Differential Proteomics Identification of HSP90 as Potential Serum Biomarker in Hepatocellular Carcinoma by Two-dimensional Electrophoresis and Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Sample Collection
2.2. Serum High-Abundance Protein Depletion
2.3. Two-dimensional Gel Electrophoresis
2.4. Image Analysis
2.5. Tryptic Digestion
2.6. Protein Identification by MALDI-TOF
2.7. ELISA for HSP90
3. Results
3.1. Differential Protein Expression in HCC Patients Included in the Study
3.2. Validation of the Significantly Differentially-Expressed Protein HSP90 by ELISA
4. Conclusions
References and Notes
- Parkin, DM. Global cancer statistics in the year 2000. Lancet Oncol 2001, 2, 533–543. [Google Scholar]
- Cha, C; DeMatteo, RP; Blumgart, LH. Surgery and ablative therapy for hepatocellular carcinoma. J. Clin. Gastroenterol 2002, 35, S130–S137. [Google Scholar]
- Shariff, MI; Cox, IJ; Gomaa, AI; Khan, SA; Gedroyc, W; Taylor-Robinson, SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev. Gastroenterol Hepatol 2009, 3, 353–367. [Google Scholar]
- Müller, C. Hepatocellular carcinoma-rising incidence, changing therapeutic strategies. Wien Med. Wochenschr 2006, 156, 404–409. [Google Scholar]
- Mazzanti, R; Gramantieri, L; Bolondi, L. Hepatocellular carcinoma: Epidemiology and clinical aspects. Mol. Aspects Med 2008, 29, 130–143. [Google Scholar]
- Pandey, A; Mann, M. Proteomics to study genes and genomes. Nature 2000, 405, 837–846. [Google Scholar]
- Alessandro, R; Fontana, S; Kohn, E; De Leo, G. Proteomic strategies and their application in cancer research. Tumori 2005, 91, 447–455. [Google Scholar]
- Koomen, JM; Haura, EB; Bepler, G; Sutphen, R; Remily-Wood, ER; Benson, K; Hussein, M; Hazlehurst, LA; Yeatman, TJ; Hildreth, LT; Sellers, TA; Jacobsen, PB; Fenstermacher, DA; Dalton, WS. Proteomic contributions to personalized cancer care. Mol. Cell. Proteomics 2008, 7, 1780–1794. [Google Scholar]
- Peng, XQ; Wang, F; Geng, X; Zhang, WM. Current advances in tumor proteomics and candidate biomarkers for hepatic cancer. Expert Rev. Proteomics 2009, 6, 551–561. [Google Scholar]
- Petricoin, EF; Zoon, KC; Kohn, EC; Barrett, JC; Liotta, LA. Clinical proteomics: Translating benchside promise into bedside reality. Nat. Rev. Drug. Discov 2002, 1, 683–695. [Google Scholar]
- Li, Z; Zhao, X; Bai, S; Wang, Z; Chen, L; Wei, Y; Huang, C. Proteomics identification of cyclophilin a as a potential prognostic factor and therapeutic target in endometrial carcinoma. Mol. Cell. Proteomics 2008, 7, 1810–1823. [Google Scholar]
- Sagynaliev, E; Steinert, R; Nestler, G; Lippert, H; Knoch, M; Reymond, MA. Web-based data warehouse on gene expression in human colorectal cancer. Proteomics 2005, 5, 3066–3078. [Google Scholar]
- Rabilloud, T. Two-dimensional gel electrophoresis in proteomics: Old, old fashioned, but it still climbs up the mountains. Proteomics 2002, 2, 3–10. [Google Scholar]
- Mathivanan, S; Lim, JW; Tauro, BJ; Ji, H; Moritz, RL; Simpson, RJ. Proteomic analysis of A33-immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol. Cell. Proteomics 2010, 9, 197–208. [Google Scholar]
- Schulz, DM; Böllner, C; Thomas, G; Atkinson, M; Esposito, I; Höfler, H; Aubele, M. Identification of differentially expressed proteins in triple-negative breast carcinomas using DIGE and mass spectrometry. J. Proteome Res 2009, 8, 3430–3438. [Google Scholar]
- Hongsachart, P; Huang-Liu, R; Sinchaikul, S; Pan, FM; Phutrakul, S; Chuang, YM; Yu, CJ; Chen, ST. Glycoproteomic analysis of WGA-bound glycoprotein biomarkers in sera from patients with lung adenocarcinoma. Electrophoresis 2009, 30, 1206–1220. [Google Scholar]
- Cho, HJ; Baek, KE; Park, SM; Kim, IK; Choi, YL; Cho, HJ; Nam, IK; Hwang, EM; Park, JY; Han, JY; Kang, SS; Kim, DC; Lee, WS; Lee, MN; Oh, GT; Kim, JW; Lee, CW; Yoo, J. RhoGDI2 expression is associated with tumor growth and malignant progression of gastric cancer. Clin. Cancer Res 2009, 15, 2612–2619. [Google Scholar]
- Vellaichamy, A; Sreekumar, A; Strahler, JR; Rajendiran, T; Yu, J; Varambally, S; Li, Y; Omenn, GS; Chinnaiyan, AM; Nesvizhskii, AI. Proteomic interrogation of androgen action in prostate cancer cells reveals roles of aminoacyl tRNA synthetases. PLoS One 2009, 4, e7075. [Google Scholar]
- Ono, M; Matsubara, J; Honda, K; Sakuma, T; Hashiguchi, T; Nose, H; Nakamori, S; Okusaka, T; Kosuge, T; Sata, N; Nagai, H; Ioka, T; Tanaka, S; Tsuchida, A; Aoki, T; Shimahara, M; Yasunami, Y; Itoi, T; Moriyasu, F; Negishi, A; Kuwabara, H; Shoji, A; Hirohashi, S; Yamada, T. Prolyl 4-hydroxylation of alpha-fibrinogen: A novel protein modification revealed by plasma proteomics. J. Biol. Chem 2009, 284, 29041–29049. [Google Scholar]
- Roessler, M; Rollinger, W; Mantovani-Endl, L; Hagmann, ML; Palme, S; Berndt, P; Engel, AM; Pfeffer, M; Karl, J; Bodenmüller, H; Rüschoff, J; Henkel, T; Rohr, G; Rossol, S; Rösch, W; Langen, H; Zolg, W; Tacke, M. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol. Cell. Proteomics 2006, 5, 2092–2101. [Google Scholar]
- Sun, Y; Mi, W; Cai, J; Ying, W; Liu, F; Lu, H; Qiao, Y; Jia, W; Bi, X; Lu, N; Liu, S; Qian, X; Zhao, X. Quantitative proteomic signature of liver cancer cells: Tissue transglutaminase 2 could be a novel protein candidate of human hepatocellular carcinoma. J. Proteome Res 2008, 7, 3847–3859. [Google Scholar]
- Fujiyama, S; Tanaka, M; Maeda, S; Ashihara, H; Hirata, R; Tomita, K. Tumor markers in early diagnosis, follow-up and management of patients with hepatocellular carcinoma. Oncology 2002, 62, 57–63. [Google Scholar]
- Spangenberg, HC; Thimme, R; Blum, HE. Serum markers of hepatocellular carcinoma. Semin. Liver Dis 2006, 26, 385–390. [Google Scholar]
- Marrero, JA. Hepatocellular carcinoma. Curr. Opin. Gastroenterol 2003, 19, 243–249. [Google Scholar]
- Chromy, BA; Gonzales, AD; Perkins, J; Choi, MW; Corzett, MH; Chang, BC; Corzett, CH; McCutchen-Maloney, SL. Proteomic analysis of human serum by two-dimensional differential gel electrophoresis after depletion of high-abundant proteins. J. Proteome Res 2004, 3, 1120–1127. [Google Scholar]
- Corzett, TH; Fodor, IK; Choi, MW; Walsworth, VL; Chromy, BA; Turteltaub, KW; McCutchen-Maloney, SL. Statistical analysis of the experimental variation in the proteomic characterization of human plasma by two-dimensional difference gel electrophoresis. J. Proteome Res 2006, 5, 2611–2619. [Google Scholar]
- Hochstrasser, DF; Frutiger, S; Paquet, N; Bairoch, A; Ravier, F; Pasquali, C; Sanchez, JC; Tissot, JD; Bjellqvist, B; Vargas, R. Human liver protein map: A reference database established by microsequencing and gel comparison. Electrophoresis 1992, 13, 992–1001. [Google Scholar]
- Zimmermann-Ivol, CG; Burkhard, PR; Le Floch-Rohr, J; Allard, L; Hochstrasser, DF; Sanchez, JC. Fatty acid binding protein as a serum marker for the early diagnosis of stroke: A pilot study. Mol. Cell. Proteomics 2004, 3, 66–72. [Google Scholar]
- Görg, A; Obermaier, C; Boguth, G; Weiss, W. Recent developments in two-dimensional gel electrophoresis with immobilized pH gradients: Wide pH gradients up to pH 12, longer separation distances and simplified procedures. Electrophoresis 1999, 20, 712–717. [Google Scholar]
- Görg, A; Obermaier, C; Boguth, G; Harder, A; Scheibe, B; Wildgruber, R; Weiss, W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 2000, 21, 1037–1053. [Google Scholar]
- Stasyk, T; Morandell, S; Bakry, R; Feuerstein, I; Huck, CW; Stecher, G; Bonn, GK; Huber, LA. Quantitative detection of phosphoproteins by combination of two-dimensional difference gel electrophoresis and phosphospecific fluorescent staining. Electrophoresis 2005, 26, 2850–2854. [Google Scholar]
- Huang, HL; Stasyk, T; Morandell, S; Dieplinger, H; Falkensammer, G; Griesmacher, A; Mogg, M; Schreiber, M; Feuerstein, I; Huck, CW; Stecher, G; Bonn, GK; Huber, LA. Biomarker discovery in breast cancer serum using 2-D differential gel electrophoresis / MALDI-TOF/TOF and data validation by routine clinical assays. Electrophoresis 2006, 27, 1641–1650. [Google Scholar]
- Sung, WK; Lu, Y; Lee, CW; Zhang, D; Ronaghi, M; Lee, CG. Deregulated direct targets of the hepatitis B virus (HBV) protein, HBx, identified through chromatin immunoprecipitation and expression microarray profiling. J. Biol. Chem 2009, 284, 21941–21954. [Google Scholar]
- Kanda, M; Nomoto, S; Okamura, Y; Nishikawa, Y; Sugimoto, H; Kanazumi, N; Takeda, S; Nakao, A. Detection of metallothionein 1G as a methylated tumor suppressor gene in human hepatocellular carcinoma using a novel method of double combination array analysis. Int. J. Oncol 2009, 35, 4774–4783. [Google Scholar]
- Motallebipour, M; Enroth, S; Punga, T; Ameur, A; Koch, C; Dunham, I; Komorowski, J; Ericsson, J; Wadelius, C. Novel genes in cell cycle control and lipid metabolism with dynamically regulated binding sites for sterol regulatory element-binding protein 1 and RNA polymerase II in HepG2 cells detected by chromatin immunoprecipitation with microarray detection. FEBS J 2009, 276, 187818–187890. [Google Scholar]
- Chau, BN; Diaz, RL; Saunders, MA; Cheng, C; Chang, AN; Warrener, P; Bradshaw, J; Linsley, PS; Cleary, MA. Identification of SULF2 as a novel transcriptional target of p53 by use of integrated genomic analyses. Cancer Res 2009, 69, 1368–1374. [Google Scholar]
- Wilkins, MR; Sanchez, JC; Gooley, AA; Appel, RD; Humphery-Smith, I; Hochstrasser, DF; Williams, KL. Progress with proteome projects: Why all proteins expressed by a genome should be identified and how to do it. Biotechnol. Genet. Eng. Rev 1996, 13, 19–50. [Google Scholar]
- Anderson, L; Seilhamer, J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis 1997, 18, 533–537. [Google Scholar]
- Hanash, SM. Biomedical applications of two-dimensional electrophoresis using immobilized pH gradients: Current status. Electrophoresis 2000, 21, 1202–1209. [Google Scholar]
- Reymond, MA; Sanchez, JC; Hughes, GJ; Gunther, K; Riese, J; Tortola, S; Peinado, MA; Kirchner, T; Hohenberger, W; Hochstrasser, DF; Kockerling, F. Standardized characterization of gene expression in human colorectal epithelium by two-dimensional electrophoresis. Electrophoresis 1997, 18, 2842–2848. [Google Scholar]
- Zhou, G; Li, H; DeCamp, D; Chen, S; Shu, H; Gong, Y; Flaig, M; Gillespie, JW; Hu, N; Taylor, PR; Emmert-Buck, MR; Liotta, LA; Petricoin, EF, III; Zhao, Y. 2D differential in-gel electrophoresis for the identification of esophageal scans cell cancer-specific protein markers. Mol. Cell. Proteomics 2002, 1, 117–124. [Google Scholar]
- Isaacs, JS; Xu, W; Neckers, L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell 2003, 3, 213–217. [Google Scholar]
- Gooljarsingh, LT; Fernandes, C; Yan, K; Zhang, H; Grooms, M; Johanson, K; Sinnamon, RH; Kirkpatrick, RB; Kerrigan, J; Lewis, T; Arnone, M; King, AJ; Lai, Z; Copeland, RA; Tummino, PJ. A biochemical rationale for the anticancer effects of Hsp90 inhibitors: Slow, tight binding inhibition by geldanamycin and its analogues. Proc. Natl. Acad. Sci. USA 2006, 103, 7625–7630. [Google Scholar]
- Pascale, RM; Simile, MM; Calvisi, DF; Frau, M; Muroni, MR; Seddaiu, MA; Daino, L; Muntoni, MD; De Miglio, MR; Thorgeirsson, SS; Feo, F. Role of HSP90, CDC37, and CRM1 as modulators of P16(INK4A) activity in rat liver carcinogenesis and human liver cancer. Hepatology 2005, 42, 1310–1319. [Google Scholar]
- Lang, SA; Moser, C; Fichnter-Feigl, S; Schachtschneider, P; Hellerbrand, C; Schmitz, V; Schlitt, HJ; Geissler, EK; Stoeltzing, O. Targeting heat-shock protein 90 improves efficacy of rapamycin in a model of hepatocellular carcinoma in mice. Hepatology 2009, 49, 523–532. [Google Scholar]
- Breinig, M; Caldas-Lopes, E; Goeppert, B; Malz, M; Rieker, R; Bergmann, F; Schirmacher, P; Mayer, M; Chiosis, G; Kern, MA. Targeting heat shock protein 90 with non-quinone inhibitors: A novel chemotherapeutic approach in human hepatocellular carcinoma. Hepatology 2009, 50, 102–112. [Google Scholar]
- Zhang, H; Burrows, F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J. Mol. Med 2004, 82, 488–499. [Google Scholar]
- Pearl, LH; Prodromou, C; Workman, P. The Hsp90 molecular chaperone: An open and shut case for treatment. Biochem. J 2008, 410, 439–453. [Google Scholar]
- Ma, Y; Peng, J; Huang, L; Liu, W; Zhang, P; Qin, H. Searching for serum tumor markers for colorectal cancer using a 2-D DIGE approach. Electrophoresis 2009, 30, 2591–1599. [Google Scholar]
- Hinestrosa, MC; Dickersin, K; Klein, P; Mayer, M; Noss, K; Slamon, D; Sledge, G; Visco, FM. Shaping the future of biomarker research in breast cancer to ensure clinical relevance. Nat. Rev. Cancer 2007, 7, 309–315. [Google Scholar]
- Ma, Y; Peng, J; Liu, W; Zhang, P; Huang, L; Gao, B; Shen, T; Zhou, Y; Chen, H; Chu, Z; Zhang, M; Qin, H. Proteomics identification of desmin as a potential oncofetal diagnostic and prognostic biomarker in colorectal cancer. Mol. Cell. Proteomics 2009, 8, 1878–1890. [Google Scholar]
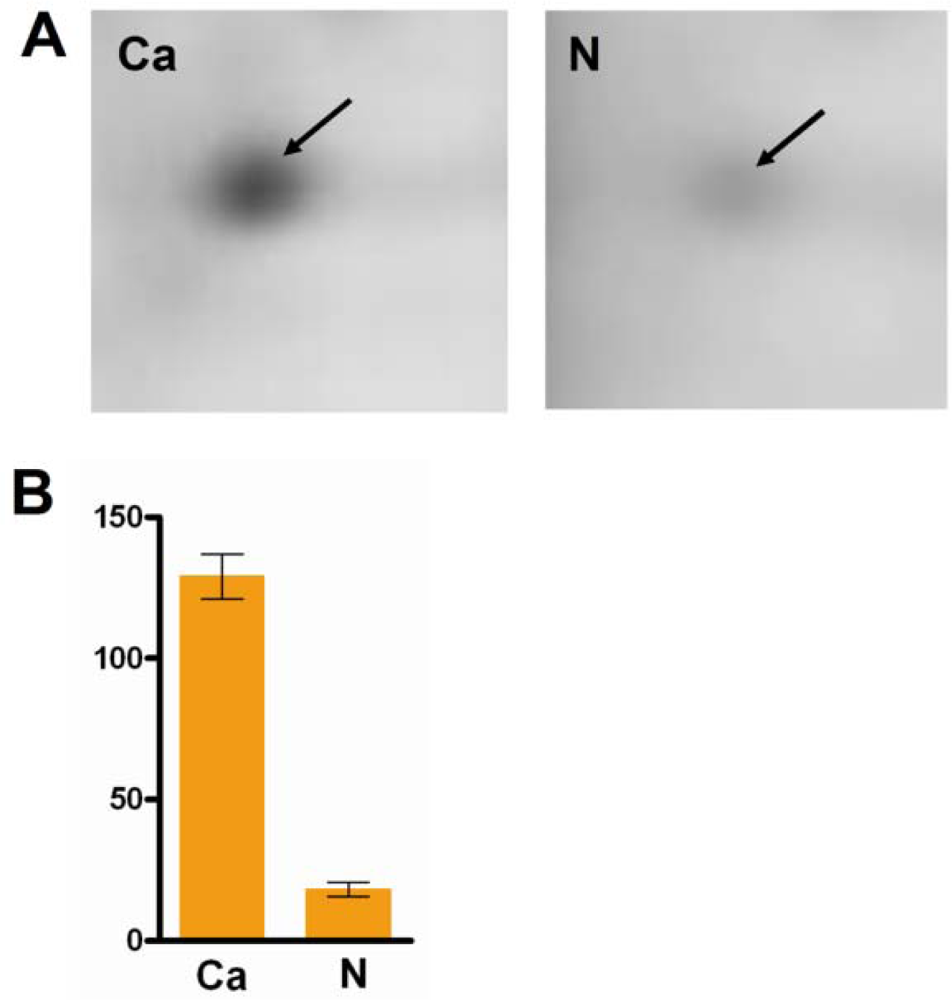

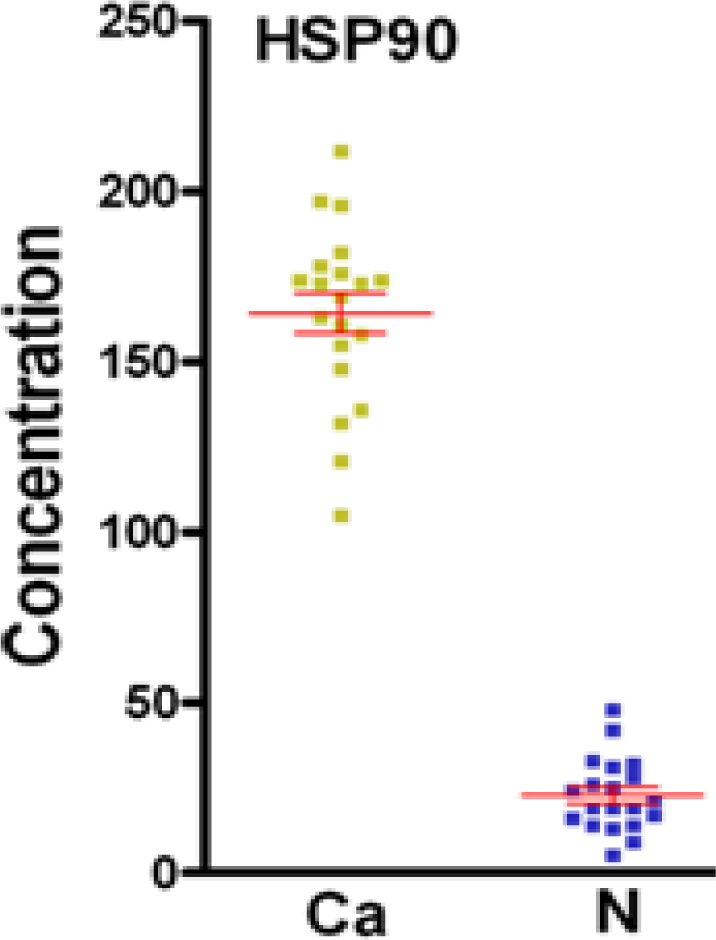
| Spot Number | Protein name | MW (kDa) | pI | Fold change (Ca/N) | P value (t test) |
|---|---|---|---|---|---|
| 1 | S-adenosylmethionine synthetase isoform type-1 | 43.6 | 5.86 | 4.21 | 0.005 |
| 2 | Glycine N-methyltransferase | 32.6 | 6.58 | 4.56 | 0.007 |
| 3 | Haptoglobin precursor | 38.9 | 6.42 | 5.10 | 0.004 |
| 4 | Serum amyloid P-component precursor | 25.4 | 6.10 | 3.84 | 0.018 |
| 5 | Glyceraldehyde-3-phosphate dehydrogenase | 35.9 | 8.58 | 5.73 | 0.003 |
| 6 | Heat shock protein 90 | 83.1 | 4.97 | 7.04 | <0.0001 |
| 7 | Annexin V | 35.8 | 4.98 | 3.89 | 0.012 |
| 8 | Carbonic anhydrase I | 28.9 | 6.59 | −6.73 | 0.002 |
| 9 | Beta-galactoside-binding lectin | 15.1 | 5.34 | −6.09 | <0.0001 |
| 10 | Vitamin D-binding protein | 52.8 | 5.20 | −5.24 | 0.004 |
| 11 | Apolipoprotein A-I | 28.1 | 5.30 | −3.59 | 0.017 |
| 12 | Annexin 4 | 36.1 | 5.80 | −4.32 | 0.006 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, Y.; Zang, Z.; Xu, X.; Zhang, Z.; Zhong, L.; Zan, W.; Zhao, Y.; Sun, L. Differential Proteomics Identification of HSP90 as Potential Serum Biomarker in Hepatocellular Carcinoma by Two-dimensional Electrophoresis and Mass Spectrometry. Int. J. Mol. Sci. 2010, 11, 1423-1433. https://doi.org/10.3390/ijms11041423
Sun Y, Zang Z, Xu X, Zhang Z, Zhong L, Zan W, Zhao Y, Sun L. Differential Proteomics Identification of HSP90 as Potential Serum Biomarker in Hepatocellular Carcinoma by Two-dimensional Electrophoresis and Mass Spectrometry. International Journal of Molecular Sciences. 2010; 11(4):1423-1433. https://doi.org/10.3390/ijms11041423
Chicago/Turabian StyleSun, Yiyi, Zhihe Zang, Xiaohong Xu, Zhonglin Zhang, Ling Zhong, Wang Zan, Yan Zhao, and Lin Sun. 2010. "Differential Proteomics Identification of HSP90 as Potential Serum Biomarker in Hepatocellular Carcinoma by Two-dimensional Electrophoresis and Mass Spectrometry" International Journal of Molecular Sciences 11, no. 4: 1423-1433. https://doi.org/10.3390/ijms11041423




