Sequential Events in the Irreversible Thermal Denaturation of Human Brain-Type Creatine Kinase by Spectroscopic Methods
Abstract
:1. Introduction
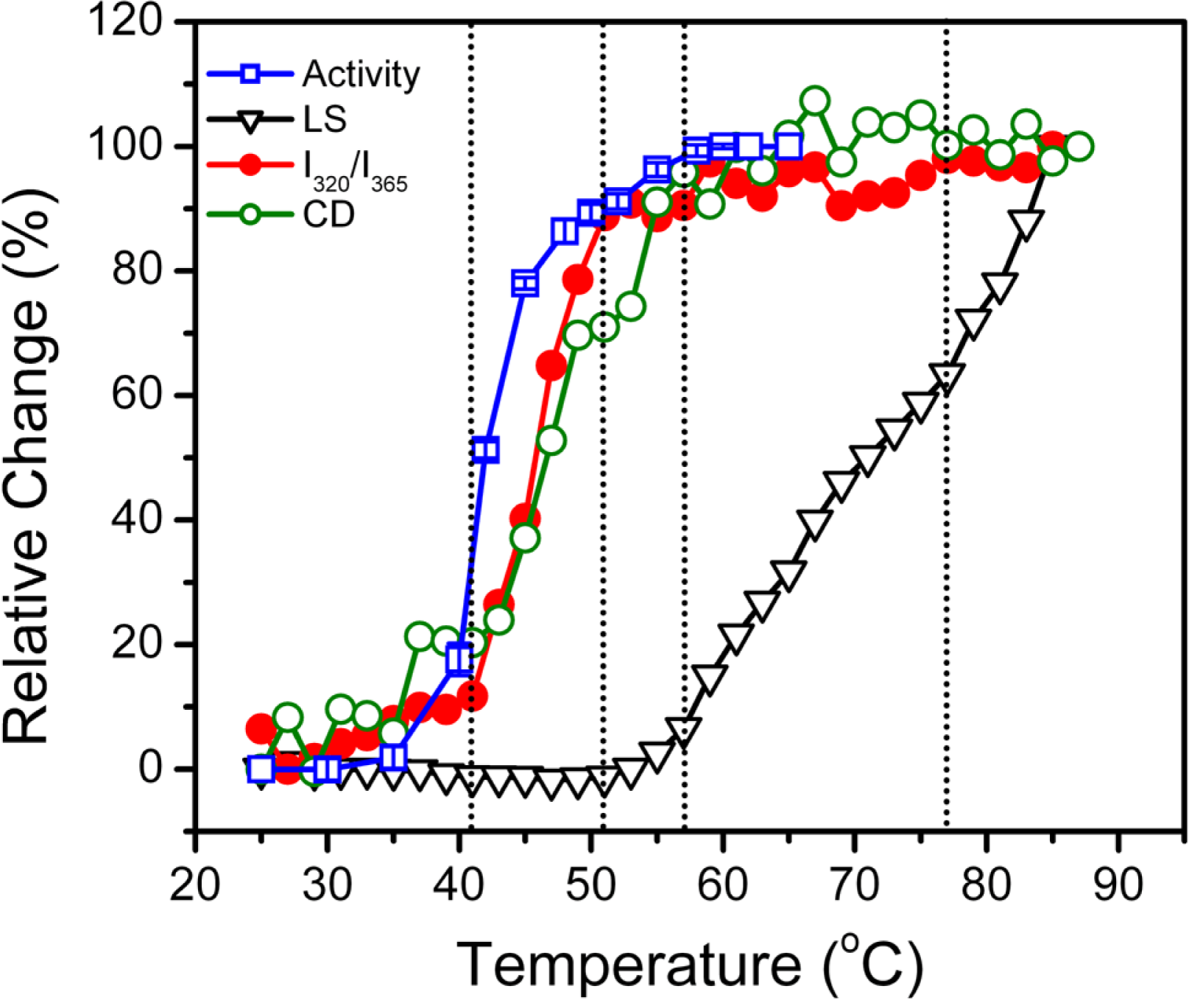
2. Results and Discussion
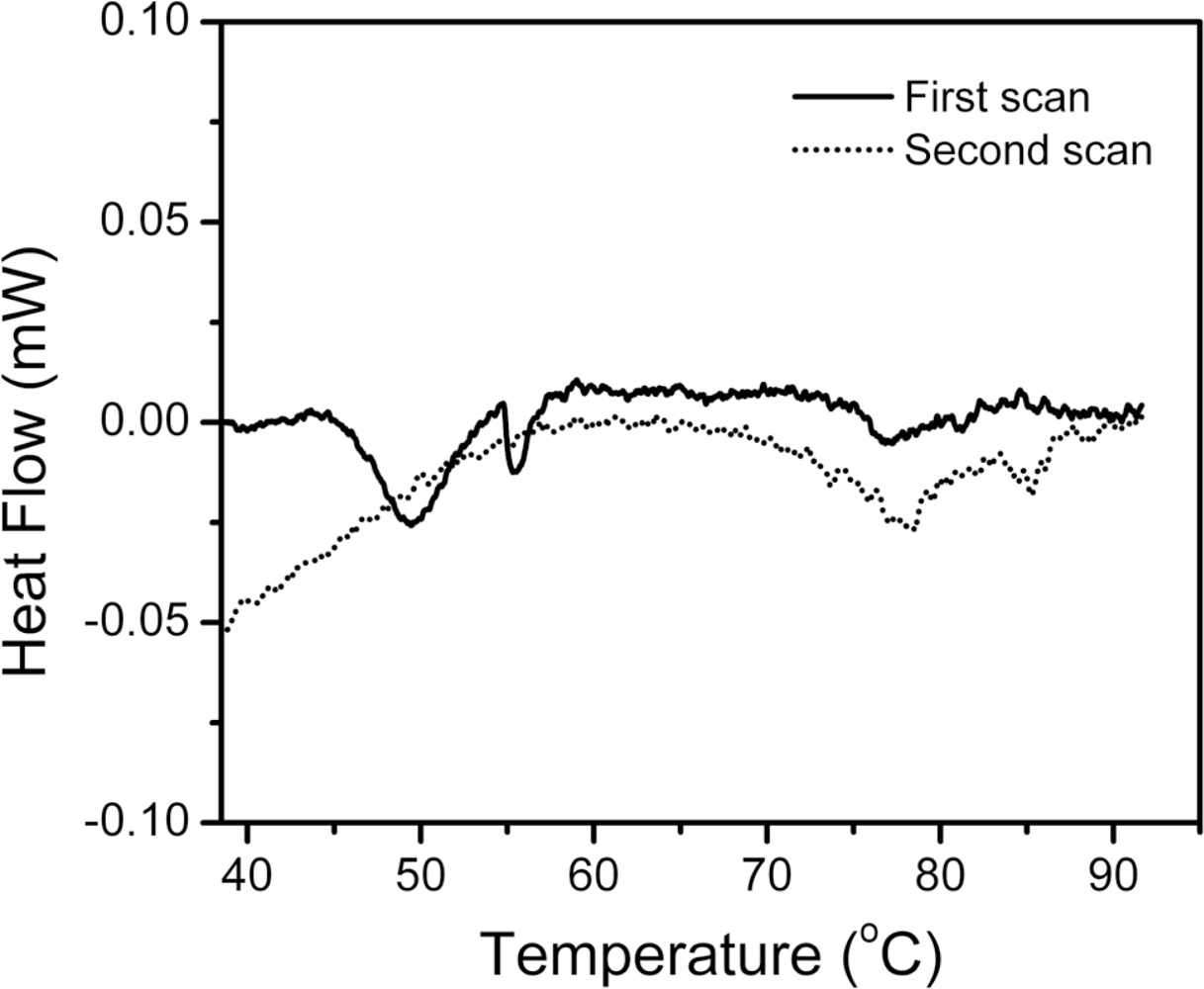
2.1. Thermal Denaturation of hBBCK Is Calorimetrically Irreversible
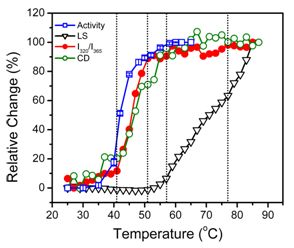
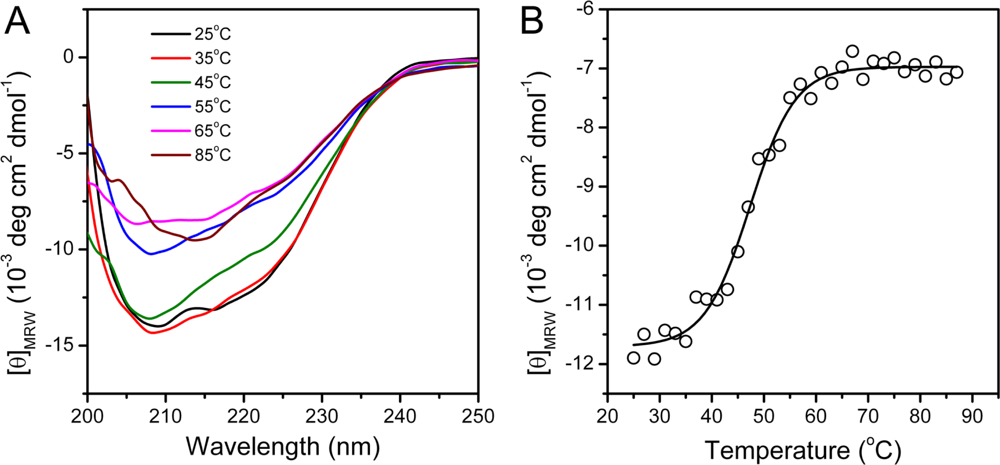
2.2. hBBCK Thermal Denaturation Monitored by CD Spectroscopy
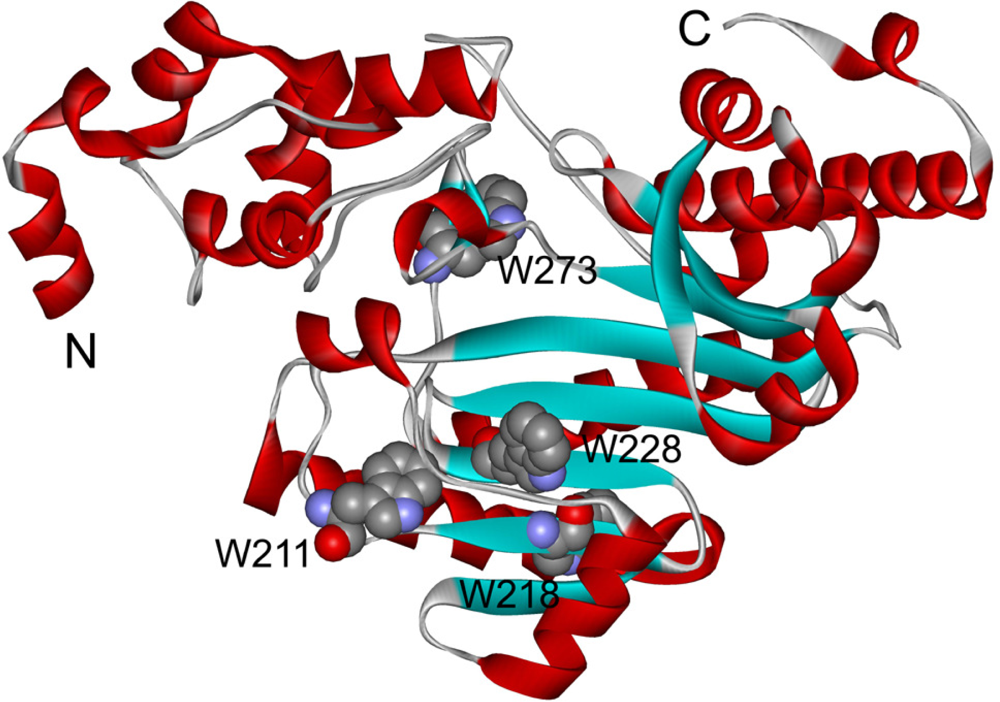
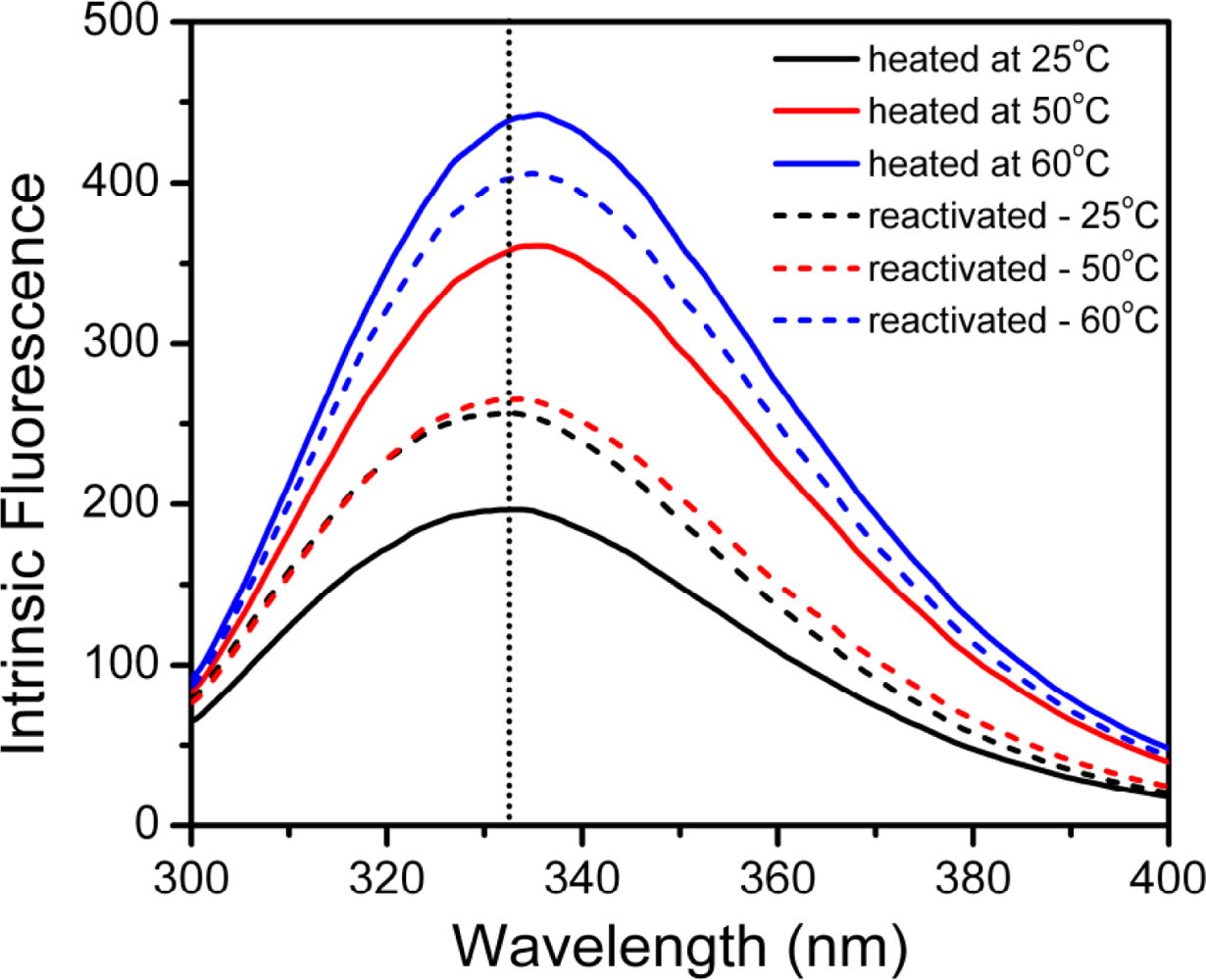
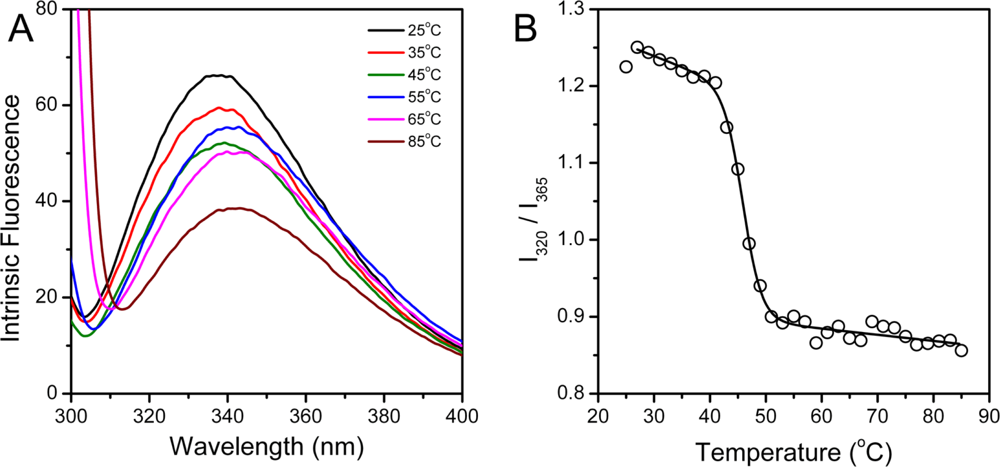
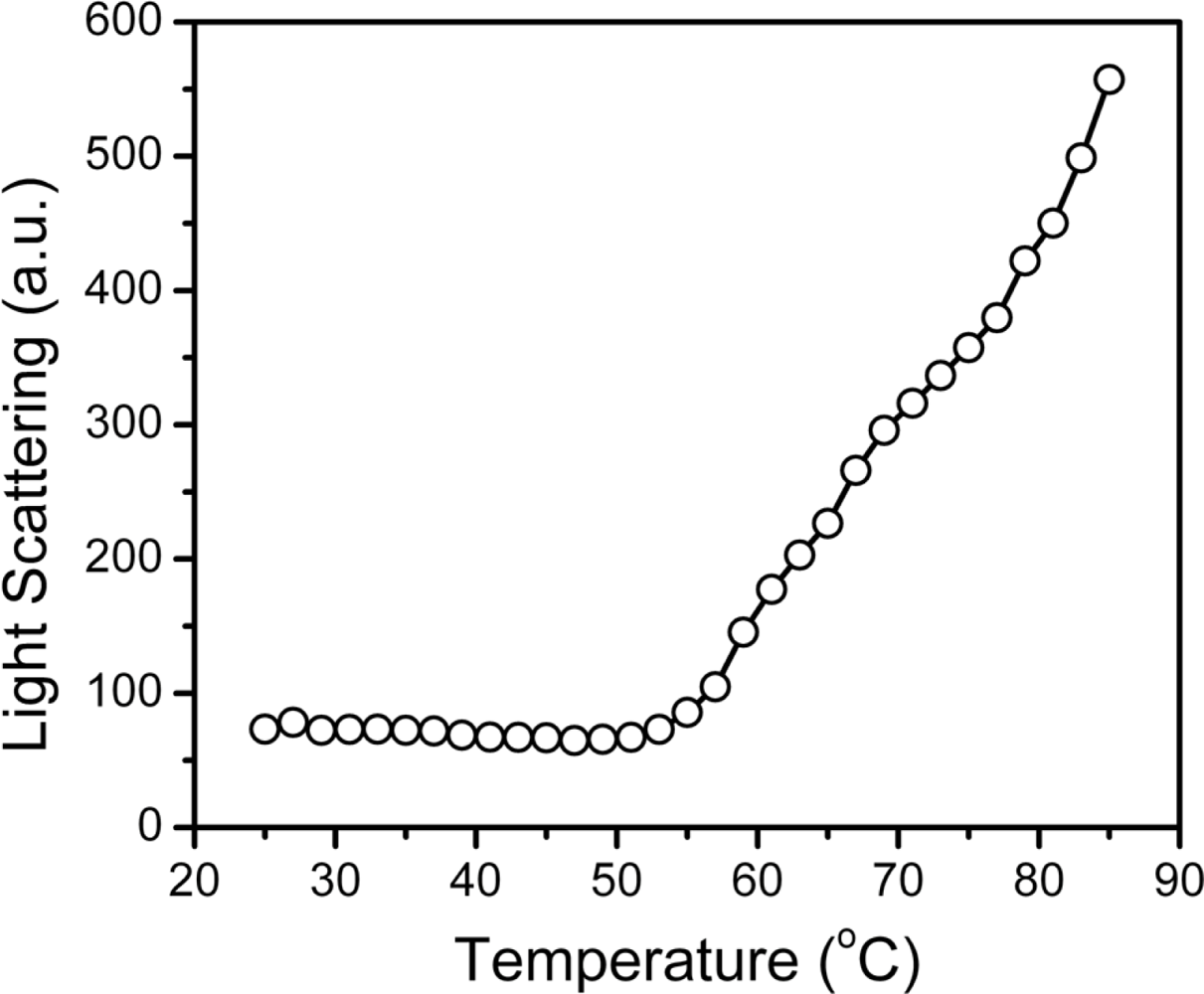
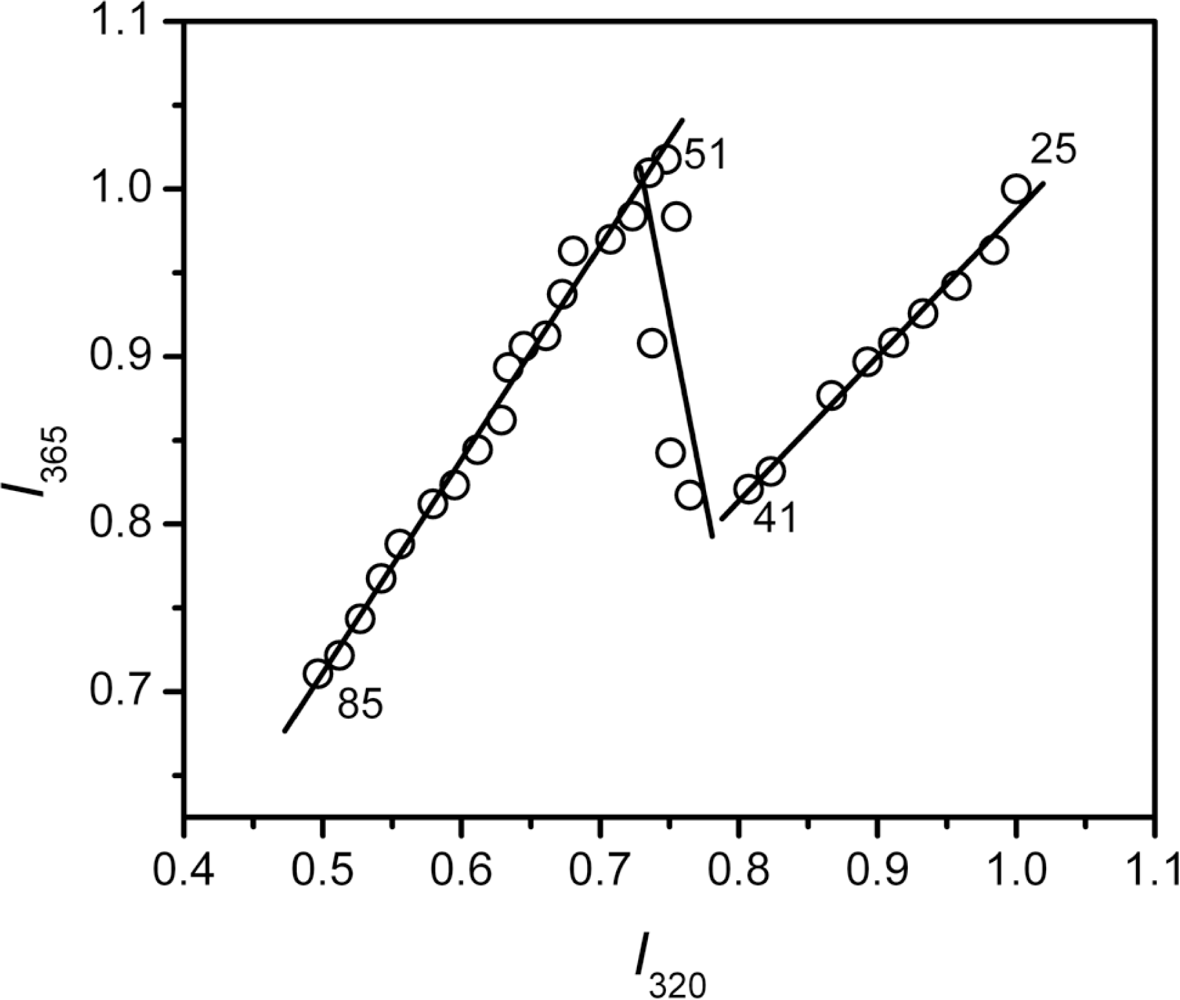
2.3. hBBCK Thermal Denaturation Monitored by Intrinsic Fluorescence Spectroscopy
3. Experimental Section
3.1. Materials
3.2. Protein Expression and Purification
3.3. Activity Assay
3.4. DSC Experiments
3.5. Spectroscopy
4. Conclusions
Acknowledgments
References
- Jaenicke, R. Stability and folding of domain proteins. Prog. Biophys. Mol. Biol 1999, 71, 155–241. [Google Scholar]
- Jaenicke, R; Lilie, H; Matthews, CR. Folding and association of oligomeric and multimeric proteins. Adv. Protein Chem 2000, 53, 329–362. [Google Scholar]
- Burgess, AW; Scheraga, HA. A hypothesis for the pathway of the thermally-induced unfolding of bovine pancreatic ribonuclease. J. Theor. Biol 1975, 53, 403–420. [Google Scholar]
- Matheson, RR, Jr; Scheraga, HA. Steps in the pathway of the thermal unfolding of ribonuclease A. A nonspecific photochemical surface-labeling study. Biochemistry 1979, 18, 2437–2445. [Google Scholar]
- Matheson, RR, Jr; Scheraga, HA. Steady-state kinetic study of action of ribonuclease A, involving a conformational change between 30 and 40 degrees C. Biochemistry 1979, 18, 2446–2450. [Google Scholar]
- Navon, A; Ittah, V; Laity, JH; Scheraga, HA; Haas, E; Gussakovsky, EE. Local and long-range interactions in the thermal unfolding transition of bovine pancreatic ribonuclease A. Biochemistry 2001, 40, 93–104. [Google Scholar]
- Stelea, SD; Keiderling, TA. Pretransitional structural changes in the thermal denaturation of ribonuclease S and S protein. Biophys. J 2002, 83, 2259–2269. [Google Scholar]
- Stelea, SD; Pancoska, P; Benight, AS; Keiderling, TA. Thermal unfolding of ribonuclease A in phosphate at neutral pH: Deviations from the two-state model. Protein Sci 2001, 10, 970–978. [Google Scholar]
- Vermeer, AW; Norde, W. The thermal stability of immunoglobulin: Unfolding and aggregation of a multi-domain protein. Biophys. J 2000, 78, 394–404. [Google Scholar]
- Paquet, MJ; Laviolette, M; Pezolet, M; Auger, M. Two-dimensional infrared correlation spectroscopy study of the aggregation of cytochrome c in the presence of dimyristoylphosphatidylglycerol. Biophys. J 2001, 81, 305–312. [Google Scholar]
- Dong, A; Randolph, TW; Carpenter, JF. Entrapping intermediates of thermal aggregation in ahelical proteins with low concentration of guanidine hydrochloride. J. Biol. Chem 2000, 275, 27689–27693. [Google Scholar]
- Fabian, H; Mantsch, HH; Schultz, CP. Two-dimensional IR correlation spectroscopy: Sequential events in the unfolding process of the l cro-V55C repressor protein. Proc. Natl. Acad. Sci. USA 1999, 96, 13153–13158. [Google Scholar]
- Yan, Y-B; Wang, Q; He, H-W; Hu, X-Y; Zhang, R-Q; Zhou, H-M. Two-dimensional infrared correlation spectroscopy study of sequential events in the heat-induced unfolding and aggregation process of myoglobin. Biophys. J 2003, 85, 1959–1967. [Google Scholar]
- He, H-W; Zhang, J; Zhou, H-M; Yan, Y-B. Conformational change in the C-terminal domain is responsible for the initiation of creatine kinase thermal aggregation. Biophys. J 2005, 89, 2650–2658. [Google Scholar]
- Zhang, J; Yan, Y-B. Probing conformational changes of proteins by quantitative second-derivative infrared spectroscopy. Anal. Biochem 2005, 340, 89–98. [Google Scholar]
- Yan, Y-B; Zhang, J; He, H-W; Zhou, H-M. Oligomerization and aggregation of bovine pancreatic ribonuclease A: Characteristic events observed by FTIR spectroscopy. Biophys. J 2006, 90, 2525–2533. [Google Scholar]
- Zhang, J; He, H-W; Wang, Q; Yan, Y-B. Sequential events in ribonuclease A thermal unfolding characterized by two-dimensional infrared correlation spectroscopy. Protein Pep. Lett 2006, 13, 33–40. [Google Scholar]
- Su, J-T; Kim, S-H; Yan, Y-B. Dissecting the pretransitional conformational changes in aminoacylase I thermal denaturation. Biophys. J 2007, 92, 578–587. [Google Scholar]
- Stadler, AM; Digel, I; Artmann, GM; Embs, JP; Zaccai, G; Buldt, G. Hemoglobin dynamics in red blood cells: Correlation to body temperature. Biophys. J 2008, 95, 5449–5461. [Google Scholar]
- Yan, Y-B; Wang, Q; He, H-W; Zhou, H-M. Protein thermal aggregation involves distinct regions: Sequential events in the heat-induced unfolding and aggregation of hemoglobin. Biophys. J 2004, 86, 1682–1690. [Google Scholar]
- Digel, I; Maggakis-Kelemen, C; Zerlin, KF; Linder, P; Kasischke, N; Kayser, P; Porst, D; Artmann, AT; Artmann, GM. Body temperature-related structural transitions of monotremal and human hemoglobin. Biophys. J 2006, 91, 3014–3021. [Google Scholar]
- Zerlin, KFT; Kasischke, N; Digel, I; Maggakis-Kelemen, C; Artmann, AT; Porst, D; Kayser, P; Linder, P; Artmann, GM. Structural transition temperature of hemoglobins correlates with species’ body temperature. Eur. Biophys. J 2007, 37, 1–10. [Google Scholar]
- Wallimann, T; Wyss, M; Brdiczka, D; Nicolay, K; Eppenberger, HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J 1992, 281, 21–40. [Google Scholar]
- Wallimann, T. Bioenergetics: Dissecting the role of creatine kinase. Curr. Biol 1994, 4, 42–46. [Google Scholar]
- Wallimann, T; Hemmer, W. Creatine-kinase in nonmuscle tissues and cells. Mol. Cell. Biochem 1994, 133, 193–220. [Google Scholar]
- McLeish, MJ; Kenyon, GL. Relating structure to mechanism in creatine kinase. Crit. Rev. Biochem. Mol. Biol 2005, 40, 1–20. [Google Scholar]
- Bong, SM; Moon, JH; Nam, KH; Lee, KS; Chi, YM; Hwang, KY. Structural studies of human brain-type creatine kinase complexed with the ADP-Mg2+-NO3-creatine transition-state analogue complex. FEBS Lett 2008, 582, 3959–3965. [Google Scholar]
- Kuby, SA; Noda, L; Lardy, HA. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J. Biol. Chem 1954, 209, 191–201. [Google Scholar]
- Yao, QZ; Zhou, HM; Hou, LX; Zou, CG. A comparison of denaturation and inactivation rates of creatine kinase in guanidine solutions. Sci. Sin. B 1982, 25, 1296–1802. [Google Scholar]
- Morris, GE; Frost, LC; Newport, PA; Hudson, N. Monoclonal antibody studies of creatine kinase: Antibody-binding sites in the N-terminal region of creatine kinase and effects of antibody onenzyme refolding. Biochem. J 1987, 248, 53–59. [Google Scholar]
- Webb, T; Jackson, PJ; Morris, GE. Protease digestion studies of an equilibrium intermediate in the unfolding of creatine kinase. Biochem. J 1997, 321, 83–88. [Google Scholar]
- Fan, YX; Zhou, JM; Kihara, H; Tsou, CL. Unfolding and refolding of dimeric creatine kinase equilibrium and kinetic studies. Protein Sci 1998, 7, 2631–2641. [Google Scholar]
- Leydier, C; Clottes, E; Couthon, F; Marcillat, O; Ebel, C; Vial, C. Evidence for kinetic intermediate states during the refolding of GdnHCl-denatured MM-creatine kinase. Characterization of a trapped monomeric species. Biochemistry 1998, 37, 17579–17589. [Google Scholar]
- Li, S; Bai, JH; Park, YD; Zhou, HM. Aggregation of creatine kinase during refolding and chaperonin-mediated folding of creatine kinase. Int. J. Biochem.: Cell Biol 2001, 33, 279–286. [Google Scholar]
- Park, YD; Ou, WB; Yu, TW; Zhou, HM. Folding pathway for partially folded rabbit muscle creatine kinase. Biochem. Cell Biol 2001, 79, 479–487. [Google Scholar]
- Mazon, H; Marcillat, O; Vial, C; Clottes, E. Role of C-terminal sequences in the folding of muscle creatine kinase. Biochemistry 2002, 41, 9646–9653. [Google Scholar]
- Mazon, H; Marcillat, O; Forest, E; Smith, DL; Vial, C. Conformational dynamics of the GdmHCl-induced molten globule state of creatine kinase monitored by hydrogen exchange and mass spectrometry. Biochemistry 2004, 43, 5045–5054. [Google Scholar]
- Zhao, TJ; Ou, WB; Xie, Q; Liu, Y; Yan, YB; Zhou, HM. Catalysis of creatine kinase refolding by protein disulfide isomerase involves disulfide cross-link and dimer to tetramer switch. J. Biol. Chem 2005, 280, 13470–13476. [Google Scholar]
- Li, S; Bai, JH; Park, YD; Zhou, HM. Capture of monomeric refolding intermediate of human muscle creatine kinase. Protein Sci 2006, 15, 171–181. [Google Scholar]
- Ou, WB; Luo, W; Park, YD; Zhou, HM. Chaperone-like activity of peptidyl-prolyl cis-trans isomerase during creatine kinase refolding. Protein Sci 2001, 10, 2346–2353. [Google Scholar]
- Lyubarev, AE; Kurganov, BI; Orlov, VN; Zhou, HM. Two-state irreversible thermal denaturation of muscle creatine kinase. Biophys. Chem 1999, 79, 199–204. [Google Scholar]
- Meng, FG; Hong, YK; He, HW; Lyubarev, AE; Kurganov, BI; Yan, YB; Zhou, HM. Osmophobic effect of glycerol on irreversible thermal denaturation of rabbit creatine kinase. Biophys. J 2004, 87, 2247–2254. [Google Scholar]
- Gao, Y-S; Zhao, T-J; Chen, Z; Li, C; Wang, Y; Yan, Y-B; Zhou, H-M. Isoenzyme-specific thermostability of human cytosolic creatine kinase. Int. J. Biol. Macromol 2010, 47, 27–32. [Google Scholar]
- Wallimann, T; Moser, H; Eppenberger, HM. Isozyme-specific localization of M-line bound creatine kinase in myogenic cells. J. Muscle Res. Cell Motil 1983, 4, 429–441. [Google Scholar]
- Hornemann, T; Stolz, M; Wallimann, T. Isoenzyme-specific interaction of muscle-type creatine kinase with the sarcomeric M-line is mediated by NH2-terminal lysine charge-clamps. J. Cell Biol 2000, 149, 1225–1234. [Google Scholar]
- Pelton, JT; McLean, LR. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem 2000, 277, 167–176. [Google Scholar]
- Turoverov, KK; Haitlina, SY; Pinaev, GP. Ultra-violet fluorescence of actin. Determination of native actin content in actin preparations. FEBS Lett 1976, 62, 4–6. [Google Scholar]
- Bushmarina, NA; Kuznetsova, IM; Biktashev, AG; Turoverov, KK; Uversky, VN. Partially folded conformations in the folding pathway of bovine carbonic anhydrase II: A fluorescence spectroscopic analysis. ChemBioChem 2001, 2, 813–821. [Google Scholar]
- Feng, S; Xu, Z; Yan, Y-B. Blocking creatine kinase refolding by trace amounts of copper ions. J. Inorg. Biochem 2008, 102, 928–935. [Google Scholar]
- He, H-W; Feng, S; Pang, M; Zhou, H-M; Yan, Y-B. Role of the linker between the N- and Cterminal domains in the stability and folding of rabbit muscle creatine kinase. Int. J. Biochem. Cell Biol 2007, 39, 1816–1827. [Google Scholar]
- Zhao, T-J; Feng, S; Wang, Y-L; Liu, Y; Luo, X-C; Zhou, H-M; Yan, Y-B. Impact of intrasubunit domain-domain interactions on creatine kinase activity and stability. FEBS Lett 2006, 580, 3835–3840. [Google Scholar]
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- He, G-J; Zhang, A; Liu, W-F; Cheng, Y; Yan, Y-B. Conformational stability and multistate unfolding of poly(A)-specific ribonuclease. FEBS J 2009, 276, 2849–2860. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gao, Y.-S.; Su, J.-T.; Yan, Y.-B. Sequential Events in the Irreversible Thermal Denaturation of Human Brain-Type Creatine Kinase by Spectroscopic Methods. Int. J. Mol. Sci. 2010, 11, 2584-2596. https://doi.org/10.3390/ijms11072584
Gao Y-S, Su J-T, Yan Y-B. Sequential Events in the Irreversible Thermal Denaturation of Human Brain-Type Creatine Kinase by Spectroscopic Methods. International Journal of Molecular Sciences. 2010; 11(7):2584-2596. https://doi.org/10.3390/ijms11072584
Chicago/Turabian StyleGao, Yan-Song, Jing-Tan Su, and Yong-Bin Yan. 2010. "Sequential Events in the Irreversible Thermal Denaturation of Human Brain-Type Creatine Kinase by Spectroscopic Methods" International Journal of Molecular Sciences 11, no. 7: 2584-2596. https://doi.org/10.3390/ijms11072584
APA StyleGao, Y.-S., Su, J.-T., & Yan, Y.-B. (2010). Sequential Events in the Irreversible Thermal Denaturation of Human Brain-Type Creatine Kinase by Spectroscopic Methods. International Journal of Molecular Sciences, 11(7), 2584-2596. https://doi.org/10.3390/ijms11072584