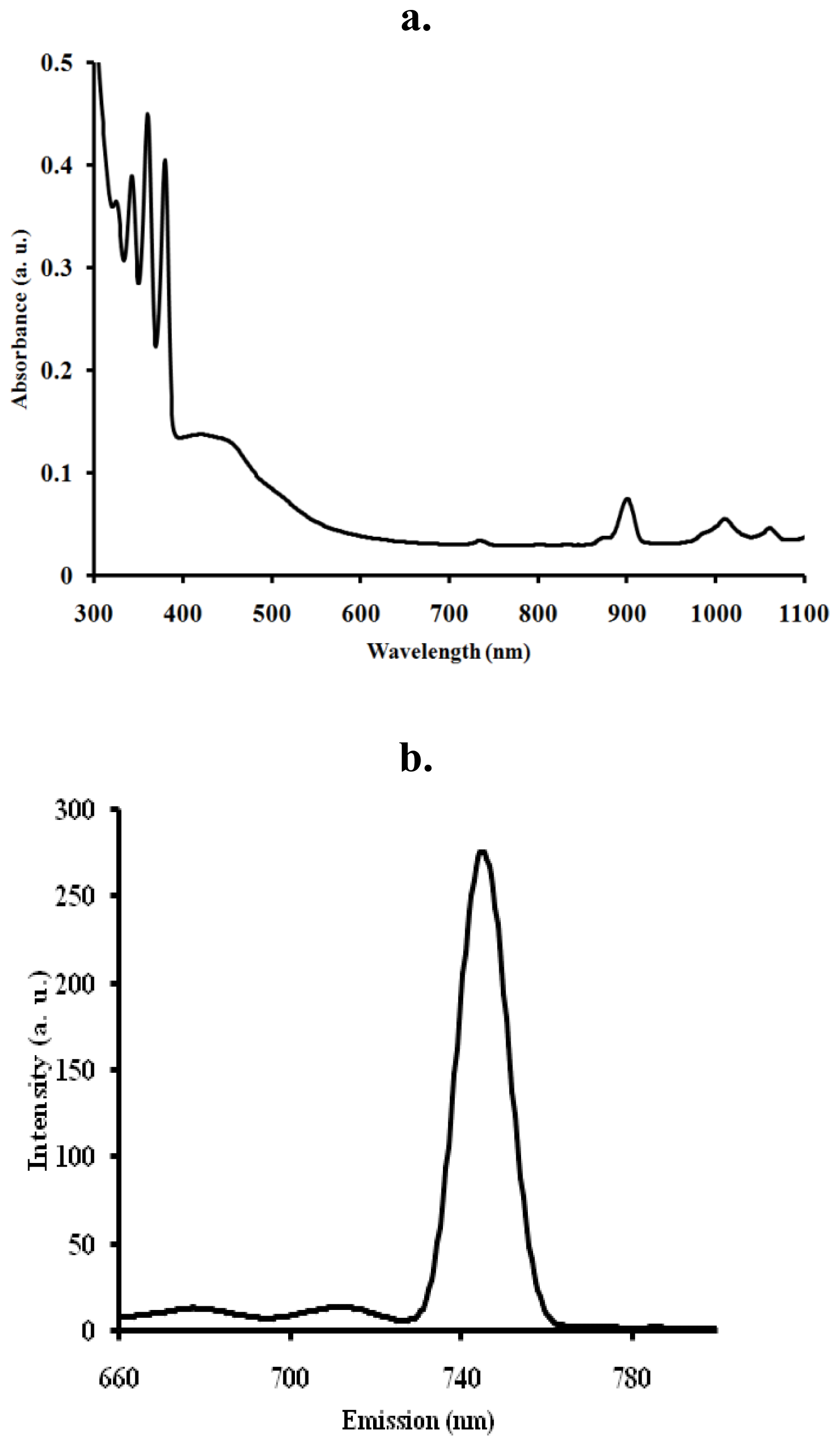
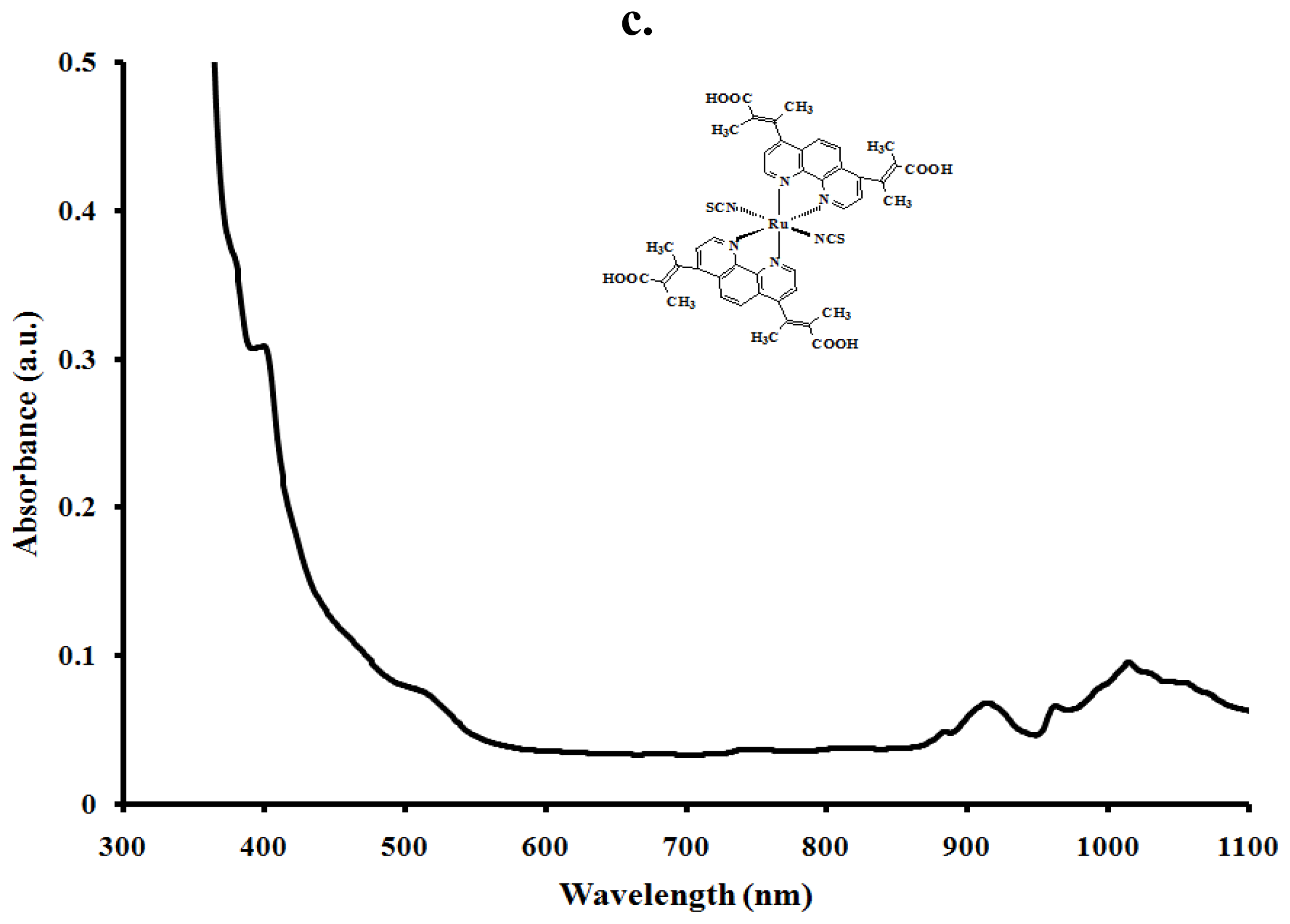
2.4. Electronic and Emission Spectra
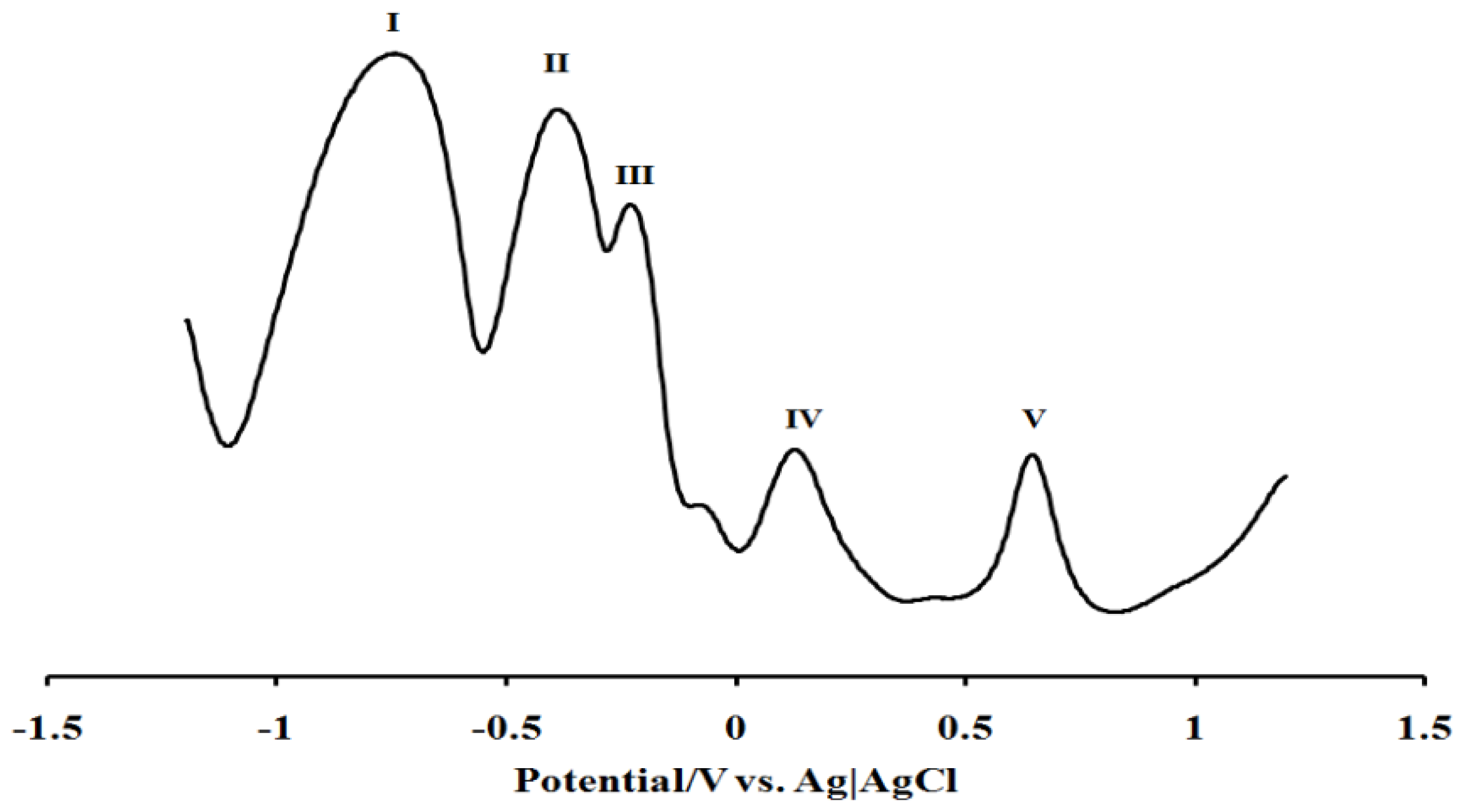
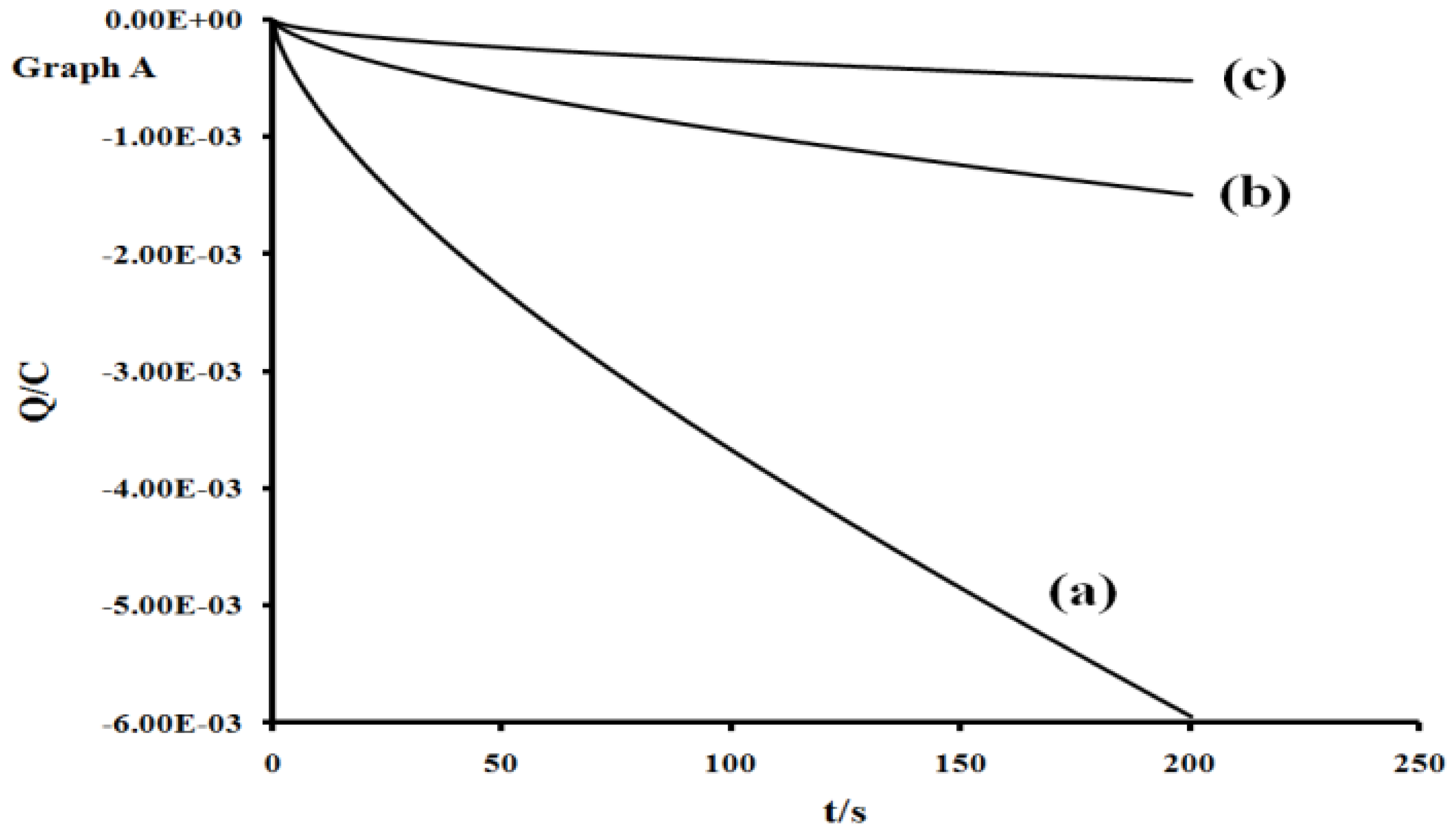
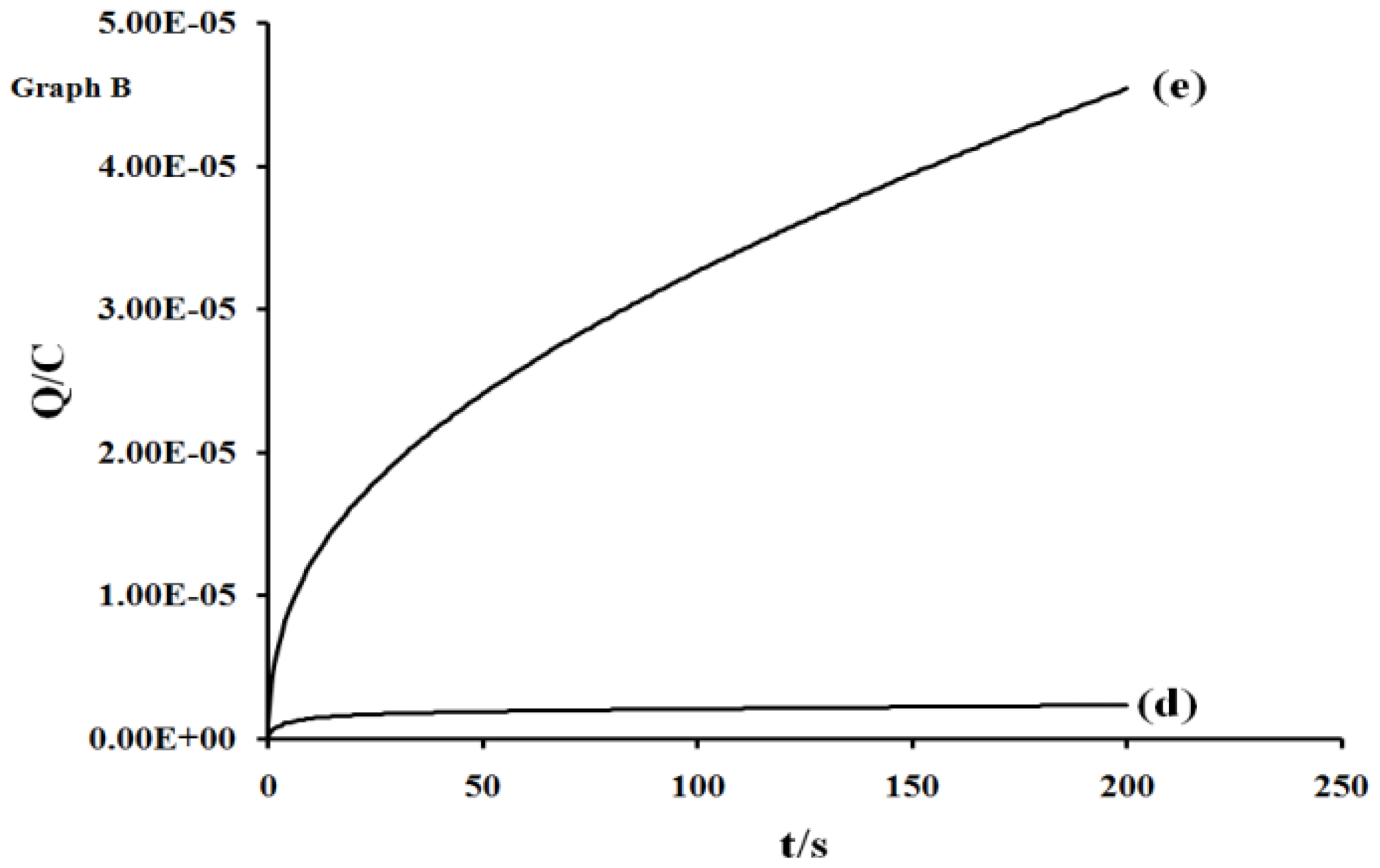
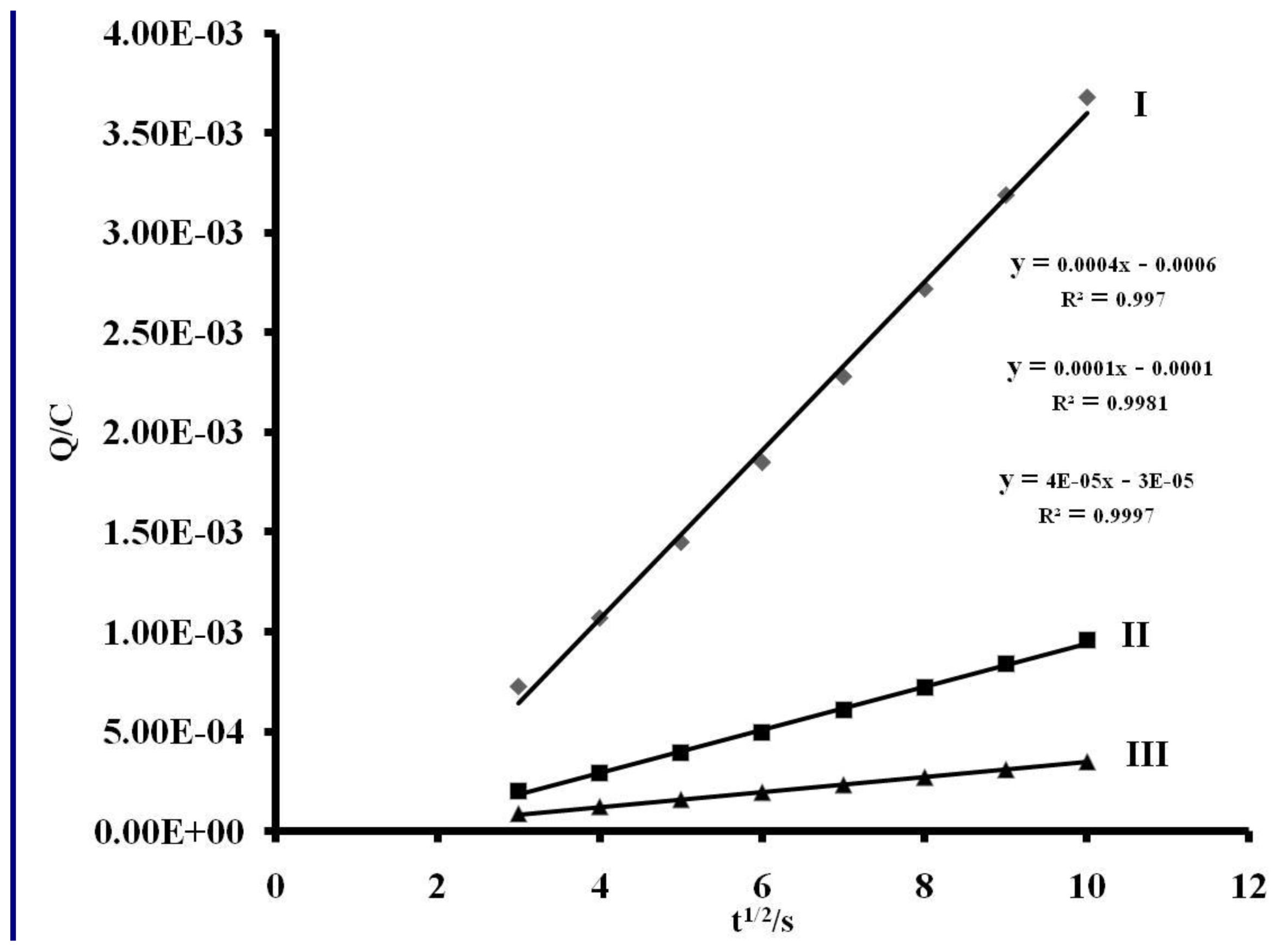
2.5. Electrochemistry
3. Experimental Section
3.1. Materials and General Physical Measurements
3.2. Synthesis of Ligand 1
3.3. Synthesis of Ligand 2
3.4. Synthesis of [Ru(II)-L1L2(NCS)2]
4. Conclusions
Supporting Information
Acknowledgements
References
- Juris, A; Balzani, V; Barigelletti, F; Campagna, S; Belser, P; Zelewsky, AV. Ruthenium(II) polypyridine complexes: photophysics, photochemistry, electrochemistry, and chemiluminescence. Coord. Chem. Rev 1988, 84, 85–277. [Google Scholar]
- Juris, A; Campagna, S; Balzani, V; Gremaud, G; Zelewsky, AV. Absorption spectra, luminescence properties, and electronchemical behaviour of tris-heteroleptic ruthenium(II) polypyridine complexes. Inorg. Chem 1988, 3652–3655. [Google Scholar]
- Prasanna de Silva, A; Fox, DB; Moody, TS; Weir, SM. Luminescent sensors and photonic switches. Pure Appl. Chem 2001, 73, 503–511. [Google Scholar]
- Robertson, N; McGowan, CA. A comparison of potential molecular wires as components for molecular electronics. Chem. Soc. Rev 2003, 32, 96–130. [Google Scholar]
- Balzani, V; Bolletta, F; Ciano, M; Maestri, M. Electron transfer reactions involving light. J. Chem. Ed 1983, 60, 447. [Google Scholar]
- Sun, L; Hammarstrom, L; Norrby, T; Berglund, H; Davydov, R; Andersson, M; Borje, A; Korall, P; Philouze, C; Almgren, M; Styring, S; Akermark, B. Intramolecular electron transfer from coordinated manganese(II) to photogenerated ruthenium(III). Chem. Commun 1997, 607–608. [Google Scholar]
- Leroy-lhez, S; Belin, C; D’aleo, A; Williams, RM; De Cola, L; Fages, F. Extending excitedstate lifetimes by interchromophoric triplet-state equilibration in a pyrene-Ru(II) diimine dyad system. Supramol. Chem 2003, 15, 627–637. [Google Scholar]
- Tyson, DS; Luman, CR; Zhou, X; Castellano, FN. New Ru(II) Chromophores with extended excited state lifetimes. Inorg. Chem 2001, 40, 4063. [Google Scholar]
- Harriman, A; Hissler, M; Khatyr, A; Ziessel, R. The photophysical properties of hybrid metal complexes containing both 2,2′-bipyridine and 2,2′:6′,2″-terpyridine units. Eur. J. Inorg. Chem 2003, 2003, 955–959. [Google Scholar]
- Juris, A; Barigelleti, F; Balzani, V; Belser, P; Zelewsky, AV. New photosensitizers of the ruthenium-polypyridine family for the water splitting reaction. Isr. J. Chem 1982, 22, 87. [Google Scholar]
- Prasanna de Silva, A; Fox, DB; Moody, TS; Weir, SM. Luminescent sensors and photonic switches. Pure Appl. Chem 2001, 73, 503–511. [Google Scholar]
- Jin, Y; Hua, J; Wu, W; Ma, X; Meng, F. Synthesis, characterization and photovoltaic properties of two novel near-infrared perylene dyes containing benzo[e] indole for dye-sensitized solar cells. Synthet. Metal 2008, 158, 64–71. [Google Scholar]
- Tian, FM; Wang, P; Zhu, GY. Synthesis and properties of three tris(1,10-phenanthroline) ruthenium(II) derivatives. Chin. Chem. Lett 1999, 10, 881–884. [Google Scholar]
- Ocakoglu, K; Zafer, C; Cetinkaya, B; Icli, S. Synthesis, characterization and spectroscopic studies of two new heteroleptic Ru(II) polypyridyl complexes. Dye. Pigment 2007, 75, 385–394. [Google Scholar]
- Meyer, TJ. Photochemistry of metal coordination complexes: metal to ligand charge transfer excited state. Pure Appl. Chem 1986, 58, 1193–1206. [Google Scholar]
- Bolletta, F; Maestri, M; Moggi, L; Jamieson, MA; Serpone, N; Henry, MS; Hoffman, MZ. Photochemical, photophysical and thermal behaviour of the tris-(1,10-phenanthroline) chromium(II) ion in aqueous solution. Inorg. Chem 1983, 22, 2502. [Google Scholar]
- Kalyanasundaram, K. Photochemistry of Polypyridine and Porphyrin Complexes; Academic Press Limited: London, UK, 1992; pp. 87–212. [Google Scholar]
- Polo, AS; Itokazu, MK; Iha, NYM. Metal complex sensitizers in dye-sensitized solar cells. Coord. Chem. Rev 2004, 248, 1343–1361. [Google Scholar]
- Wang, P; Zakeeruddin, SM; Moser, JE; Baker, RH; Comte, P; Aranyos, V. Stable new sensitizer with improved light harvesting for nanocrystalline dye sensitized solar cells. Adv. Mater 2004, 16, 1806–1811. [Google Scholar]
- Chen, CY; Lu, HC; Wu, CG; Chen, JG; Ho, KC. New ruthenium complexes containing oligoalkylthiophene-substituted 1,10-phenanthroline for nanocrystalline dye sensitized solar cells. Adv. Funct. Mater 2007, 17, 29–36. [Google Scholar]
- Goze, C; Kozlov, DV; Castellano, FN; Suffert, J; Ziessel, R. Synthesis of bipyridine and terpyridine based ruthenium metallosynthons for grafting of multiple pyrene auxiliaries. Tetrahedron 2003, 44, 8713–8716. [Google Scholar]
- Jiang, KJ; Masaki, N; Xia, JB; Noda, S; Yanagida, S. A novel ruthenium sensitizer with a hydrophobic 2-thiophen-2-yl-vinyl-conjugated bipyridyl ligand for effective dye sensitized TiO2 solar cells. Chem. Commun 2006, 2460–2462. [Google Scholar]
- Kukrek, A; Wang, D; Hou, Y; Zong, R; Thummel, R. Photosensitizers containing the 1,8-naphthyridyl moiety and their use in dye-sensitized solar cells. Inorg. Chem 2006, 45, 10131–10317. [Google Scholar]
- Nazeeruddin, MK; Kay, A; Rodicio, I; Humphry-Baker, R; Muller, E; Liska, P; Vlachopoulos, N; Grätzel, M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate) ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, NCS-) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc 1993, 115, 6382–6390. [Google Scholar]
- Yanagida, M; Yamaguchi, T; Kurashige, M; Hara, K; Katoh, R; Sugihara, H; Arakawa, H. Panchromatic sensitization of nanocrystalline TiO2 with cis-bis(4-carboxy-2-[2′-(4′-carboxypyridyl)]quinoline) bis(thiocyanato-N) ruthenium(II). Inorg. Chem 2003, 42, 7921–7931. [Google Scholar]
- Sauve, G; Cass, ME; Coia, G; Doig, SJ; Lauermann, I; Pomykal, KE; Lewis, NS. Dyesensitization of nanocrystalline titanium dioxide with osmium and ruthenium polypyridyl complexes. J. Phys. Chem 2000, 104, 6831–6836. [Google Scholar]
- Argazzi, R; Bignozzi, CA; Heimer, TA; Castellano, FN; Meyer, GJ. Enhanced spectral sensitivity from Ru(II) polypyridyl photovoltaic devices. Inorg. Chem 1994, 33, 5741–5749. [Google Scholar]
- Heimer, TA; Heilweil, EJ; Bignozzi, CA; Meyer, GJ. Electron injection, recombination and halide oxidation dynamics at dye-sensitized TiO2 interfaces. J. Phys. Chem. A 2000, 104, 4256–4262. [Google Scholar]
- Yamamoto, T; Naoyuki, K; Hiroki, F. Method for Producing Polymerized Hydrocarbon. US Patent 7138484 B2, 26 November 2006. [Google Scholar]
- Yamamoto, T; Ito, T; Kubota, K. A soluble poly(arylene) with large degree of depolarization. poly(2,5-pyridinediyl) prepared by dehalogenation polycondensation of 2,5-dibromopyridine with Ni (0)- complexes. Chem. Lett 1988, 153–154. [Google Scholar]
- Yamamoto, T; Morita, A; Miyazaki, Y; Maruyama, T; Wakayama, H; Zhou, ZH; Nakamura, Y; Kanbara, T; Sasaki, S; Kubota, K. Preparation of π-conjugated poly(thiophene-2,5-diyl), poly(p-phenylene), and related polymers using zerovalent nickel complexes. Linear structure and properties of the π-conjugated polymers. Macromolecules 1992, 25, 1214–1223. [Google Scholar]
- Khatyr, A; Ziessel, R. Stepwise construction of pyrene bridged polytopic ligands carrying acetylenic tethers. Tetrahedron Lett 2002, 43, 7431–7434. [Google Scholar]
- Lipshutz, BL; Chung, DW; Rich, B. Sonogashira couplings of aryl bromides: room temperature, water only, no copper. Org. Lett 2008, 10, 3793–3796. [Google Scholar]
- Evans, IP; Spencer, A; Wilkinson, G. Dichlorotetrakis(dimethyl sulphoxide) ruthenium(II) and its use as a source material for some new Ruthenium(II) complexes. J. Chem. Soc. Dalton Trans 1973, 204–208. [Google Scholar]
- Vyas, P; Bhatt, AK; Ramachandraiah, G; Bedekar, AV. Environmentally benign chlorination and bromination of aromatic amines, hydrocarbons and naphthols. Tetrahedron Lett 2003, 44, 4085–4088. [Google Scholar]
- Mitsopoulou, CA; Veroni, I; Philippopoulos, AI; Falaras, P. Synthesis, characterization and sensitization properties of two novel mono and bis carboxyl-dipyrido-phenazine ruthenium(II) charge transfer complexes. J. Photochem. Photobiol. A: Chem 2007, 191, 6–12. [Google Scholar]
- Chen, CY; Wu, SJ; Wu, CG; Chen, JG; Ho, KC. A ruthenium complex with super high light-harvesting capacity for dye-sensitized solar cells. Angew. Chem 2006, 118, 5954–5957. [Google Scholar]
- Chen, CY; Wu, SJ; Li, JY; Wu, CG; Chen, JG; Ho, KC. A new route to enhance the lightharvesting capability of ruthenium complexes for dye-sensitized solar cells. Adv. Mat 2007, 19, 3888–3891. [Google Scholar]
- Brown, DW; Floyd, AJ; Sainsbury, M. Organic Spectroscopy; John Wiley & Sons: New York, NY, USA, QD: 272.S6 B76; November 1988; pp. 24–53. [Google Scholar]
- Cooper, JW. Spectroscopic Techniques for Organic Chemists; John Wiley & Sons: New York, NY, USA, 1980; pp. 53–117. [Google Scholar]
- Nazeeruddin, MK; Zakeeruddin, SM; Humphry-Baker, R; Gorelsky, SI; Lever, ABP; Gratzel, M. Synthesis, spectroscopic and a ZINDO study of cis- and trans- (X2) Bis(4,4′-dicarboxylic acid-2,2′-bipyridine)Ruthenium(II) complexes (X = Cl−, H2O, NCS−). Coord. Chem. Rev 2000, 208, 213–226. [Google Scholar]
- Li, X; Gui, J; Yang, H; Wu, W; Li, F; Tian, H; Huang, C. A new carbozole-based phenanthrenyl ruthenium complex as sensitizer for a dye-sensitized solar cell. Inorg. Chim. Acta 2008, 361, 2835–2840. [Google Scholar]
- Nazeeruddin, MK; Zakeeruddin, SM; Humphry-Baker, R; Gorelsky, SI; Lever, ABP; Gratzel, M. Synthesis, spectroscopic and a ZINDO study of cis- and trans-(X2) Bis(4,4′-dicarboxylic acid-2,2′-bipyridine)Ruthenium(II) complexes (X = Cl-, H2O, NCS-). Coord. Chem. Rev 2000, 208, 213–226. [Google Scholar]
- Wehrli, FW; Marchand, AP; Wehrli, S. Interpretation of Carbon-13 NMR Spectra, 2nd ed; John Wiley & Sons Ltd: Tiptree, Essex, UK, 1988; pp. 164–184. [Google Scholar]
- Wu, SJ; Chen, CY; Chen, JG; Li, JY; Tung, YL; Ho, KC; Wu, CG. An efficiency lightharvesting ruthenium dye for solar cell application. Dye. Pigment 2009, 84, 1–7. [Google Scholar]
- Campbell, WM; Jolley, KW; Wagner, P; Wagner, K; Walsh, PJ; Gordon, KC. Highly efficient porphyrin sensitizers for dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 11760–11762. [Google Scholar]
- Balzani, V; Juris, A. Photochemistry and photophysics of Ru(II) polypyridine complexes in the Bologna group. From early studies to recent developments. Coord. Chem. Rev 2001, 211, 97–115. [Google Scholar]
- Nazeeruddin, MK; Klein, C; Liska, P; Gratzel, M. Synthesis of novel ruthenium sensitizers and their application in dye-sensitized solar cells. Coord. Chem. Rev 2005, 249, 1460–1467. [Google Scholar]
- Jeong, M; Nam, H; Sohn, OJ; Rhee, J, II; Kim, HJ; Cho, CW; Lee, S. Synthesis of phenanthroline derivatives by Sonogashira reaction and the use of their ruthenium complexes as optical sensors. Inorg. Chem. Commun 2008, 11, 97–100. [Google Scholar]
- Xie, PH; Hou, VJ; Wei, TX; Zhang, BW; Cao, Y; Huang, CH. Synthesis and photoelectric studies of Ru(II) polypyridyl sensitizers. Inorg. Chim. Acta 2000, 308, 73–79. [Google Scholar]
- Onggo, D; Scudder, ML; Craig, DC; Goodwin, HA. The influence of ortho-substitution within the ligand on the geometry of the tris(2,2′-bipyridine)ruthenium(II) and tris(1,10- phenathroline)ruthenium(II) ions. J. Mol. Struct 2005, 738, 129–136. [Google Scholar]
- Zhang, J; Lee, JK; Wu, Y; Royce, WM. Photoluminescence and electronic interaction of anthracene derivatives adsorbed on sidewalls of single-walled carbon nanotubes. Nano. Lett 2003, 3, 403–407. [Google Scholar]
- Li, X; Gui, J; Yang, H; Wu, W; Li, F; Tian, H; Huang, C. A new carbazole-based phenanthrenyl ruthenium complex as sensitizer for a dye-sensitized solar cell. Inorg. Chim. Acta 2008, 361, 2835–2840. [Google Scholar]
- Zhang, P; Xia, B; Sun, Y; Yang, B; Tian, W; Wang, Y; Zhang, G. Electronic structures and optical properties of two anthracene derivatives. Chin. Sci. Bull 2006, 51, 2444–2450. [Google Scholar]
- Sumby, CJ; Steel, PJ. Mono- and dinuclear ruthenium complexes of bridging ligands incorporating two di-2-pyridylamine motifs: Synthesis, spectroscopy and electrochemistry. Polyhedron 2007, 26, 5370–5381. [Google Scholar]
- Aranyos, V; Hagfeldt, A; Greenberg, H; Figgemeier, E. Electropolymerisable bipyridine ruthenium(II) complexes: synthesis, spectroscopic and electrochemical characterisation of 4-((2- thienyl) ethenyl)- and 4,4′-di((2-thienyl) ethenyl)-2,2′-bipyridine ruthenium complexes. Polyhedron 2004, 23, 589–598. [Google Scholar]
- Byabartta, P; Jasimuddin, SK; Mostafa, G; Lu, TH; Sinha, C. The synthesis, spectra studies and electrochemistry of 1,10-(phenanthroline-bis-{1-alkyl-2-(arylazo)imidazole}ruthenium(II) perchlorate. Single crystal X-ray structure of [Ru(phen)(HaaiMe)2](ClO4)2 [phen = 1,10- phenanthroline, HaaiMe = 1-methyl-2-(phenylazo)imidazole]. Polyhedron 2003, 22, 849–859. [Google Scholar]
- Roundhill, DM. Photochemistry and Photophysics of Metal Complexes; Plenum Address: New York, NY, USA, 1993. [Google Scholar]
- Calogero, G; Di Marco, G; Cazzanti, S; Caramori, S; Argazzi, R; Di Carlo, A; Bignozzi, CA. Efficient dye-sensitized solar cells using red turnip and purple wild silician prickly pear fruits. Int. J. Mol. Sci 2010, 11, 254–267. [Google Scholar]
- Wei, D. Dye sensitized solar cells. Int. J. Mol. Sci 2010, 11, 1103–1113. [Google Scholar]
- Koyama, Y; Miki, T; Wang, XF; Nagae, H. Dye-sensitized solar cells based on the principles and materials of photosynthesis: mechanisms of suppression and enhancement of photocurrent and conversion efficiency. Int. J. Mol. Sci 2009, 10, 4575–4622. [Google Scholar]
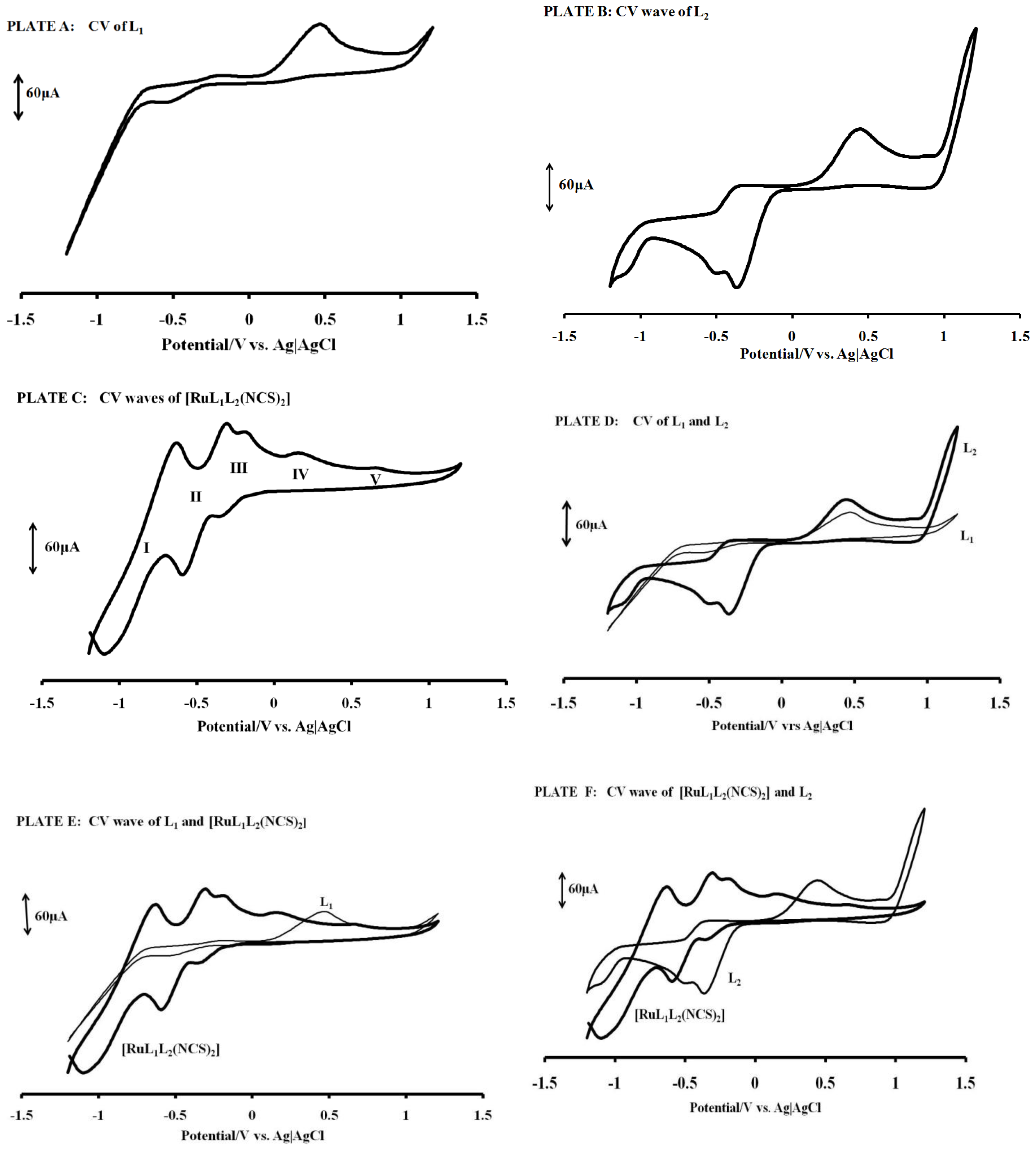
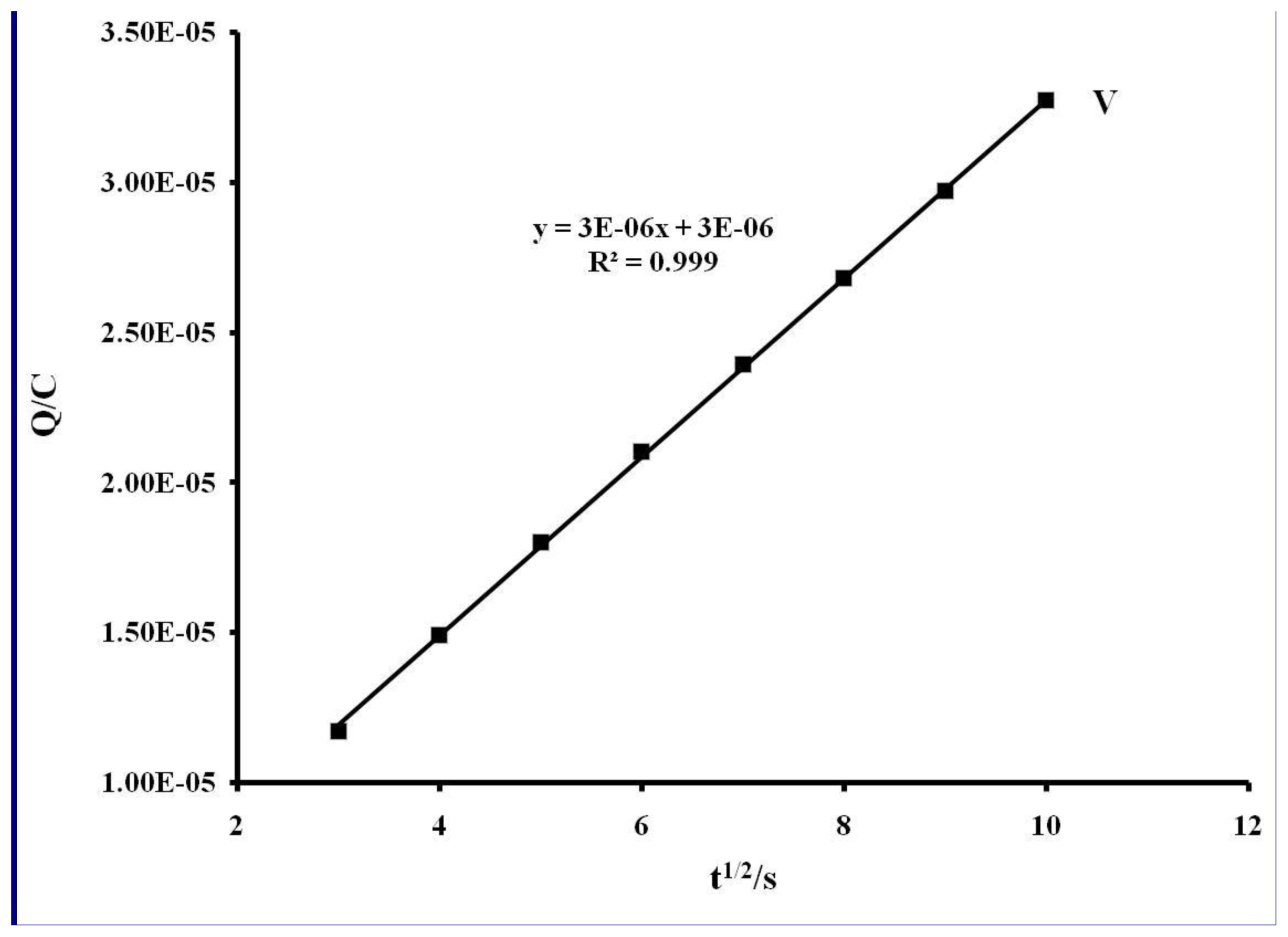
| Compound | Epa/V | E1/2/V |
|---|---|---|
| L1 | 0.45 | −0.23, −0.59 |
| L2 | 0.48 | −0.38, −0.57, 0.94 |
| [RuL1L2(NCS)2] | 0.17, 0.63 | −0.27, −0.45, −0.86 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Adeloye, A.O.; Ajibade, P.A. Synthesis and Characterization of a Heteroleptic Ru(II) Complex of Phenanthroline Containing Oligo-Anthracenyl Carboxylic Acid Moieties. Int. J. Mol. Sci. 2010, 11, 3158-3176. https://doi.org/10.3390/ijms11093158
Adeloye AO, Ajibade PA. Synthesis and Characterization of a Heteroleptic Ru(II) Complex of Phenanthroline Containing Oligo-Anthracenyl Carboxylic Acid Moieties. International Journal of Molecular Sciences. 2010; 11(9):3158-3176. https://doi.org/10.3390/ijms11093158
Chicago/Turabian StyleAdeloye, Adewale O., and Peter A. Ajibade. 2010. "Synthesis and Characterization of a Heteroleptic Ru(II) Complex of Phenanthroline Containing Oligo-Anthracenyl Carboxylic Acid Moieties" International Journal of Molecular Sciences 11, no. 9: 3158-3176. https://doi.org/10.3390/ijms11093158




