Synthesis of Hydrophilic and Amphiphilic Acryl Sucrose Monomers and Their Copolymerisation with Styrene, Methylmethacrylate and α- and β-Pinenes
Abstract
:1. Introduction
2. Results and Discussion
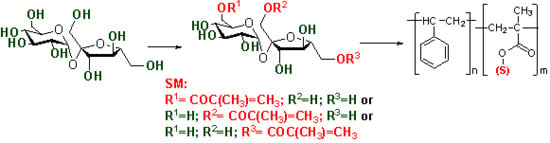
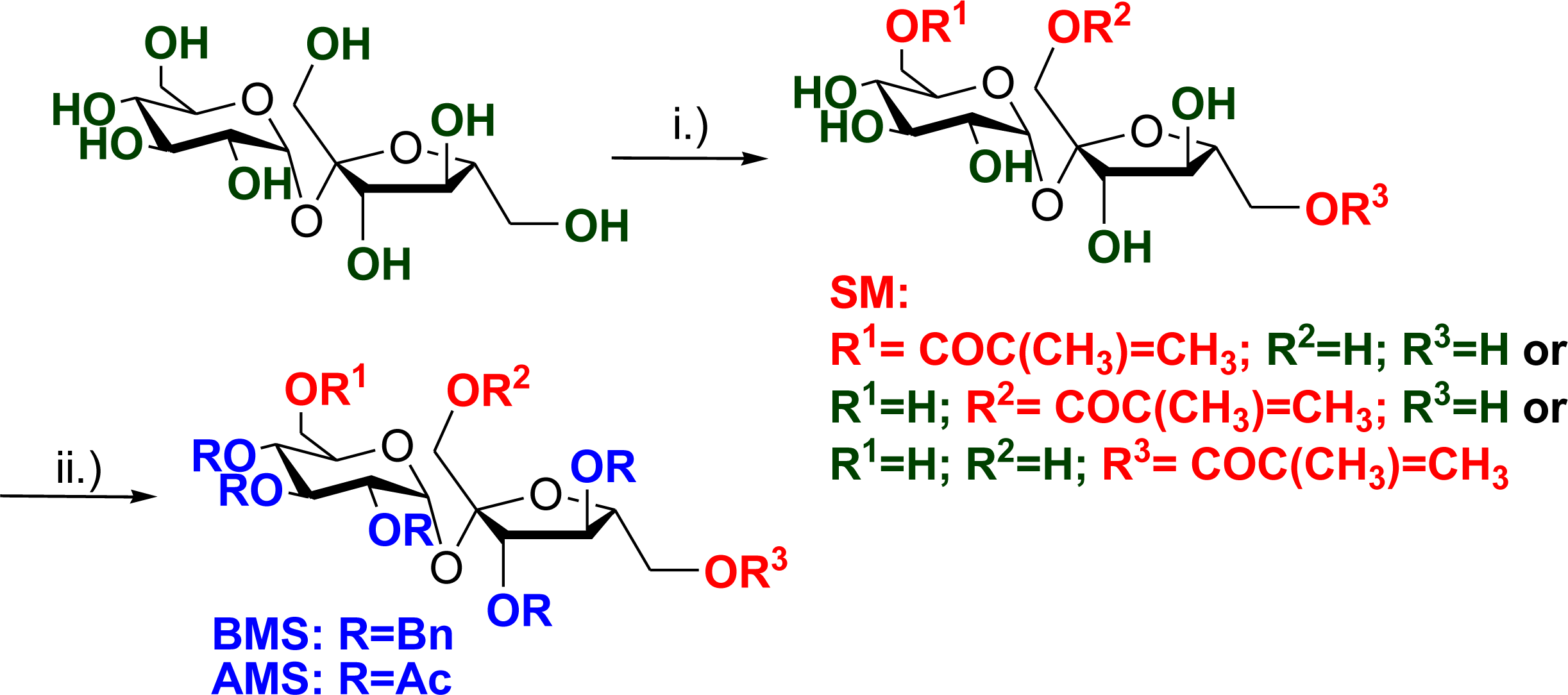
2.1. Synthesis of Unsaturated Sucrose Esters
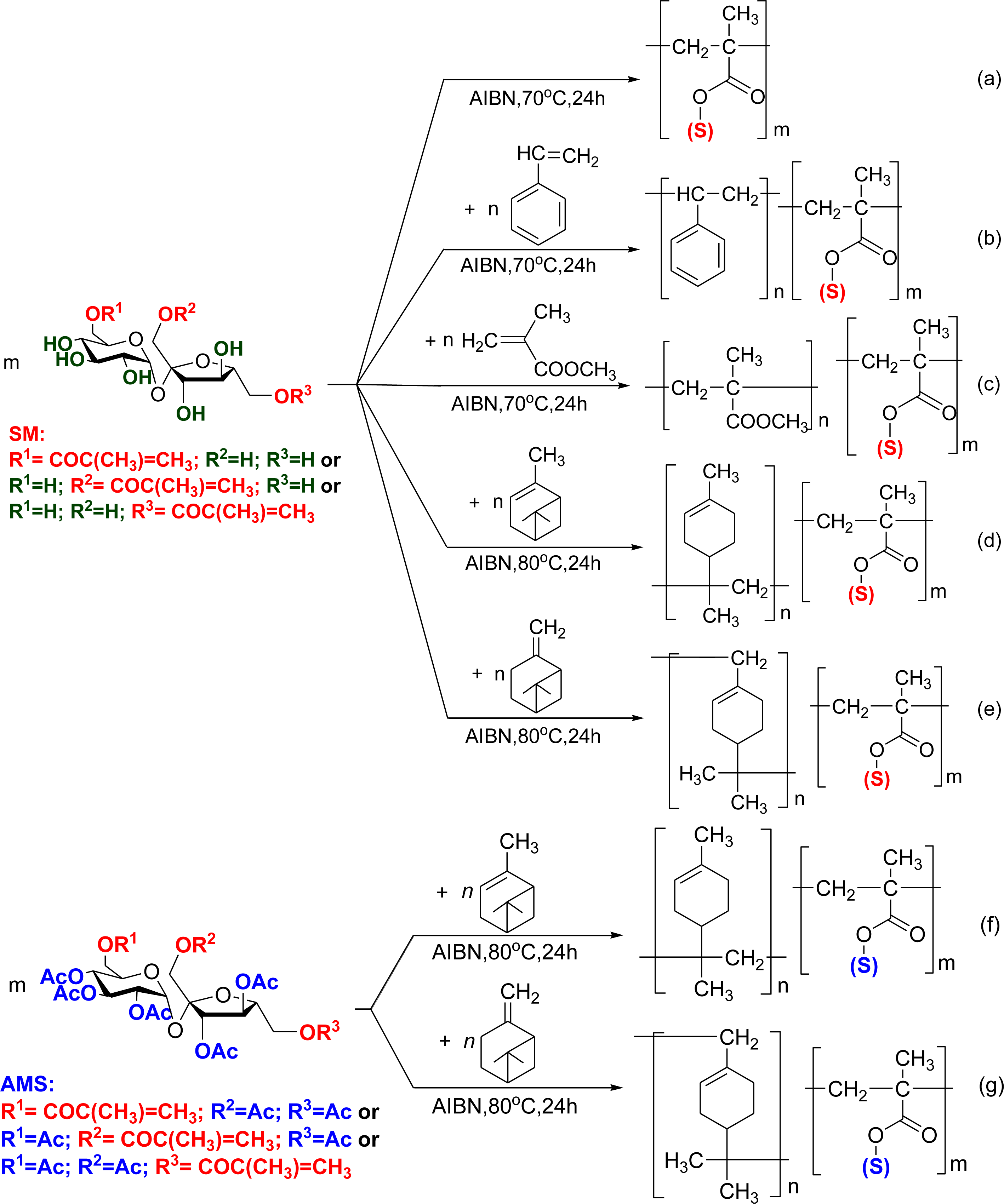
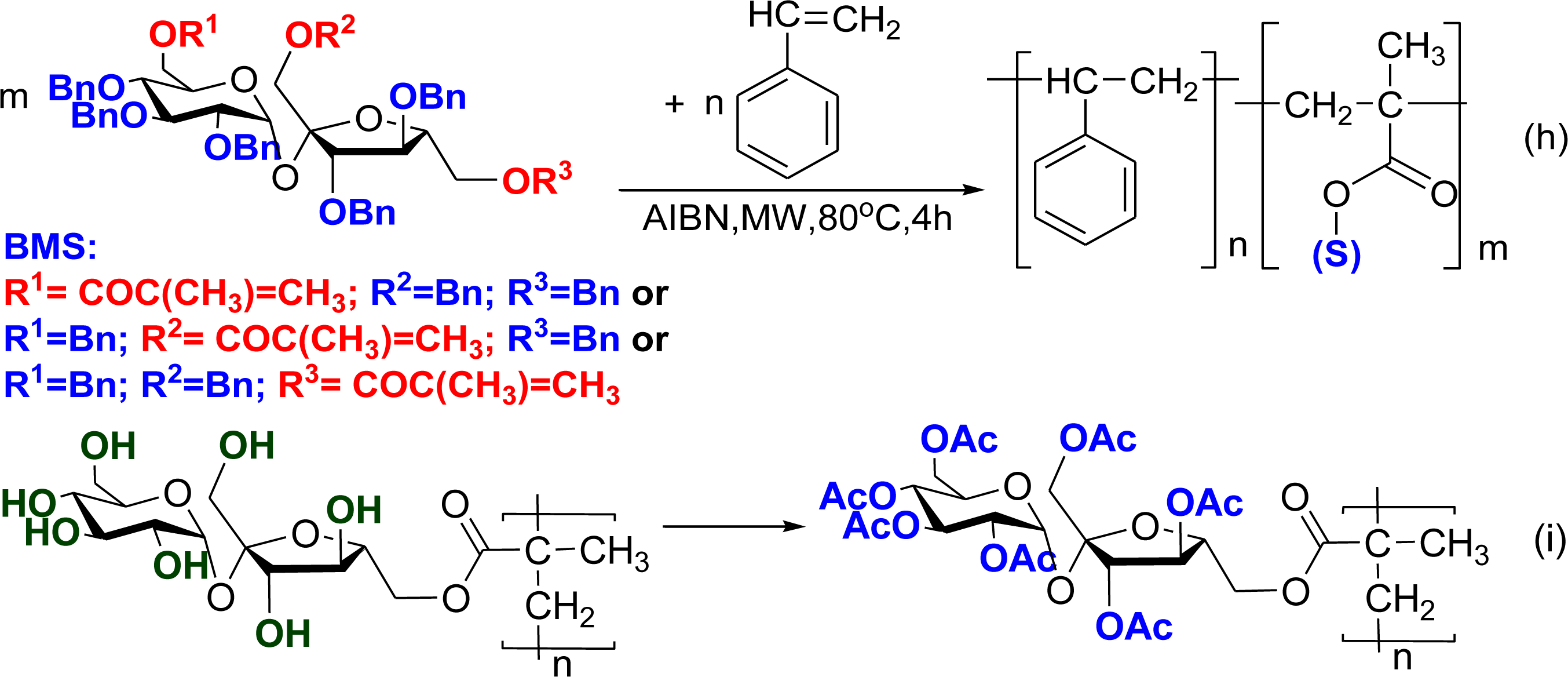
2.2. Synthesis of Amphiphilic Copolymers − Copolymerisation of Vinyl Sugar Monomers with Styrene, Methylmethacrylate, α- and β-Pinene by Free Radical Polymerisation
2.3. Biodegradation Studies: Incubation in Culture
3. Experimental Section
3.1. General Methods
3.2. Synthesis of Monomers
3.3. General Procedure for Copolymerisation under Mild Conditions
3.4. General Procedure for Copolymerisation in an Autoclave
3.5. General Procedure for Copolymerisation in Aqueous Media
3.6. General Procedure for Polymerisation and Copolymerisation in Focused Microwave Oven
3.7. General Procedure for Cationic Homopolymerisation of α- and β-Pinene
3.8. General Procedure for Radical Copolymerisation in Solution
3.9. General Procedure for Polymer-Analogous Reactions
3.10. Culture Incubation
4. Conclusions
Electronic Supplementary Information (ESI) Available:
Proton and carbon NMR spectra of reported compounds; and results from: Size Exclusion Chromatography (SEC); Differential Scanning Calorimetry (DSC); Atomic Force Microscopy (AFM); X-ray photoelectron spectroscopy (XPS); Time of Flight – Secondary Ion Mass Spectroscopy (TOF-SIMS); Biodegradation studies - incubation in culture.Acknowledgments
References and Notes
- Haworth, WN; Gregory, H; Wiggins, KF. Some derivatives of simple carbohydrates containing unsaturated substituents. J Chem Soc 1946, 488–491. [Google Scholar]
- Goddard, ED; Ananthapadmanabhan, KP. Interactions of Surfactants with Polymers and Proteins; CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Akiyoshi, K; Deguchi, S; Moriguchi, N; Yamaguchi, S; Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromolecules 1993, 26, 3062–3068. [Google Scholar]
- Park, K; Waleed, S; Shalaby, W. Biodegradable Hydrogels for Drug Delivery; CRC Press: New York, NY, USA, 1993; pp. 50–51. [Google Scholar]
- Pachence, JM; Kohn, J. Principles of Tissue Engineering; Academic Press: New York, NY, USA, 2000. [Google Scholar]
- Satomi, M; Sogawa, S. Hard coating layer for coated products useful in pharmaceuticals and foodstuffs, contains sugar, and reduced amylolysis product containing sugar composition of hydrogenated saccharides with varying polymerization degree. Japan Patent 2007-053-952-A 2007. [Google Scholar]
- Popplewell, LM; Henson, L. Method useful to deliver active ingredient to cleaning composition involves preparing structured material of hydrophilic particle, hydrophobic active ingredient and polyhydric material; and providing the structured material into composition. US Patent 2007-048-339-A1 2007. [Google Scholar]
- Singh, RP; Pandey, JK; Rutot, D; Degee, P; Dubois, P. Biodegradation of poly(E-caprolactone)/starch blends and composites in composting and culture environments; the effect of compatibilization on the inherent biodegradability of the host polymer. Carbohydr. Res 2003, 338, 1759–1769. [Google Scholar]
- Galgali, P; Varma, AJ; Puntambekar, US; Gokhale, DV. Towards biodegradable polyolefines: Strategy of anchoring minute quantities of monosaccharides and disaccharides onto functionalized polystyrene, and their effect on facilitating polymer biodegradation. Chem. Commun 2002, 23, 2884–2885. [Google Scholar]
- Tokiwa, Y; Fan, H; Hiraguri, Y; Kurane, R. Biodegradation of a sugar branched polymer consisting of sugar, fatty acid, and poly(vinyl alcohol). Macromolecules 2000, 33, 1636–1639. [Google Scholar]
- Varma, AJ; Kennedy, JF; Galgali, P. Synthetic polymers functionalized by carbohydrates: A review. Carbohydr. Polym 2004, 56, 429–445. [Google Scholar]
- Kobayashi, K; Sumitomo, H; Ina, Y. Synthesis and Functions of Polystyrene Derivatives Having Pendant Oligosaccharides. Polym. J 1985, 17, 567–575. [Google Scholar]
- Khan, R. Chemistry and new uses of sucrose: How important? Pure Appl. Chem 1984, 56, 833–844. [Google Scholar]
- Zoete, MS; Kneepkens, MF; Waard, P; Osterom, MW; Gotlieb, KF; Slagnek, TM. Enzymatic synthesis and NMR studies of acylated sucrose acetates. Green Chem 1999, 1, 153–156. [Google Scholar]
- Breuil, VD; Pinel, C; Gallezot, P. Green approach to substituted carbohydrates: Telomerisation of butadiene with sucrose. Green Chem 2001, 3, 175–177. [Google Scholar]
- Barros, MT; Petrova, K; Ramos, AM. Regioselective copolymerization of acryl sucrose monomers. J. Org. Chem 2004, 69, 7772–7775. [Google Scholar]
- Barros, MT; Petrova, KT; Ramos, AM. Biodegradable polymers based on α- or β-pinene and sugar derivatives or styrene, obtained under normal conditions and microwave irradiation. Eur. J. Org. Chem 2007, 8, 1357–1363. [Google Scholar]
- Crucho, CC; Petrova, KT; Pinto, RC; Barros, MT. Novel unsaturated sucrose ethers and their application as monomers. Molecules 2008, 13, 762–770. [Google Scholar]
- Barros, MT; Petrova, KT. Ziegler-Natta catalysed polymerisation for the preparation of potentially biodegradable copolymers with pendant sucrose moieties. Eur. Polym. J 2009, 45, 295–301. [Google Scholar]
- Russo, A; Maschio, G; Ampelli, C. Reaction inhibition as a method for preventing thermal runaway in industrial processes. Macromol. Symp 2007, 259, 365–370. [Google Scholar]
- Jhurry, D; Deffieux, A; Fontanille, M. Sucrose based polymers. Linear polymers with sucrose side-chains. Makromol. Chem 1992, 193, 2997–3007. [Google Scholar]
- Sheldon, RA. E factors, green chemistry and catalysis: An odyssey. Chem. Commun 2008, 29, 3352–3365. [Google Scholar]
- Simonsen, JL. The Terpenes, 2nd ed; Cambridge University Press: Cambridge, UK, 1949; Volume 2, pp. 105–191. [Google Scholar]
- Ruckel, ER; Arlt, HG. Polyterpene resins. In Naval Stores Production, Chemistry, Utilization; Zinkel, DF, Ed.; Pulp Chemicals Association: New York, NY, USA, 1989; ; Chapter 13. [Google Scholar]
- Ramos, AM; Lobo, SL; Bordado, JM. Polymers from pine gum components: Radical and coordination homo and copolymerization of pinenes. Macromol. Symp 1998, 127, 43–50. [Google Scholar]
- Grima, S; Bellon-Maurel, V; Feuilloley, P; Silvestre, F. Aerobic Biodegradation of Polymers in Solid-State Conditions: A Review of Environmental and Physicochemical Parameter Settings in Laboratory Simulations. J. Polym. Environ 2000, 8, 183–195. [Google Scholar]
- Perrin, DD; Armagedo, WLF; Perrin, DR. Purification of Laboratory Chemicals, 2nd ed; Pergamon Press Ltd: New York, NY, USA, 1980; pp. 74–465. [Google Scholar]
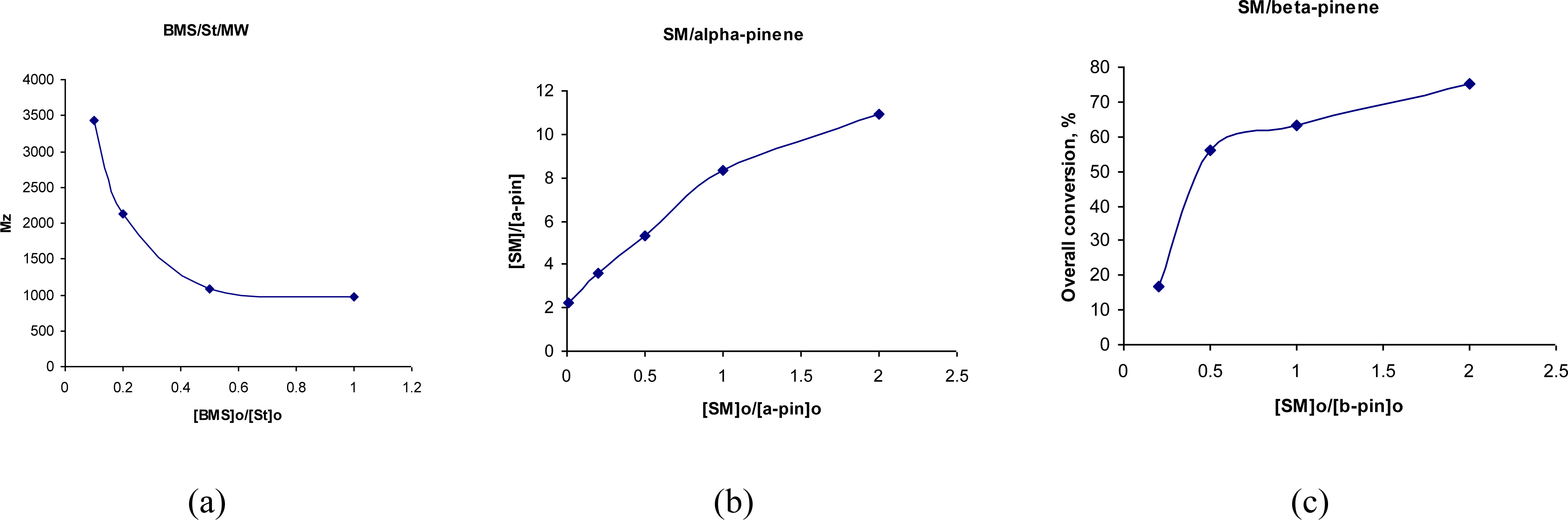

| № | Sugar | Alkene | a | b | Solvent, initiator | Reaction temp. | Reaction time [h] | Yieldc [%] | Mnd [g/mol] | MWd [g/mol] | Mn/MWd | φ e [Å] | Df [cm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SM | St | 1 | 0.06 | DMF, AIBN | 70 °C | 48 | 26.5 | 33970 | 40760 | 1.2 | 745 | 4.90 × 10−9 |
| 2 | SM | St | 1 | 0.34 | DMF, AIBN | 150°C | 48 | 25.0 | 7800 | 12470 | 1.6 | 401 | 9.09 × 10−9 |
| 3g | SM | MM | 1 | 0.96 | DMF, AIBN | 70 °C | 48 | 32.0 | 69180 | 131450 | 1.9 | 1370 | 2.66 × 10−9 |
| 4 | SM | MM | 1 | 0.35 | DMF, AIBN | 150 °C | 48 | 23.2 | 19200 | 29795 | 1.5 | 631 | 5.77 × 10−9 |
| 5 | SM | _ | 100% | 100% | DMF, AIBN | 70 °C | 48 | 59.4 | 246800 | 468920 | 1.9 | 2648 | 1.37 × 10−9 |
| 6 | SM | _ | 100% | 100% | DMF, AIBN | 150 °C | 48 | 44.7 | insoluble | ||||
| 7g | SM | _ | 100% | 100% | _ | r.t. | 24 | 100 | 29370 | 38180 | 1.3 | 718 | 5.07 × 10−9 |
| 8 | SM | _ | 100% | 100% | DMF | r.t. | 48 | 12.8 | 53400 | 85460 | 1.6 | 1092 | 3.33 × 10−9 |
| 9 | SM | _ | 100% | 100% | H2O | r.t. | 48 | 13.9 | insoluble | ||||
| 10 | SM | _ | 100% | 100% | pyridine | r.t. | 24 | 23.7 | 20400 | 28550 | 1.4 | 617 | 5.90 × 10−9 |
| 11 | SM | _ | 100% | 100% | H2O, AIBN | 60 °C | 48 | 27.8 | 48030 | 81650 | 1.7 | 1066 | 3.41 × 10−9 |
| 12 | SM | St | 0.3 | 0.07 | H2O+ acetone, AIBN | 60 °C | 48 | 71.4 | 17770 | 23095 | 1.3 | 553 | 6.69 × 10−9 |
| 13 | SM | MM | 0.3 | 0.12 | H2O+ acetone, AIBN | 60 °C | 48 | 59.0 | 12150 | 17430 | 1.4 | _ | _ |
| № | Sugar | Alkene | a | b | Solvent, initiator | Reaction temp. | Reaction time [h] | Yieldc [%] | Mnd [g/mol] | MWd [g/mol] | Mn/MWd | φ e [Å] | Df [cm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14a | _ | St | 0 | 0 | toluene, AIBN | MW, 80 °C | 4 | 87 | 5300 | 9370 | 1.8 | _ | _ |
| 14b | BMS | _ | 100% | 100% | toluene, AIBN | MW, 80 °C | 4 | 0.7 | 33400 | 56820 | 1.7 | 883 | 4.12 × 10−9 |
| 15 | BMS | St | 1 | 0.29 | toluene, AIBN | MW, 80 °C | 4 | 55.7 | 880 | 970 | 1.1 | 106 | 3.44 × 10−8 |
| 16 | BMS | St | 0.5 | 3.57 | toluene, AIBN | MW, 80 °C | 4 | 7.4 | 980 | 1080 | 1.1 | 112 | 3.25 × 10−8 |
| 17 | BMS | St | 0.2 | 0.31 | toluene, AIBN | MW, 80 °C | 4 | 34.9 | 1760 | 2120 | 1.2 | 159 | 2.29 × 10−8 |
| 18 | BMS | St | 0.1 | 0.11 | toluene, AIBN | MW, 80 °C | 4 | 62.8 | 3130 | 3440 | 1.1 | 205 | 1.78 × 10−8 |
| № | Sugar | Alkene | a | b | Solvent, initiator | Reaction temp. | Reaction time [h] | Yieldc [%] | Mnd [g/mol] | MWd [g/mol] | Mn/MWd | φ e [Å] | Df [cm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | SM | α-P | 2 | 10.9 | py, AIBN | 80 °C | 48 | 13.7 | 12250 | 19600 | 1.6 | _ | _ |
| 20 | SM | α-P | 1 | 8.31 | py, AIBN | 80 °C | 48 | 72.6 | 26600 | 45300 | 1.7 | _ | _ |
| 21 | SM | α-P | 0.5 | 5.29 | py, AIBN | 80 °C | 48 | 60.7 | 21330 | 33000 | 1.6 | _ | _ |
| 22 | SM | α-P | 0.2 | 3.57 | py, AIBN | 80 °C | 48 | 39.8 | 15600 | 23400 | 1.5 | _ | _ |
| 23 | SM | α-P | 0.01 | 2.22 | py, AIBN | 80 °C | 48 | 25.0 | 12450 | 18700 | 1.5 | _ | _ |
| № | Sugar | Alkene | a | b | Solvent, initiator | Reaction temp. | Reaction time [h] | Yieldc [%] | Mnd [g/mol] | MWd [g/mol] | Mn/MWd | φ e [Å] | Df [cm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | SM | β-P | 2 | 7.54 | py, AIBN | 80 °C | 48 | 75.0 | 5980 | 10760 | 1.8 | _ | _ |
| 25 | SM | β-P | 1 | 7.32 | py, AIBN | 80 °C | 48 | 63.1 | 8110 | 12980 | 1.6 | _ | _ |
| 26 | SM | β-P | 0.5 | 8.34 | py, AIBN | 80 °C | 48 | 56.1 | 5140 | 9760 | 1.9 | _ | _ |
| 27 | SM | β-P | 0.2 | 3.06 | py, AIBN | 80 °C | 48 | 17.0 | 10800 | 17230 | 1.6 | _ | _ |
| 28 | SM | β-P | 0.01 | 6.13 | py, AIBN | 80 °C | 48 | 18.3 | 11650 | 19800 | 1.7 | _ | _ |
| № | Sugar | Alkene | a | b | Solvent, initiator | Reaction temp. | Reaction time [h] | Yieldc [%] | Mnd [g/mol] | MWd [g/mol] | Mn/MWd | φ e [Å] | Df [cm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 29 | SM | α-P | 0.1 | 0.14 | DMF, AlCl3 | r.t. | 24 | 35.0 | 4400 | 7504 | 1.7 | _ | _ |
| 30 | SM | β-P | 0.1 | 0.12 | DMF, AlCl3 | r.t. | 24 | 29.1 | 3700 | 5560 | 1.5 | _ | _ |
| № | Sugar | Alkene | a | b | Solvent, initiator | Reaction temp. | Reaction time [h] | Yieldc [%] | Mnd [g/mol] | MWd [g/mol] | Mn/MWd | φ e [Å] | Df [cm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 31 | AMS | α-P | 2 | 1.53 | pyridine, AIBN | 80 °C | 48 | 9.37 | 1970 | 2170 | 1.1 | _ | _ |
| 32 | AMS | α-P | 1 | 0.63 | pyridine, AIBN | 80 °C | 48 | 19.8 | 2150 | 2370 | 1.1 | _ | _ |
| 33 | AMS | α-P | 0.5 | 0.35 | pyridine, AIBN | 80 °C | 48 | 8.02 | 2290 | 2750 | 1.2 | _ | _ |
| 34 | AMS | α-P | 0.2 | 0.12 | pyridine, AIBN | 80 °C | 48 | 5.72 | 2380 | 2620 | 1.1 | _ | _ |
| 35 | AMS | α-P | 0.1 | 0.07 | pyridine, AIBN | 80 °C | 48 | 13.2 | 2080 | 2500 | 1.2 | _ | _ |
| № | Sugar | Alkene | a | b | Solvent, initiator | Reaction temp. | Reaction time [h] | Yieldc [%] | Mnd [g/mol] | MWd [g/mol] | Mn/MWd | φ e [Å] | Df [cm2/s] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 36 | AMS | β-P | 2 | 1.21 | pyridine, AIBN | 80 °C | 48 | 21.4 | 1700 | 1880 | 1.1 | _ | _ |
| 37 | AMS | β-P | 1 | 0.34 | pyridine, AIBN | 80 °C | 48 | 13.4 | 1850 | 2400 | 1.3 | _ | _ |
| 38 | AMS | β-P | 0.5 | 0.22 | pyridine, AIBN | 80 °C | 48 | 24.6 | 2200 | 2640 | 1.2 | _ | _ |
| 39 | AMS | β-P | 0.2 | 0.95 | pyridine, AIBN | 80 °C | 48 | 8.38 | 2150 | 2370 | 1.1 | _ | _ |
| 40 | AMS | β-P | 0.1 | 0.02 | pyridine, AIBN | 80 °C | 48 | 3.89 | 1970 | 2170 | 1.1 | _ | _ |
| № | Copolymer | Reaction time [h] | Yielda (%) | Mnb [g/mol] | MWb [g/mol] | Mn/MWb | φ c [Å] | Dd [cm2/s] |
|---|---|---|---|---|---|---|---|---|
| 41 | Table 1, pol. 7 | 48 | 40.1 | 98700 | 148040 | 1.5 | 1454 | 2.50 × 10−9 |
| 42 | Table 1, pol. 1 | 120 | 67.3 | 38200 | 49660 | 1.3 | 824 | 4.42 × 10−9 |
| 43 | Table 1, pol. 3 | 120 | 67.1 | 123000 | 233740 | 1.9 | 1852 | 1.97 × 10−9 |
| 44 | Table 3, pol. 22 | 120 | 81.8 | 11800 | 18830 | 1.6 | 497 | 7.33 × 10−9 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barros, M.T.; Petrova, K.T.; Singh, R.P. Synthesis of Hydrophilic and Amphiphilic Acryl Sucrose Monomers and Their Copolymerisation with Styrene, Methylmethacrylate and α- and β-Pinenes. Int. J. Mol. Sci. 2010, 11, 1792-1807. https://doi.org/10.3390/ijms11041792
Barros MT, Petrova KT, Singh RP. Synthesis of Hydrophilic and Amphiphilic Acryl Sucrose Monomers and Their Copolymerisation with Styrene, Methylmethacrylate and α- and β-Pinenes. International Journal of Molecular Sciences. 2010; 11(4):1792-1807. https://doi.org/10.3390/ijms11041792
Chicago/Turabian StyleBarros, Maria Teresa, Krasimira T. Petrova, and Raj P. Singh. 2010. "Synthesis of Hydrophilic and Amphiphilic Acryl Sucrose Monomers and Their Copolymerisation with Styrene, Methylmethacrylate and α- and β-Pinenes" International Journal of Molecular Sciences 11, no. 4: 1792-1807. https://doi.org/10.3390/ijms11041792