Cytotoxic Effects of 2-Bromopropane on Embryonic Development in Mouse Blastocysts
Abstract
:1. Introduction
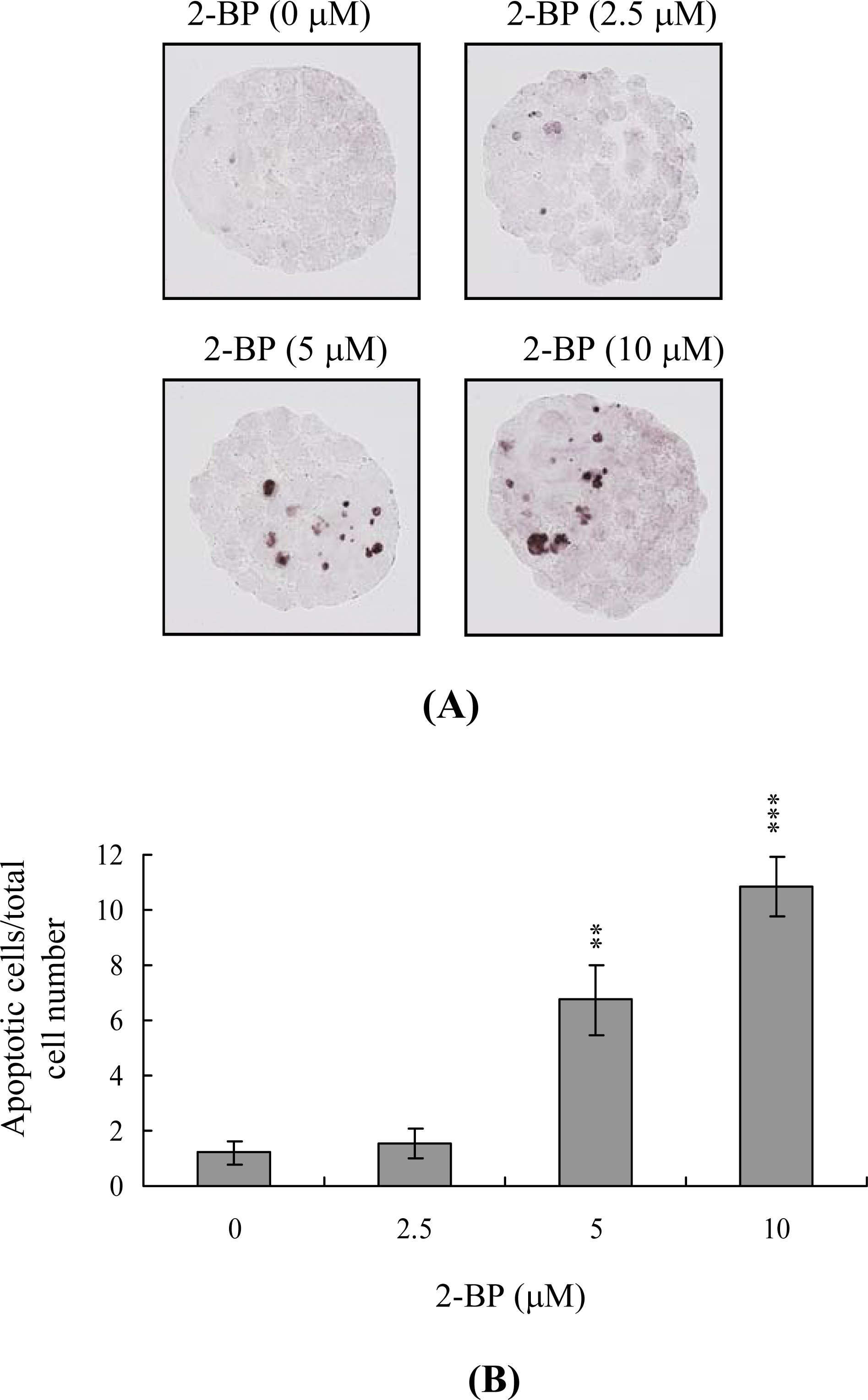
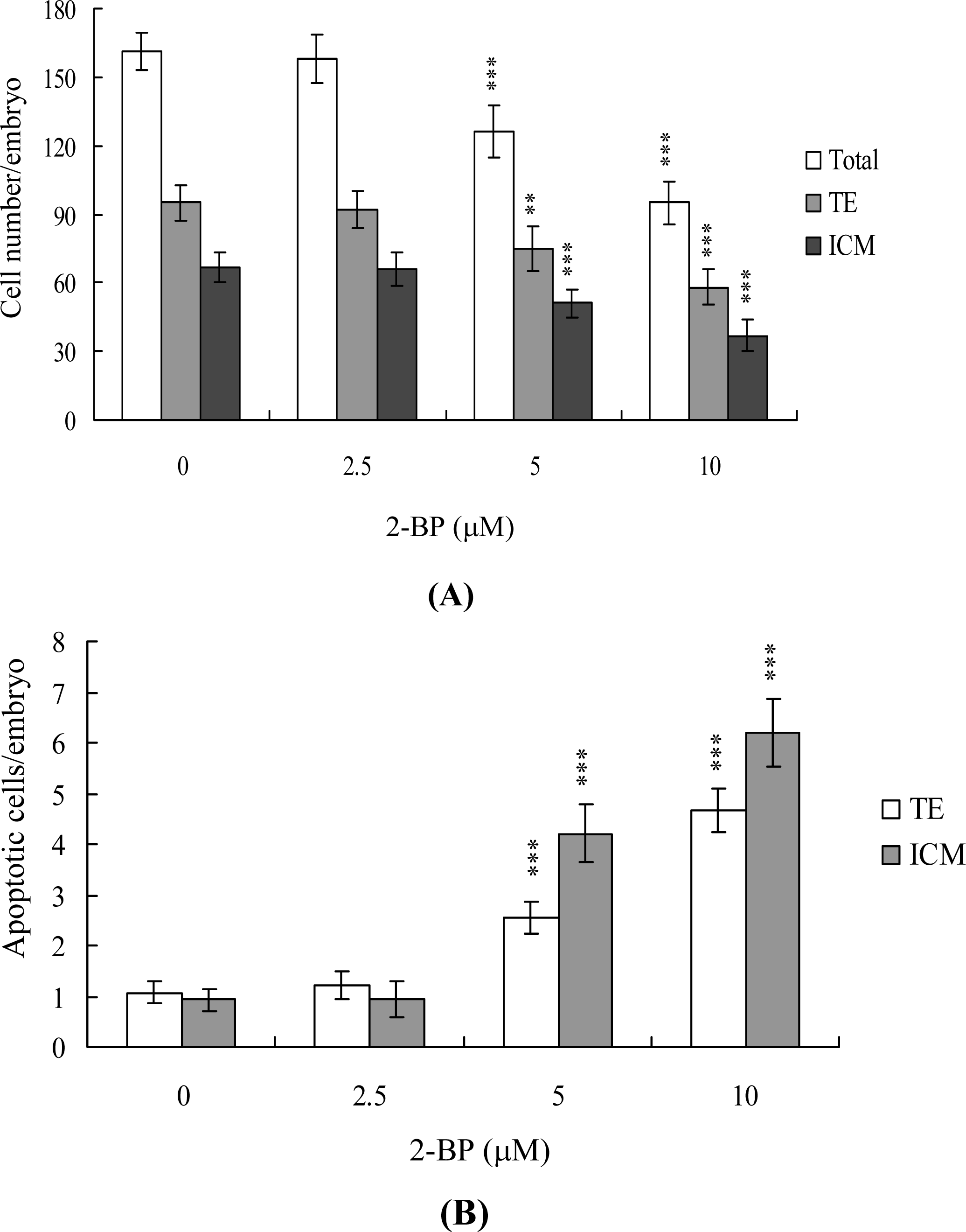
2. Results and Discussion
3. Experimental Section
3.1. Chemicals
3.2. Collection of Mouse Morulas and Blastocysts
3.3. 2-BP Treatment and Tunel Assay
3.4. 2-BP Treatment and Cell Proliferation
3.5. Annexin V Staining
3.6. Morphological Analysis of Embryonic Development
3.7. Blastocyst Development Following Embryo Transfer
3.8. Statistics
4. Conclusions
Acknowledgments
References and Notes
- Kim, Y; Jung, K; Hwang, T; Jung, G; Kim, H; Park, J; Kim, J; Park, J; Park, D; Park, S; Choi, K; Moon, Y. Hematopoietic and reproductive hazards of Korean electronic workers exposed to solvents containing 2-bromopropane. Scand. J. Work Environ. Health 1996, 22, 387–391. [Google Scholar]
- Li, GX; Kang, KS; Lee, YS. 2-Bromopropane induced germ cell apoptosis during spermatogenesis in male rat. J. Vet. Med. Sci 2001, 63, 373–382. [Google Scholar]
- Park, JS; Kim, YH; Park, DW; Choi, KS; Park, SH; Moon, YH. An outbreak of hematopoietic and reproductive disorders due to solvents containing 2-bromopropane in an electronic factory, South Korea: Epidemiological survey. J. Occup. Health 1997, 39, 138–143. [Google Scholar]
- Omura, M; Romero, Y; Zhao, M; Inoue, N. Histopathological evidence that spermatogonia are the target cells of 2-bromopropane. Toxicol. Lett 1999, 104, 19–26. [Google Scholar]
- Zhao, LX; Kim, EK; Lim, HT; Moon, YS; Kim, NH; Kim, TH; Choi, H; Chae, W; Jeong, TC; Lee, ES. Synthesis, characterization and in vitro identification of N7-guanine adduct of 2-bromopropane. Arch. Pharm. Res 2002, 25, 39–44. [Google Scholar]
- Yu, X; Kamijima, M; Ichihara, G; Li, W; Kitoh, J; Xie, Z; Shibata, E; Hisanaga, N; Takeuchi, Y. 2-Bromopropane causes ovarian dysfunction by damaging primordial follicles and their oocytes in female rats. Toxicol. Appl. Pharmacol 1999, 159, 185–193. [Google Scholar]
- Son, HY; Kim, YB; Kang, BH; Cho, SW; Ha, CS; Roh, JK. Effects of 2-bromopropane on spermatogenesis in the Sprague-Dawley rat. Reprod. Toxicol 1999, 13, 179–187. [Google Scholar]
- Wu, X; Faqi, AS; Yang, J; Pang, BP; Ding, X; Jiang, X; Chahoud, I. 2-Bromopropane induces DNA damage, impairs functional antioxidant cellular defenses, and enhances the lipid peroxidation process in primary cultures of rat Leydig cells. Reprod. Toxicol 2002, 16, 379–384. [Google Scholar]
- Yu, X; Ichihara, G; Kitoh, J; Xie, Z; Shibata, E; Kamijima, M; Asaeda, N; Hisanaga, N; Takeuchi, Y. Effect of inhalation exposure to 2-bromopropane on the nervous system in rats. Toxicology 1999, 135, 87–93. [Google Scholar]
- Kim, JC; Kim, SH; Shin, DH; Ahn, TH; Kim, HC; Kim, YB; Jiang, CZ; Han, J; Chung, MK. Effects of prenatal exposure to the environmental pollutant 2-bromopropane on embryo-fetal development in rats. Toxicology 2004, 196, 77–86. [Google Scholar]
- Ichihara, G; Asaeda, N; Kumazawa, T; Tagawa, T; Kamijima, M; Yu, X; Kondo, H; Nakajima, T; Kitoh, J; Yu, IJ; Moon, YH; Hisanaga, N; Takeuchi, Y. Testicular and haematopoietic toxicity of 2-bromopropane, a substitute for ozone layer-depleting chlorofluorocarbons. J. Occup. Health 1997, 39, 57–63. [Google Scholar]
- Ishikawa, H; Tian, Y; Yamauchi, T. Induction of micronuclei formation in preimplantation mouse embryos after maternal treatment with 2-bromopropane. Reprod. Toxicol 2001, 15, 81–85. [Google Scholar]
- Kang, KS; Li, GX; Che, JH; Lee, YS. Impairment of male rat reproductive function in F1 offspring from dams exposed to 2-bromopropane during gestation and lactation. Reprod. Toxicol 2002, 16, 151–159. [Google Scholar]
- Thompson, CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar]
- Brill, A; Torchinsky, A; Carp, H; Toder, V. The role of apoptosis in normal and abnormal embryonic development. J. Assist. Reprod. Genet 1999, 16, 512–519. [Google Scholar]
- Lotz, K; Proff, P; Bienengraeber, V; Fanghaenel, J; Gedrange, T; Weingaertner, J. Apoptosis as a creative agent of embryonic development of bucca, mentum and nasolacrimal duct. An in vivo study in rats. J Craniomaxillofac Surg 2006, 34(Suppl 2), 8–13. [Google Scholar]
- Weingaertner, J; Proff, P; Bienengraeber, V; Gedrange, T; Fanghaenel, J; Lotz, K. In vivo study of apoptosis as a creative agent of embryonic development of the primary nasal duct in rats. J Craniomaxillofac Surg 2006, 34(Suppl 2), 3–7. [Google Scholar]
- Huang, FJ; Shen, CC; Chang, SY; Wu, TC; Hsuuw, YD. Retinoic acid decreases the viability of mouse blastocysts in vitro. Hum. Reprod 2003, 18, 130–136. [Google Scholar]
- Hsuuw, YD; Chang, CK; Chan, WH; Yu, JS. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell Physiol 2005, 205, 379–386. [Google Scholar]
- Chan, WH. Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts. Hum. Reprod 2006, 21, 2985–2995. [Google Scholar]
- Shang, EH; Wu, RS. Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ. Sci. Technol 2004, 38, 4763–4767. [Google Scholar]
- Detmar, J; Rabaglino, T; Taniuchi, Y; Oh, J; Acton, BM; Benito, A; Nunez, G; Jurisicova, A. Embryonic loss due to exposure to polycyclic aromatic hydrocarbons is mediated by Bax. Apoptosis 2006, 11, 1413–1425. [Google Scholar]
- Chan, WH; Lu, HY; Shiao, NH. Effect of genistein on mouse blastocyst development in vitro. Acta Pharmacol. Sin 2007, 28, 238–245. [Google Scholar]
- Chan, WH. Ginkgolides induce apoptosis and decrease cell numbers in mouse blastocysts. Biochem. Biophys. Res. Commun 2005, 338, 1263–1267. [Google Scholar]
- Chan, WH. Citrinin induces apoptosis via a mitochondria-dependent pathway and inhibition of survival signals in embryonic stem cells, and causes developmental injury in blastocysts. Biochem. J 2007, 404, 317–326. [Google Scholar]
- Chan, WH. Citrinin induces apoptosis in mouse embryonic stem cells. IUBMB Life 2008, 60, 171–179. [Google Scholar]
- Chan, WH. Impact of genistein on maturation of mouse oocytes, fertilization, and fetal development. Reprod. Toxicol 2009, 28, 52–58. [Google Scholar]
- Chan, WH; Shiao, NH. Effect of citrinin on mouse embryonic development in vitro and in vivo. Reprod. Toxicol 2007, 24, 120–125. [Google Scholar]
- Witschi, E. Characterization of developmental stages. Part II. Rat. In Biology Data Book, 2nd ed; Federation of American Societies of Experimental Biologies: Washington, DC, USA, 1972; pp. 178–180. [Google Scholar]
- Huang, FJ; Hsuuw, YD; Lan, KC; Kang, HY; Chang, SY; Hsu, YC; Huang, KE. Adverse effects of retinoic acid on embryo development and the selective expression of retinoic acid receptors in mouse blastocysts. Hum. Reprod 2006, 21, 202–209. [Google Scholar]
- Chan, WH; Shiao, NH. Cytotoxic effect of CdSe quantum dots on mouse embryonic development. Acta Pharmacol. Sin 2008, 29, 259–266. [Google Scholar]
- Yu, X; Kubota, H; Wang, R; Saegusa, J; Ogawa, Y; Ichihara, G; Takeuchi, Y; Hisanaga, N. Involvement of Bcl-2 family genes and Fas signaling system in primary and secondary male germ cell apoptosis induced by 2-bromopropane in rat. Toxicol. Appl. Pharmacol 2001, 174, 35–48. [Google Scholar]
- Takeuchi, T; Okuda, H; Arito, H; Nagano, K; Yamamoto, S; Matsushima, T. Developmental effects of inhalation exposure to 2-bromopropane in rats. Reprod. Toxicol 2004, 18, 431–437. [Google Scholar]
- Cross, JC; Werb, Z; Fisher, SJ. Implantation and the placenta: key pieces of the development puzzle. Science 1994, 266, 1508–1518. [Google Scholar]
- Pampfer, S; de Hertogh, R; Vanderheyden, I; Michiels, B; Vercheval, M. Decreased inner cell mass proportion in blastocysts from diabetic rats. Diabetes 1990, 39, 471–476. [Google Scholar]
- Kelly, SM; Robaire, B; Hales, BF. Paternal cyclophosphamide treatment causes postimplantation loss via inner cell mass-specific cell death. Teratology 1992, 45, 313–318. [Google Scholar]
- Tam, PP. Postimplantation development of mitomycin C-treated mouse blastocysts. Teratology 1988, 37, 205–212. [Google Scholar]
- Hardy, K. Cell death in the mammalian blastocyst. Mol. Hum. Reprod 1997, 3, 919–925. [Google Scholar]
- Hardy, K; Stark, J; Winston, RM. Maintenance of the inner cell mass in human blastocysts from fragmented embryos. Biol. Reprod 2003, 68, 1165–1169. [Google Scholar]
- Byrne, AT; Southgate, J; Brison, DR; Leese, HJ. Analysis of apoptosis in the preimplantation bovine embryo using TUNEL. J. Reprod. Fertil 1999, 117, 97–105. [Google Scholar]
- Hardy, K; Handyside, AH; Winston, RM. The human blastocyst: cell number, death and allocation during late preimplantation development in vitro. Development 1989, 107, 597–604. [Google Scholar]
- Gardner, RL; Davies, TJ. Lack of coupling between onset of giant transformation and genome endoreduplication in the mural trophectoderm of the mouse blastocyst. J. Exp. Zool 1993, 265, 54–60. [Google Scholar]
- Huang, FJ; Wu, TC; Tsai, MY. Effect of retinoic acid on implantation and post-implantation development of mouse embryos in vitro. Hum. Reprod 2001, 16, 2171–2176. [Google Scholar]



© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chan, W.-H. Cytotoxic Effects of 2-Bromopropane on Embryonic Development in Mouse Blastocysts. Int. J. Mol. Sci. 2010, 11, 731-744. https://doi.org/10.3390/ijms11020731
Chan W-H. Cytotoxic Effects of 2-Bromopropane on Embryonic Development in Mouse Blastocysts. International Journal of Molecular Sciences. 2010; 11(2):731-744. https://doi.org/10.3390/ijms11020731
Chicago/Turabian StyleChan, Wen-Hsiung. 2010. "Cytotoxic Effects of 2-Bromopropane on Embryonic Development in Mouse Blastocysts" International Journal of Molecular Sciences 11, no. 2: 731-744. https://doi.org/10.3390/ijms11020731



