Screening of Molecular Virulence Markers in Staphylococcus aureus and Pseudomonas aeruginosa Strains Isolated from Clinical Infections
Abstract
:1. Introduction
2. Results and Discussion
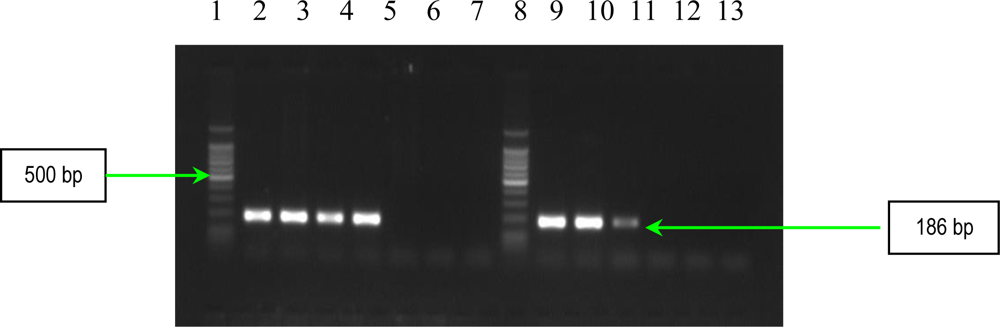
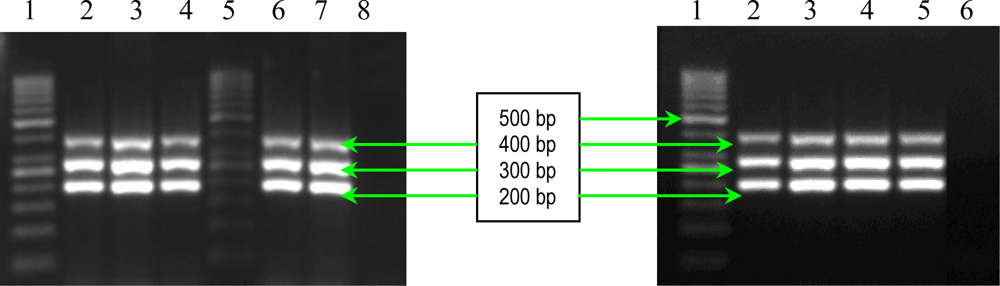
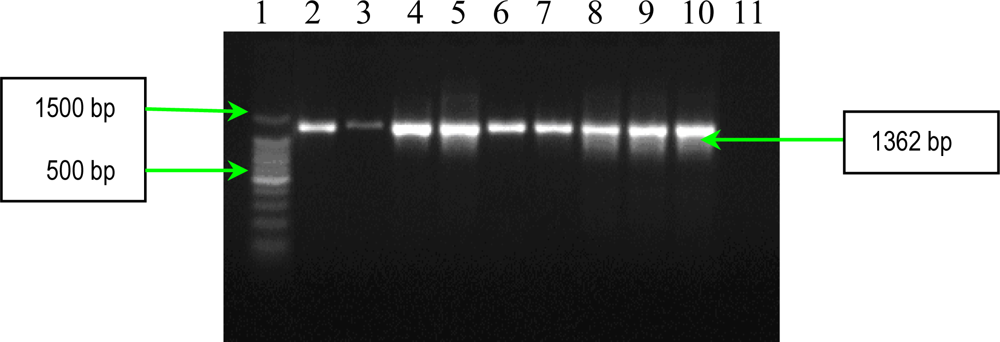
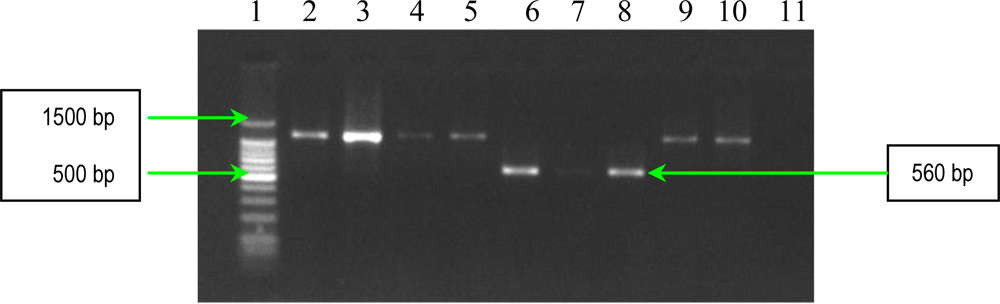
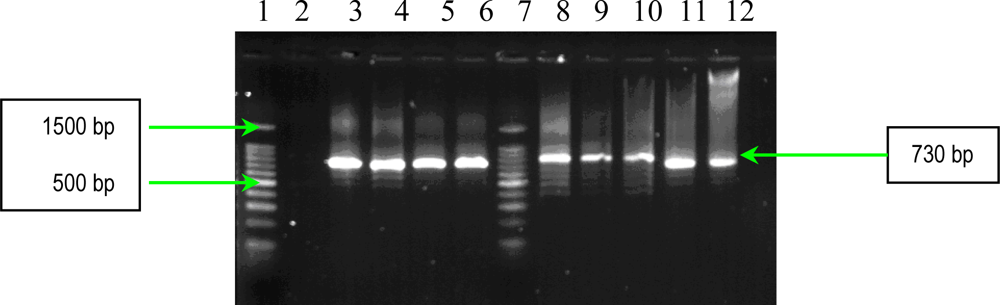
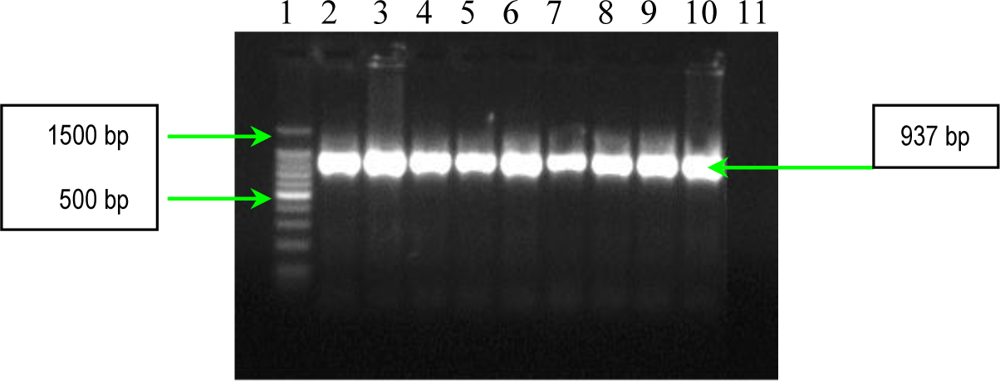
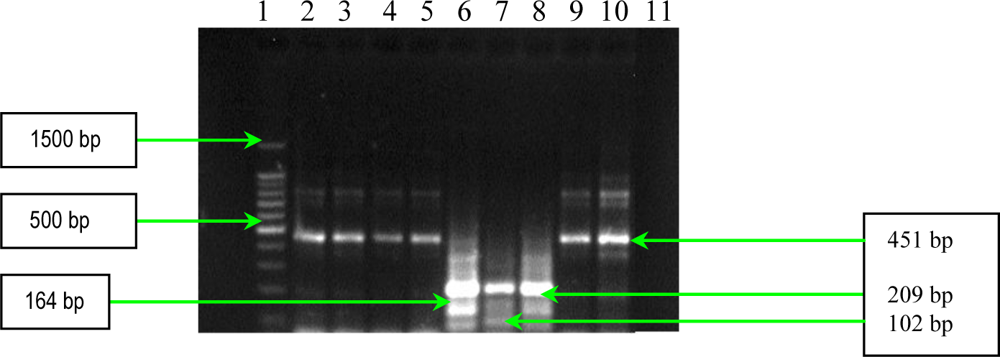
2.1. PCR Assays for S. aureus Virulence Genes Detection
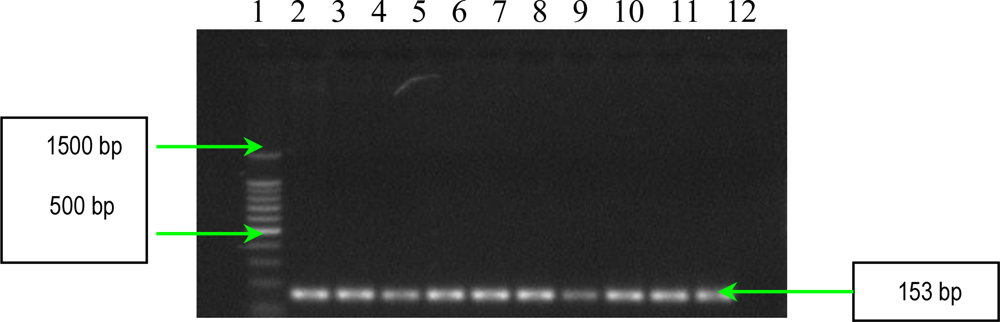
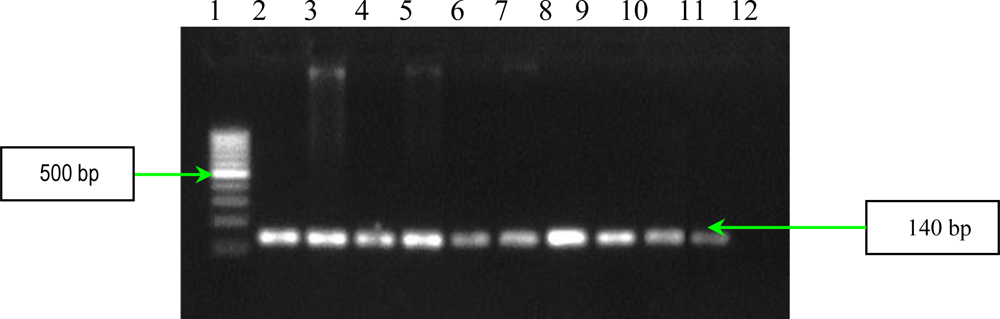
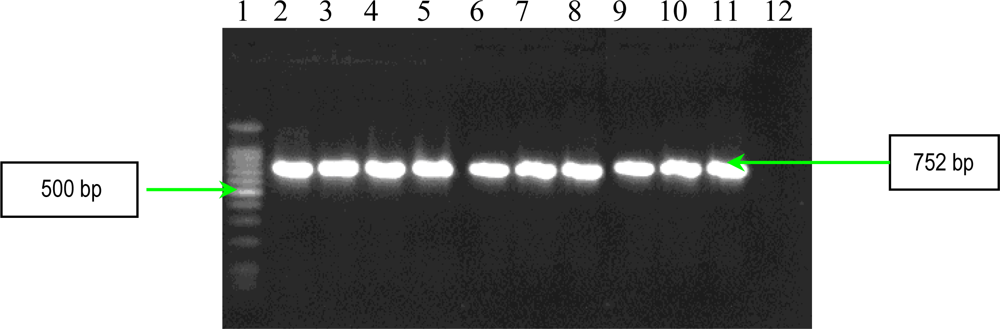
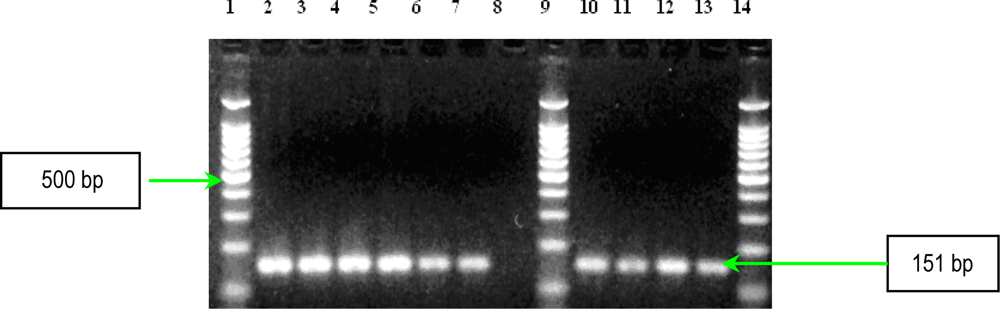
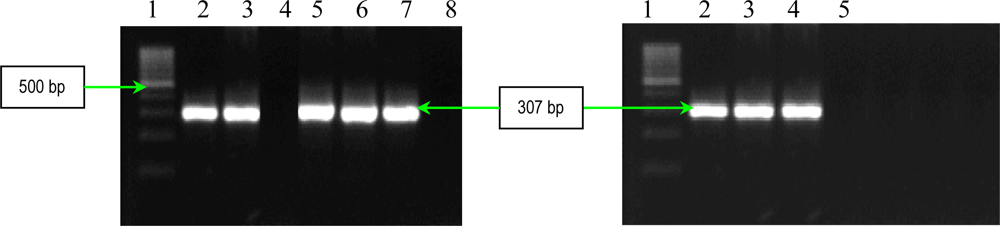
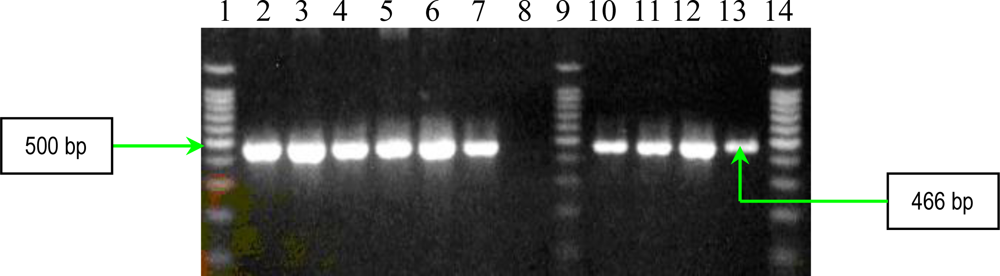
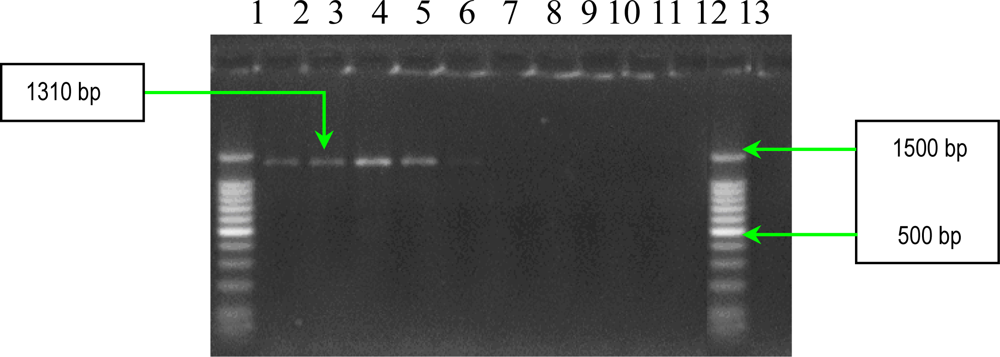
2.2. PCR Assays for Ps. aeruginosa Virulence Genes Detection
3. Experimental Section
3.1. Bacterial Strains
3.2. Detection of S. aureus Virulence Genes by PCR
3.2.1. Multiplex and Uniplex PCRs for Detection of Genes Encoding Adhesins
3.3. PCR Assays for Detection of Extracellular Proteins (Coagulase, Panton-Valentine Leukocidin and Gamma–Hemolysin)
3.4. Detection of Ps. aeruginosa Virulence Genes by PCR
4. Conclusions
Acknowledgments
References
- Jarraud, S; Mougel, C; Thioulouse, J; Lina, G; Meugnier, H; Forey, F; Nesme, X; Etienne, J; Vandenesch, F. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun 2002, 70, 631–641. [Google Scholar]
- Delden, CV; Iglewski, BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis 1998, 4, 551–558. [Google Scholar]
- Zhu, H; Bandara, R; Conibear, TCR; Thuruthyil, SJ; Rice, SA; Kjelleberg, S; Givskov, M; Willcox, MDP. Pseudomonas aeruginosa with lasI quorum-sensing deficiency during corneal infection. Invest. Ophthalmol. Vis. Sci 2004, 45, 1897–1903. [Google Scholar]
- Foster, TJ; Hook, M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol 1998, 12, 484–488. [Google Scholar]
- Entenza, JM; Foster, TJ; Ni Eidhin, D; Vaudaux, P; Francioli, P; Moreillon, P. Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect. Immun 2000, 68, 5443–5446. [Google Scholar]
- Tristan, A; Ying, L; Bes, M; Etienne, J; Vandenesch, F; Lina, G. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J. Clin. Microbiol 2003, 41, 4465–4467. [Google Scholar]
- Dinges, MM; Orwin, PM; Schlievert, PM. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev 2000, 13, 16–34. [Google Scholar]
- Peacock, SJ; Moore, CE; Justice, A; Kantzanou, M; Story, L; Mackie, K; O’Neill, G; Day, NPJ. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun 2002, 70, 4987–4996. [Google Scholar]
- Prevost, G; Cribier, B; Couppie, P. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun 1995, 63, 4121–4129. [Google Scholar]
- Lina, G; Piemont, Y; Godail-Gamot, F; Bes, M; Peter, MO; Gauduchon, V; Vandenesch, F; Etienne, J. Involvement of panton-valentine leukocidin–producing staphylococcus aureus in primary skin infections. Clin. Infect. Dis 1999, 29, 1128–1132. [Google Scholar]
- Sadikot, RT; Blackwell, TS; Christman, JW; Prince, AS. Pathogen–host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med 2005, 171, 1209–1223. [Google Scholar]
- Smith, L; Rose, B; Tingpej, P; Zhu, H; Conibear, T; Manos, J; Bye, P; Elkins, M; Willcox, M; Bell, S; Wainwright, C; Harbour, C. Protease IV production in Pseudomonas aeruginosa from the lungs of adults with cystic fibrosis. J. Med. Microbiol 2006, 55, 1641–1644. [Google Scholar]
- Lanotte, P; Watt, S; Mereghetti, L; Dartiguelongue, N; Rastegar-Lari, A; Goudeau, A; Quentin, R. Genetic features of Pseudomonas aeruginosa isolates from cystic fibrosis patients compared with those of isolates from other origins. J. Med. Microbiolog 2004, 53, 73–81. [Google Scholar]
- Ramsey, DM; Wozniak, DJ. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol 2005, 56, 309–322. [Google Scholar]
- Cotar, AI; Dinu, S; Chifiriuc, MC; Banu, O; Iordache, C; Larion, C; Bucur, M; Dracea, O; Lazar, V. Screening of molecular markers of quorum sensing in Pseudomonas aeruginosa strains isolated from clinical infections. Rom. Biotehnol. Lett 2008, 13, 3765–3770. [Google Scholar]
- Aslantas, O; Demir, C; Turutoglu, H; Cantekin, Z; Ergun, Y; Dogruer, G. Coagulase gene polymorphism of Staphylococcus aureus isolated from subclinical bovine mastitis. Turk. J. Vet. Anim. Sci 2007, 31, 253–257. [Google Scholar]
- Mehrotra, M; Wang, G; Johnson, WM. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol 2000, 38, 1032–1035. [Google Scholar]
| No. | S. aureus strain | Source of isolation | Lecithinase | Lipase | Amylase | DN-ase | Caseinase | β-hemolysin | Camp factor |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10936 | Bloodstream culture | ++ | - | - | ++ | +++ | - | + |
| 2 | 11372 | Bloodstream culture | ++ | +++ | - | +++ | +++ | ++ | + |
| 3 | 11327 | Bloodstream culture | ++ | ++ | - | +++ | +++ | - | + |
| 4 | MRSA 11325 | Bloodstream culture | ++ | +++ | - | +++ | +++ | ++ | + |
| 5 | MRSA 11573 | Bloodstream culture | - | - | - | +++ | +++ | - | + |
| 6 | MRSA 11047 | Bloodstream culture | + | - | - | +++ | +++ | - | + |
| 7 | 5/06 | Bloodstream culture | - | - | - | +++ | ++ | - | + |
| 8 | 9/06 | Bloodstream culture | +± | ++ | - | +± | +++ | + | + |
| 9 | 11323 | Bloodstream culture | ++ | +++ | - | +++ | ++ | ++ | + |
| No. | Ps. aeruginosa strain | Source of isolation | Lecithinase | Lipase | Amylase | DN-ase | Caseinase | Hemolysins |
|---|---|---|---|---|---|---|---|---|
| 1 | 101 | Tracheo-bronchic secretion | - | ++ | ++ | - | - | - |
| 2 | 1558 | Tracheo-bronchic secretion | - | - | + | - | - | - |
| 3 | 111 | Wound secretion | - | + | + | - | ++ | ++ |
| 4 | 1443 | Wound secretion | + | - | ++ | - | +++ | ++ |
| 5 | 1093 | Tracheo-bronchic secretion | ++ | - | ++ | - | +++ | |
| 6 | 1561 | Wound secretion | ++ | + | + | - | +++ | ++ |
| 7 | 20 | Sputum | + | + | ++ | - | ++ | ++ |
| 8 | 1442 | Tracheo-bronchic secretion | ++ | ++ | + | _ | ++ | ++ |
| 9 | 84 | Tracheo-bronchic secretion | ++ | + | - | - | ++ | - |
| 10 | 1562 | Wound secretion | - | + | - | - | ++ | + |
| The gene | Primer | Nucleotide sequence | Amplicon size (bp) | Multiplex PCR |
|---|---|---|---|---|
| bbp | BBP-1 BBP-2 | 5′-AACTACATCTAGTACTCAACAACAG-3′ 5′-ATGTGCTTGAATAACACCATCATCT-3′ | 575 | 1 |
| ebpS | EBP-1 EBP-2 | 5′-CATCCAGAACCAATCGAAGAC-3′ 5′-CTTAACAGTTACATCATCATGTTTATCTTTG-3′ | 186 | 1 |
| fnbB | FNBB-1 FNBB-2 | 5′-GTAACAGCTAATGGTCGAATTGATACT-3′ 5′-CAAGTTCGATAGGAGTACTATGTTC-3′ | 524 | 2 |
| fib | FIB-1 FIB-2 | 5′-CTACAACTACAATTGCCGTCAACAG-3′ 5′-GCTCTTGTAAGACCATTTTCTTCAC-3′ | 404 | 2 |
| clfA | CLFA-1 CLFA-2 | 5′-ATTGGCGTGGCTTCAGTGCT-3′ 5′-CGTTTCTTCCGTAGTTGCATTTG-3′ | 292 | 2 |
| clfB | CLFB-1 CLFB-2 | 5′-ACATCAGTAATAGTAGGGGGCAAC-3′ 5′-TTCGCACTGTTTGTGTTTGCAC-3′ | 205 | 2 |
| The gene | Primer | Nucleotide sequence | Amplicon size (bp) |
|---|---|---|---|
| fnbA | forward reverse | 5′-CACAACCAGCAAATATAG-3′ 5′-CTGTGTGGTAATCAATGTC-3′ | 1362 |
| cna | forward reverse | 5′-AGTGGTTACTAATACTG-3′ 5′-CAGGATAGATTGGTTTA-3′. | Multiple of 560 bp |
| The gene | Primer | Nucleotide sequence | Amplicon size (bp) |
|---|---|---|---|
| luk-PV | luk-PV-1 luk-PV-2 | 5′-ATCATTAGGTAAAATGTCTGGACATGATCCA-3′ 5′-GCATCAASTGTATTGGATAGCAAAAGC-3′ | 433 |
| hlg | hlg-1 hlg-2 | 5′-GCCAATCCGTTATTAGAAAATGC-3′ 5′-CCATAGACGTAGCAACGGAT-3′ | 937 |
| The gene | Primer | Nucleotide sequence | Amplicon size (bp) |
|---|---|---|---|
| sea | GSEAR-1 GSEAR-2 | 5′-GGTTATCAATGTGCGGGTGG-3′ 5′-CGGCACTTTTTTCTCTTCGG-3′ | 102 |
| seb | GSEBR-1 GSEBR-2 | 5′-GTATGGTGGTGTAACTGAGC-3′ 5′-CCAAATAGTGACGAGTTAGG-3′ | 164 |
| sec | GSECR-1 GSECR-2 | 5′-AGATGAAGTAGTTGATGTGTATGG-3′ 5′-CACACTTTTAGAATCAACCG-3′ | 451 |
| sed | GSEDR-1 GSEDR-2 | 5′-CCAATAATAGGAGAAAATAAAAG-3′ 5′-ATTGGTATTTTTTTTCGTTC-3′ | 278 |
| see | GSEER-1 GSEER-2 | 5′-AGGTTTTTTCACAGGTCATCC-3′ 5′-CTTTTTTTTCTTCGGTCAATC-3′ | 209 |
| The gene | Primer | Nucleotide sequence | Amplicon size (bp) |
|---|---|---|---|
| lasB | forward reverse | 5′-TTCTACCCGAAGGACTGATAC-3′ 5′-AACACCCATGATCGCAAC-3′ | 153 |
| aprA | forward reverse | 5′-ACCCTGTCCTATTCGTTCC-3′ 5′-GATTGCAGCGACAACTTGG-3′ | 140 |
| rhlAB | forward reverse | 5′-TCATGGAATTGTCACAACCGC-3′ 5′-ATACGGCAAAATCATGGCAAC-3′ | 151 |
| The gene | Primer | Nucleotide sequence | Amplicon size (bp) |
|---|---|---|---|
| algD | forward reverse | 5′-ATGCGAATCAGCATCTTTGGT-3′ 5′-CTACCAGCAGATGCCCTCGGC-3′ | 1310 |
| plcH | forward reverse | 5′-GAAGCCATGGGCTACTTCAA-3′ 5′-AGAGTGACGAGGAGCGGTAG-3′ | 307 |
| plcN | forward reverse | 5′-GTTATCGCAACCAGCCCTAC-3′ 5′-AGGTCGAACACCTGGAACAC-3′ | 466 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cotar, A.-I.; Chifiriuc, M.-C.; Dinu, S.; Bucur, M.; Iordache, C.; Banu, O.; Dracea, O.; Larion, C.; Lazar, V. Screening of Molecular Virulence Markers in Staphylococcus aureus and Pseudomonas aeruginosa Strains Isolated from Clinical Infections. Int. J. Mol. Sci. 2010, 11, 5273-5291. https://doi.org/10.3390/ijms11125273
Cotar A-I, Chifiriuc M-C, Dinu S, Bucur M, Iordache C, Banu O, Dracea O, Larion C, Lazar V. Screening of Molecular Virulence Markers in Staphylococcus aureus and Pseudomonas aeruginosa Strains Isolated from Clinical Infections. International Journal of Molecular Sciences. 2010; 11(12):5273-5291. https://doi.org/10.3390/ijms11125273
Chicago/Turabian StyleCotar, Ani-Ioana, Mariana-Carmen Chifiriuc, Sorin Dinu, Marcela Bucur, Carmen Iordache, Otilia Banu, Olguta Dracea, Cristina Larion, and Veronica Lazar. 2010. "Screening of Molecular Virulence Markers in Staphylococcus aureus and Pseudomonas aeruginosa Strains Isolated from Clinical Infections" International Journal of Molecular Sciences 11, no. 12: 5273-5291. https://doi.org/10.3390/ijms11125273
APA StyleCotar, A.-I., Chifiriuc, M.-C., Dinu, S., Bucur, M., Iordache, C., Banu, O., Dracea, O., Larion, C., & Lazar, V. (2010). Screening of Molecular Virulence Markers in Staphylococcus aureus and Pseudomonas aeruginosa Strains Isolated from Clinical Infections. International Journal of Molecular Sciences, 11(12), 5273-5291. https://doi.org/10.3390/ijms11125273







