The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production
Abstract
:1. Introduction
2. Results and Discussion
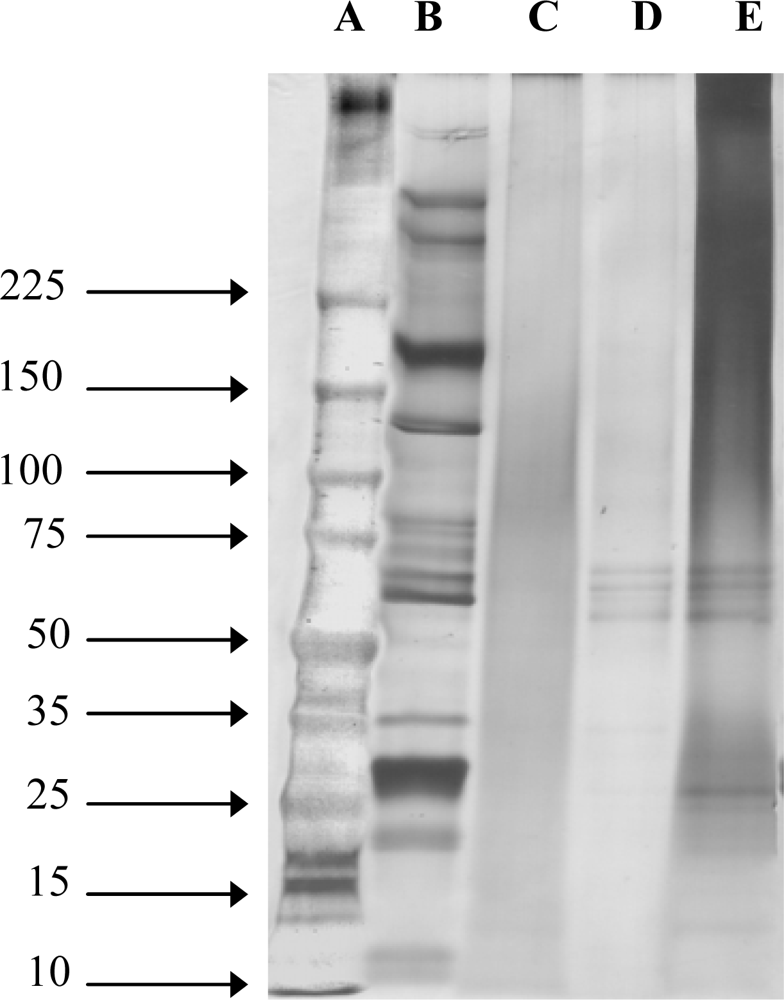
2.1. Molecular Weight of SS
2.2. Particle Size and Zeta Potential Measurement
2.3. Amino Acid Analysis
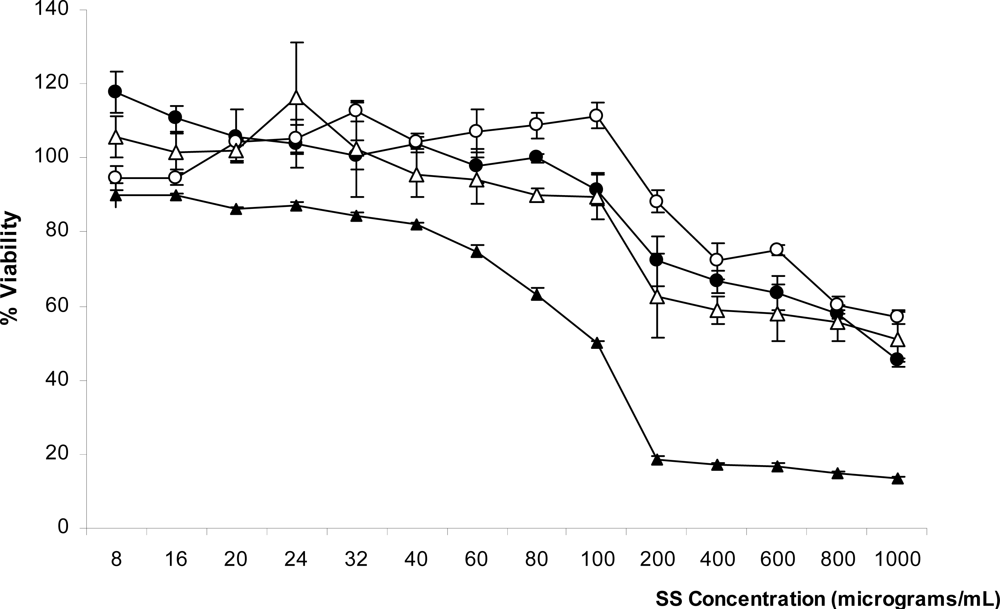
2.4. Cytotoxicity of SS Solution
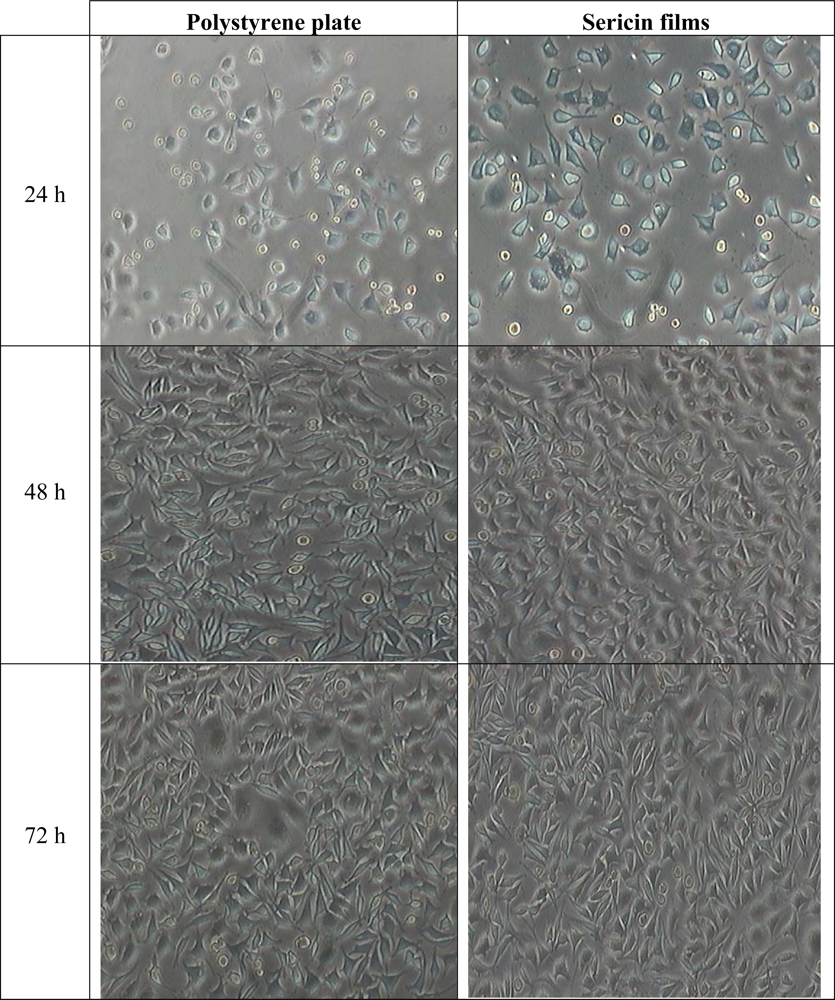
2.5. Adherence of L929 Mouse Fibroblast Cell Line to SS Films
2.6. Determination of Soluble Collagen Production Induced by SS
3. Experimental Section
3.1. Materials
3.1.1. Silkworm Cocoons
3.1.2. Fibroblast Cell Culture
3.1.3. Preparation of SS Powder Using a High Temperature and High Pressure Degumming Technique (Heat-Degraded SS Powder)
3.1.4. Preparation of SS by Citric Acid and Sodium Carbonate Solution (Acid-Degraded and Alkali-Degraded SS Powders)
3.1.5. Preparation of SS by Urea Solution
3.2. Methods
3.2.1. Molecular Weight Determination
3.2.2. Particle Size and Zeta Potential Measurement
3.2.3. Amino Acid Analysis
3.2.4. Cytotoxicity of SS Solution
3.2.5. Adherence of L929 Mouse Fibroblast Cell Line on SS Films
3.2.6. Determination of Soluble Collagen Production Induced by SS
4. Conclusions
Acknowledgments
References and Notes
- Aramwit, P; Kanokpanont, S; De-Eknamkul, W; Kamei, K; Srichana, T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J. Biomater. Sci. Polym. Ed 2009, 20, 1295–1306. [Google Scholar]
- Aramwit, P; Sangcakul, A. The effects of sericin cream on wound healing in rats. Biosci Biotechnol. Biochem 2007, 71, 2473–2477. [Google Scholar]
- Terada, S; Nishimura, T; Sasaki, M; Yamada, H; Miki, M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology 2002, 40, 3–12. [Google Scholar]
- Tsubouchi, K; Igarashi, Y; Takasu, Y; Yamada, H. Sericin enhances attachment of cultured human skin fibroblasts. Biosci. Biotechnol. Biochem 2005, 69, 403–405. [Google Scholar]
- Terada, S; Sasaki, M; Yanagihara, K; Yamada, H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J. Biosci. Bioeng 2005, 100, 667–671. [Google Scholar]
- Takahashi, M; Tsujimoto, K; Yamada, H; Takagi, H; Nakamori, S. The silk protein, sericin, protects against cell death caused by acute serum deprivation in insect cell culture. Biotechnol. Lett 2003, 25, 1805–1809. [Google Scholar]
- Sasaki, M; Kato, Y; Yamada, H; Terada, S. Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol. Appl. Biochem 2005, 42, 183–188. [Google Scholar]
- Kurioka, A; Kurioka, F; Yamazaki, M. Characterization of sericin powder prepared from citric acid-degraded sericin polypeptides of the silkworm, Bombyx Mori. Biosci. Biotechnol. Biochem 2004, 68, 774–780. [Google Scholar]
- Ogawa, A; Terada, S; Kanayama, T; Miki, M; Morikawa, M; Kimura, T; Yamaguchi, A; Sasaki, M; Yamada, H. Improvement of islet culture with sericin. J. Biosci. Bioeng 2004, 98, 217–219. [Google Scholar]
- Li, YG; Ji, DF; Lin, TB; Zhong, S; Hu, GY; Chen, S. Protective effect of sericin peptide against alcohol-induced gastric injury in mice. Chin. Med. J. (Engl.) 2008, 121, 2083–2087. [Google Scholar]
- Mason, R. Fabrics for atopic dermatitis. J. Fam. Health Care 2008, 18, 63–65. [Google Scholar]
- Koller, DY; Halmerbauer, G; Bock, A; Engstler, G. Action of a silk fabric treated with AEGIS in children with atopic dermatitis: A 3-month trial. Pediatr. Allergy Immunol 2007, 18, 335–338. [Google Scholar]
- Sprague, KU. The Bombyx mori silk proteins: Characterization of large polypeptides. Biochemistry 1975, 14, 925–931. [Google Scholar]
- Zurovec, M; Yang, C; Kodrik, D; Sehnal, F. Identification of a novel type of silk protein and regulation of its expression. J. Biol. Chem 1998, 273, 15423–15428. [Google Scholar]
- Nirmala, X; Kodrik, D; Zurovec, M; Sehnal, F. Insect silk contains both a Kunitz-type and a unique Kazal-type proteinase inhibitor. Eur. J. Biochem 2001, 268, 2064–2073. [Google Scholar]
- Zeta Potential of Colloids in Water and Waste Water; ASTM Standard D 4187-82; American Society for Testing and Materials: West Conshohocken, PA, USA, 1985.
- Franz, MG; Kuhn, MA; Nguyen, K; Wang, X; Ko, F; Wright, TE; Robson, MC. Transforming growth factor beta(2) lowers the incidence of incisional hernias. J. Surg. Res 2001, 97, 109–116. [Google Scholar]
- Henry, G; Garner, WL. Inflammatory mediators in wound healing. Surg. Clin. North. Am 2003, 83, 483–507. [Google Scholar]
- Takasu, Y; Yamada, H; Tsubouchi, K. Isolation of three main sericin components from the cocoon of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem 2002, 66, 2715–2718. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]

| Extraction method | Zeta potential (mV) | Mean size (nm) |
|---|---|---|
| Heat | −20.69 ± 2.14 | 110.42 ± 35.07 |
| Acid | −32.12 ± 5.26 | 23.80 ± 16.07 |
| Alkaline | −15.87 ± 2.89 | 824.42 ± 86.67 |
| Urea | −68.36 ± 5.67 | 4.62 ± 2.44 |
| Amino acid | Extraction method of SS | |||
|---|---|---|---|---|
| Heat | Urea | Acid | Alkaline | |
| Asp | 15.64 | 18.31 | 15.93 | 19.88 |
| Ser | 33.63 | 31.27 | 31.86 | 30.01 |
| Glu | 4.61 | 5.27 | 5.75 | 5.93 |
| Gly | 15.03 | 11.23 | 10.49 | 11.01 |
| His | 1.06 | 3.26 | 2.47 | 1.72 |
| Arg | 2.87 | 5.41 | 4.92 | 4.92 |
| Thr | 8.16 | 8.36 | 8.51 | 6.49 |
| Ala | 4.10 | 4.33 | 3.72 | 4.21 |
| Pro | 0.54 | 1.46 | 0.78 | 1.24 |
| Cys | 0.54 | 0.39 | 0.53 | 0.23 |
| Tyr | 3.45 | 0.36 | 5.56 | 5.24 |
| Val | 2.88 | 2.96 | 2.95 | 2.94 |
| Met | 3.39 | 0.12 | 0.06 | 0.15 |
| Lys | 2.35 | 3.14 | 3.48 | 2.89 |
| Ile | 0.56 | 0.96 | 0.87 | 0.75 |
| Leu | 1.00 | 1.58 | 1.43 | 1.56 |
| Phe | 0.28 | 0.60 | 0.71 | 0.81 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200-2211. https://doi.org/10.3390/ijms11052200
Aramwit P, Kanokpanont S, Nakpheng T, Srichana T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. International Journal of Molecular Sciences. 2010; 11(5):2200-2211. https://doi.org/10.3390/ijms11052200
Chicago/Turabian StyleAramwit, Pornanong, Sorada Kanokpanont, Titpawan Nakpheng, and Teerapol Srichana. 2010. "The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production" International Journal of Molecular Sciences 11, no. 5: 2200-2211. https://doi.org/10.3390/ijms11052200




