Flexible Two-Dimensional Square-Grid Coordination Polymers: Structures and Functions
Abstract
:1. Introduction
2. Gas Adsorptivity of Elastic Layer-Structured Metal-Organic Frameworks (ELMs)
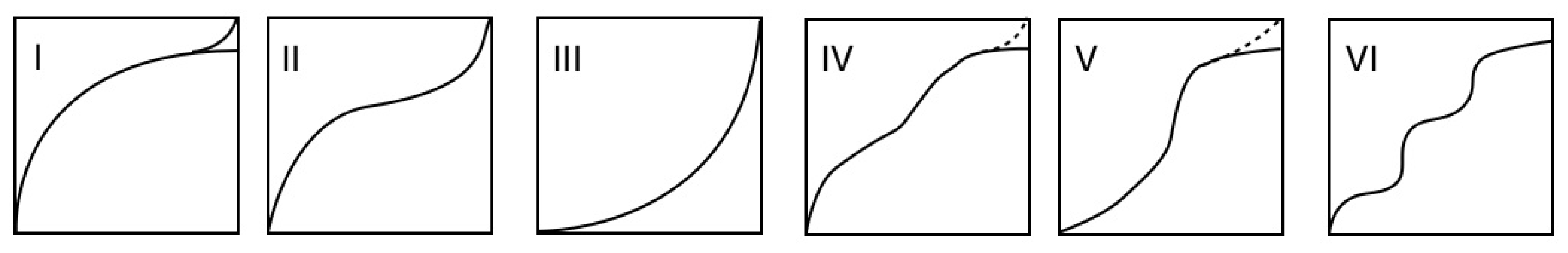
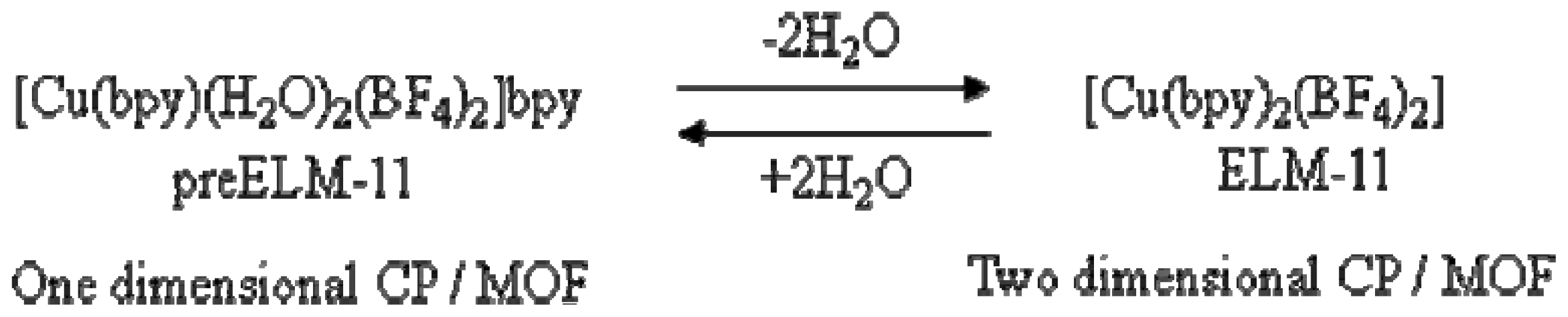
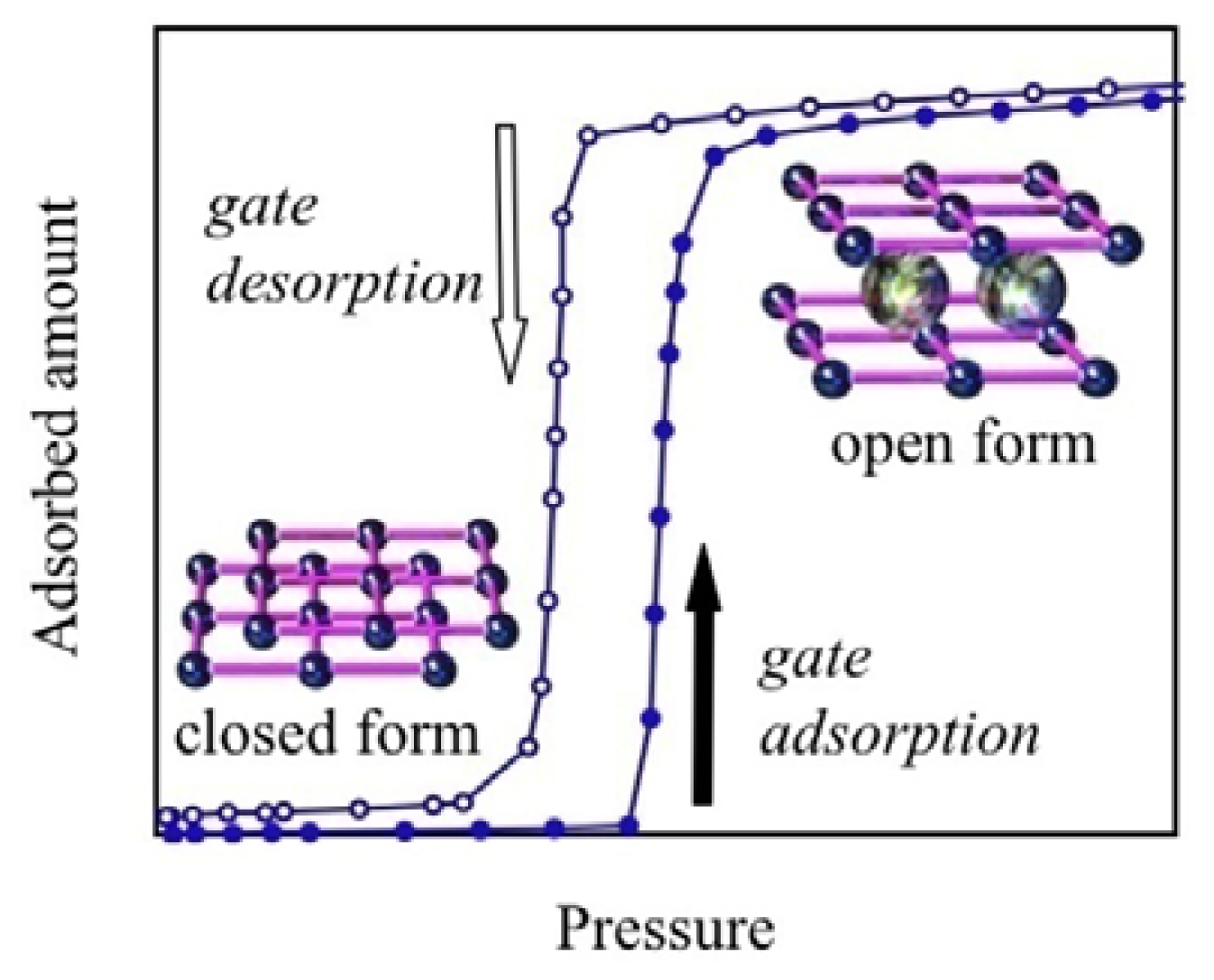
2.1. Discovery of Gate Phenomena of Coordination Polymer
2.2. Structure of Two-Dimensional Layer-Stacking Coordination Polymer
3. Elastic Layer-Structured Metal-Organic Frameworks (ELMs)
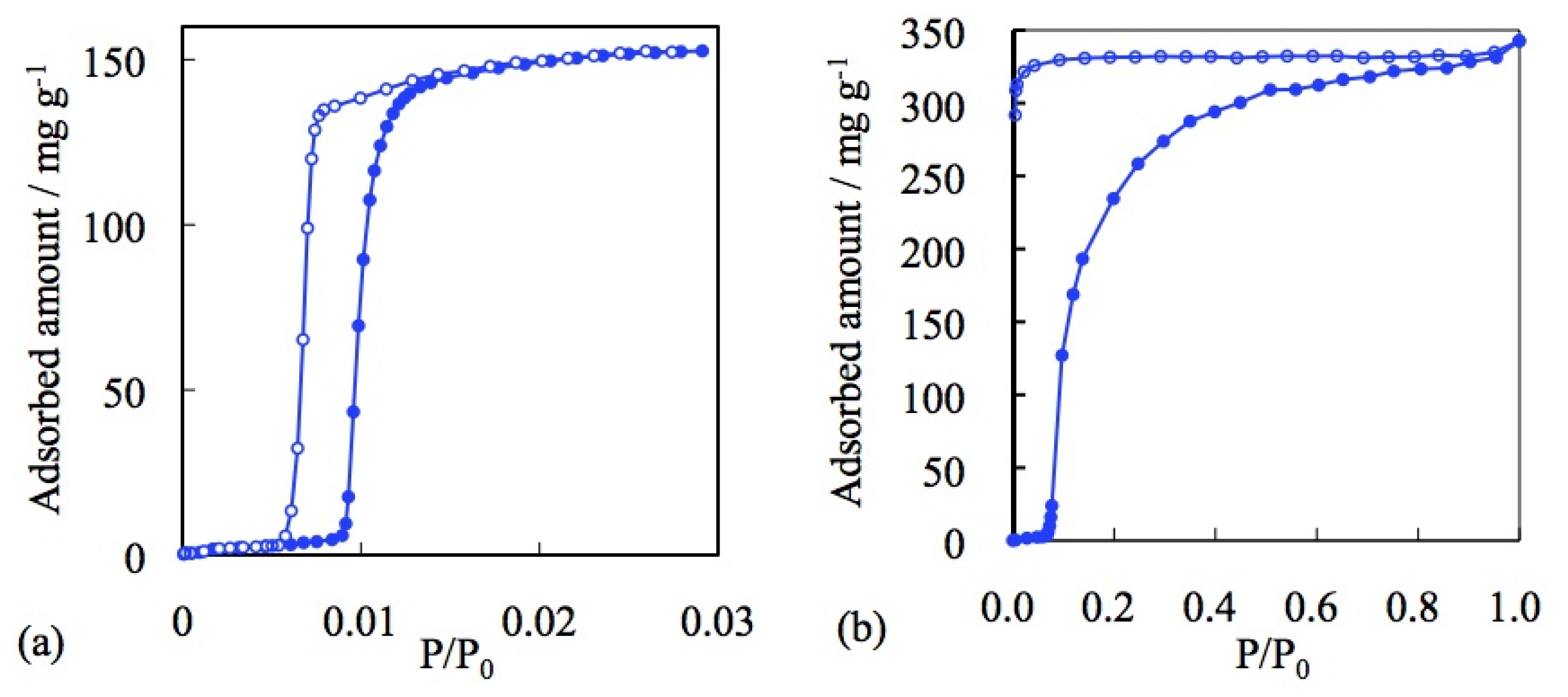
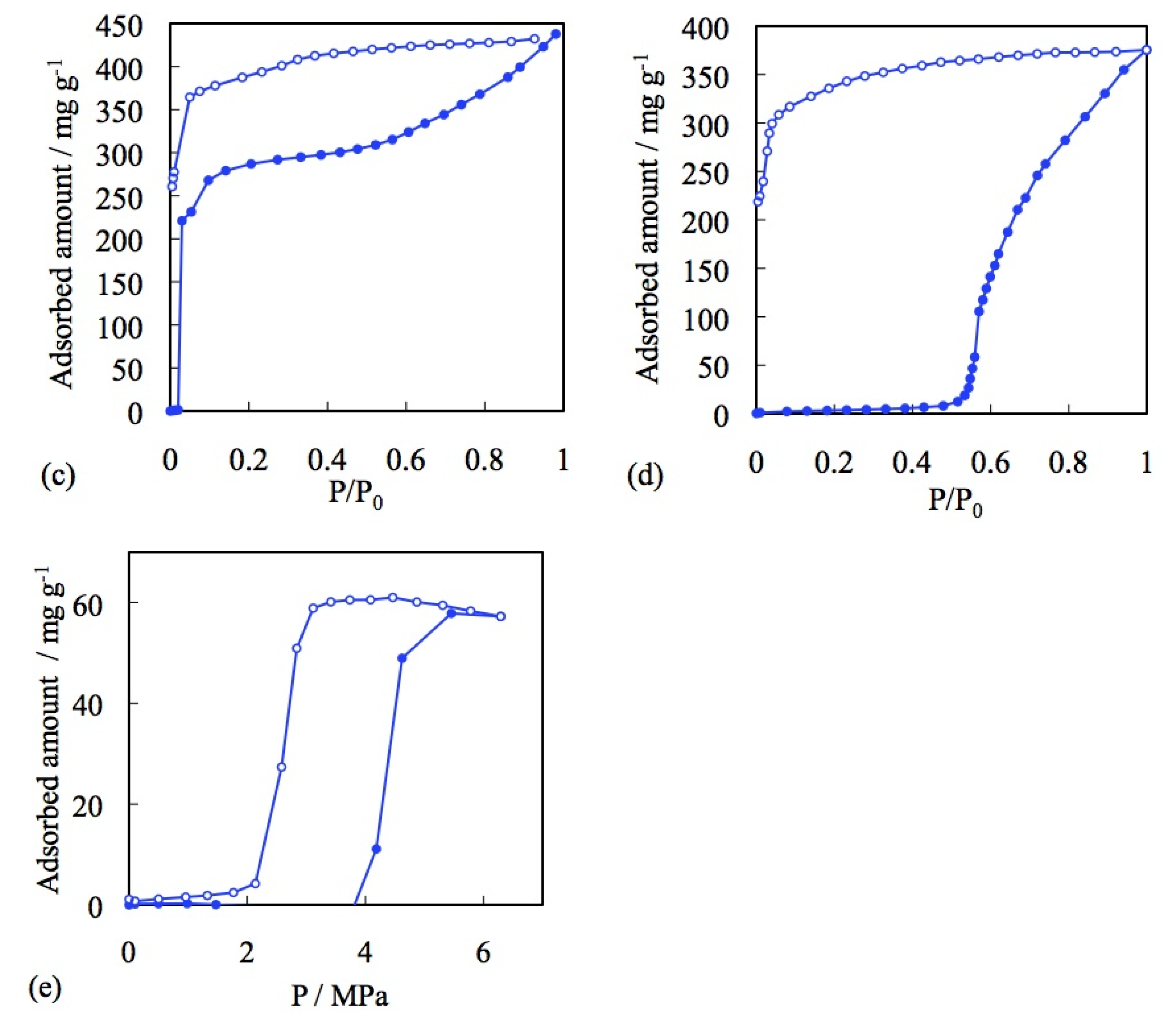
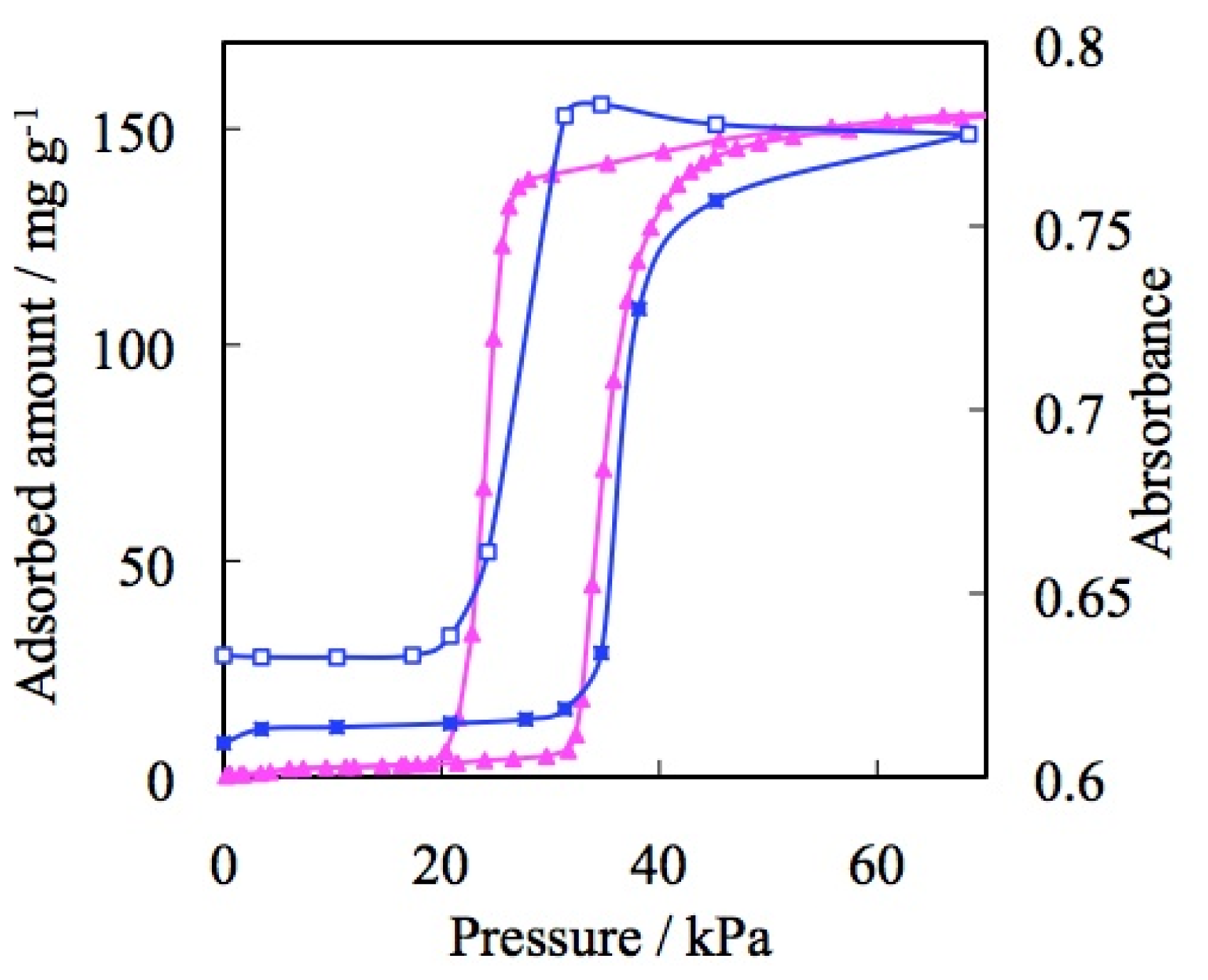
3.1. Role of the Counter Ions
3.2. Effect of Metal Cations
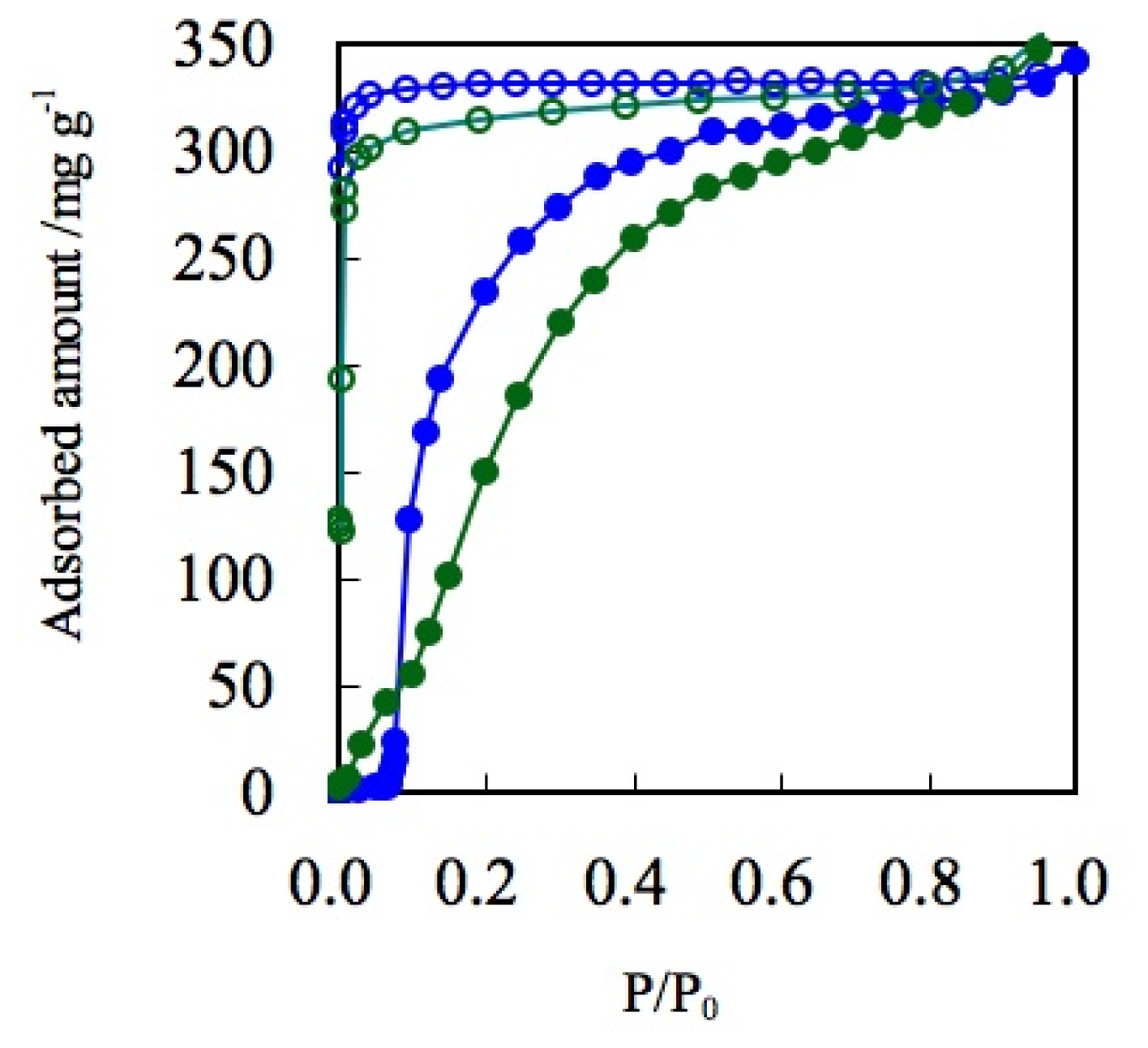
3.3. Hydrogen Adsorption
3.4. Effect of Ligand
4. Various Two-Dimensional Square Grid Stacking-Type (2DSG) CPs/MOFs
4.1. Two-Dimensional Square Grid Stacking-Type CPs/MOFs: Structure and Functions
4.2. Two-Dimensional Square Grid (2DSG) CPs/MOFs with Various Ligands Other Than bpy
4.3. The Gate Phenomena of ELMs
5. Evaluation of the Adsorptivity of ELM-11 from the Standpoint of Practical Application
5.1. Applicability of Gate Phenomena for the Energy-Saving Pressure Swing Adsorption Process
5.2. Carbon Dioxide Gas Selectivity of ELM-11
5.3. Adsorption Kinetics
5.4. Molding of the Powder of ELM-11
5.5. Temperature Elevation with the Gate Adsorption of CH4 on ELM-11
5.6. Stability of ELM-11
6. Catalytic Reaction of bpy Containing Two-Dimensional Layer PCPs/MOFs
7. Conclusions
Acknowledgements
References
- Nicolaou, KC; Sorensen, EJ. Classics in Total Synthesis: Targets, Atrategies, Methods; VCH Publishers Inc: New York, NY, USA, 1996. [Google Scholar]
- Larock, RC. Comprehensive Organic Transformations: A Guide to Functional Group Preparations, 2nd ed; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Tsuji, J. Transition Metal Reagents and Catalysts: Innovations in Organic Synthesis; John Wiley & Sons: West Sussex, UK, 2000. [Google Scholar]
- Lehn, J-M. Supramolecular chemistry-Scope and perspectives molecules, supermolecules, and molecular devices (nobel lecture). Angew. Chem. Int. Ed 1988, 27, 89–112. [Google Scholar]
- Lehn, J-M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Ruben, M; Rojo, J; Romero-Salguero, FJ; Uppadine, LH; Lehn, J-M. Grid-type metal ion architectures: Functional metallosupramolecular arrays. Angew. Chem. Int. Ed 2004, 43, 3644–3662. [Google Scholar]
- Rebek, JJ. Simultaneous encapsulation: Molecules held at close range. Angew. Chem. Int. Ed 2005, 44, 2068–2078. [Google Scholar]
- Steed, JW; Atwood, JL. Supramolecular Chemistry, 2nd ed; Jhon Wiley & Sons, Inc: West Sussex, UK, 2009. [Google Scholar]
- Roesky, HW; Andruh, M. The interplay of coordinative, hydrogen bonding and π-π stacking interactions in sustaining supramolecular solid-state architectures.: A study case of bis(4- pyridyl)- and bis(4-pyridyl-N-oxide) tectons. Coord. Chem. Rev 2003, 236, 91–119. [Google Scholar]
- Rao, CNR; Natarajan, S; Vaidhyanathan, R. Metal carboxylates with open architectures. Angew. Chem. Int. Ed 2004, 43, 1466–1496. [Google Scholar]
- Kitaura, R; Onoyama, G; Sakamoto, H; Matsuda, R; Noro, S-I; Kitagawa, S. Immobilization of a metallo schiff base into a microporous coordination polymer. Angew. Chem. Int. Ed 2004, 43, 2684–2687. [Google Scholar]
- Férey, G; Mellot-Draznieks, C; Serre, C; Millange, F; Dutour, J; Surblé, S; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar]
- Maspoch, D; Ruiz-Molina, D; Veciana, J. Old materials with new tricks: Multifunctional open-framework materials. Chem. Soc. Rev 2007, 36, 770–818. [Google Scholar]
- Kitagawa, S; Matsuda, R. Chemistry of coordination space of porous coordination polymers. Coord. Chem. Rev 2007, 251, 2490–2509. [Google Scholar]
- Banerjee, R; Phan, A; Wang, B; Knobler, C; Furukawa, H; O’Keeffe, M; Yaghi, OM. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar]
- Imaz, I; Hernando, J; Ruiz-Molina, D; Maspoch, D. Metal-organic spheres as functional systems for guest encapsulation. Angew. Chem. Int. Ed 2009, 48, 2325–2329. [Google Scholar]
- Roy, X; MacLachlan, MJ. Coordination chemistry: New routes to mesostructured materials. Chem. Eur. J 2009, 15, 6552–6559. [Google Scholar]
- O’Keeffe, M. Design of MOFs and intellectual content in reticular chemistry: A personal view. Chem. Soc. Rev 2009, 38, 1215–1217. [Google Scholar]
- Spokoyny, AM; Kim, D; Sumrein, A; Mirkin, CA. Infinite coordination polymer nano-and microparticle structures. Chem. Soc. Rev 2009, 38, 1218–1227. [Google Scholar]
- Wang, Z; Cohen, SM. Postsynthetic modification of metal-organic frameworks. Chem. Soc. Rev 2009, 38, 1315–1329. [Google Scholar]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev 2009, 38, 1353–1379. [Google Scholar]
- Zacher, D; Shekhah, O; Wöll, C; Fischer, RA. Thin films of metal-organic frameworks. Chem. Soc. Rev 2009, 38, 1418–1429. [Google Scholar]
- Yamada, T; Kitagawa, H. Protection and deprotection approach for the introduction of functional groups into metal-organic frameworks. J. Am. Chem. Soc 2009, 131, 6312–6313. [Google Scholar]
- Britt, D; Tranchemontagne, D; Yaghi, OM. Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar]
- Czaja, AU; Trukhan, N; Müller, U. Industrial applications of metal-organic frameworks. Chem. Soc. Rev 2009, 38, 1284–1293. [Google Scholar]
- Murray, LJ; Dincá, M; Long, JR. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev 2009, 38, 1294–1314. [Google Scholar]
- Uchida, S; Kawamoto, R; Tagami, H; Nakagawa, Y; Mizuno, N. Highly selective sorption of small unsaturated hydrocarbons by nonporous flexible framework with silver ion. J. Am. Chem. Soc 2008, 130, 12370–12376. [Google Scholar]
- Li, JR; Kuppler, RJ; Zhou, H-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev 2009, 38, 1477–1504. [Google Scholar]
- Lamia, N; Jorge, M; Granato, MA; Almeida Paz, FA; Chevreau, H; Rodrigues, AE. Adsorption of propane, propylene and isobutane on a metal-organic framework: Molecular simulation and experiment. Chem. Eng. Sci 2009, 64, 3246–3259. [Google Scholar]
- Cychosz, KA; Wong-Foy, AG; Matzger, AJ. Enabling cleaner fuels: Desulfurization by adsorption to microporous coordination polymers. J. Am. Chem. Soc 2009, 131, 14538–14543. [Google Scholar]
- Ohmura, T; Mori, W; Hiraga, H; Ono, M; Nishimoto, Y. Magnetic and gas-occasion properties and catalytic activity of microporous materials: Dinuclear ruthenium (II, III) dicarboxylates. Chem. Lett 2003, 32, 468–469. [Google Scholar]
- Zou, R-Q; Sakurai, H; Han, S; Zhong, R-Q; Xu, Q. Probing the lewis acid sites and co catalytic oxidation activity of the porous metal-organic polymer [Cu(5-methylisophthalate)]. J. Am. Chem. Soc 2007, 129, 8402–8403. [Google Scholar]
- Stone, MT; Moore, JS. Supramolecular chelation based on folding. J. Am. Chem. Soc 2005, 127, 5928–5935. [Google Scholar]
- Llabrés Xamena, F; Abad, A; Corma, A; Garcia, H. MOFs as catalysts: Activity, reusability and shape-selectivity of a Pd-containing MOF. J. Catal 2007, 250, 294–298. [Google Scholar]
- Horike, S; Dincá, M; Tamaki, K; Long, JR. Size-selective lewis acid catalysis in a microporous metal-organic framework with exposed Mn2+ coordination sites. J. Am. Chem. Soc 2008, 130, 5854–5855. [Google Scholar]
- Gascon, J; Aktay, U; Hernandez-Alonso, MD; van Klink, GPM; Kapteijn, F. Amino-based metal-organic frameworks as stable, highly active basic catalysts. J. Catal 2009, 261, 75–87. [Google Scholar]
- Qiu, L-G; Gu, L-N; Hu, G; Zhang, L-D. Synthesis, structural characterization and selectively catalytic properties of metal-organic frameworks with nano-sized channels: A modular design strategy. J. Solid State Chem 2009, 182, 502–508. [Google Scholar]
- Ma, L; Abney, C; Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev 2009, 38, 1248–1256. [Google Scholar]
- Cheon, YE; Suh, MP. Enhanced hydrogen storage by palladium nanoparticles fabricated in a redox-active metal-organic framework. Angew. Chem. Int. Ed 2009, 48, 2899–2903. [Google Scholar]
- Bernini, MC; Gándara, F; Iglesias, M; Snejko, N; Gutiérrez-Puebla, E; Brusau, EV; Narda, GE; Monge, MÁ. Reversible breaking and forming of metal-ligand coordination bonds: Temperature-triggered single-crystal to single-crystal transformation in a metal-organic framework. Chem. Eur. J 2009, 15, 4896–4905. [Google Scholar]
- Doonan, CJ; Morris, W; Furukawa, H; Yaghi, OM. Isoreticular metalation of metal-organic frameworks. J. Am. Chem. Soc 2009, 131, 9492–9493. [Google Scholar]
- Allendorf, MD; Bauer, CA; Bhakta, RK; Houk, RJT. Luminescent metal–organic frameworks. Chem. Soc. Rev 2009, 38, 1330–1352. [Google Scholar]
- Eddaoudi, M; Kim, J; Rosi, N; Vodak, D; Wachter, J; O’Keeffe, M; Yaghi, OM. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar]
- Koh, K; Wong-Foy, AG; Matzger, AJ. A crystalline mesoporous coordination copolymer with high microporosity. Angew. Chem. Int. Ed 2008, 47, 677–680. [Google Scholar]
- Mäkinen, SK; Melcer, NJ; Parvez, M; Shimizu, GKH. Highly selective guest uptake in a silver sulfonate network imparted by a tetragonal to triclinic shift in the solid state. Chem. Eur. J 2001, 7, 5176–5182. [Google Scholar]
- Takamizawa, S; Nakata, E-I; Yokoyama, H; Mochizuki, K; Mori, W. Carbon dioxide inclusion phases of a transformable 1D coordination polymer host [Rh2(O2CPh)4(pyz)]n. Angew. Chem. Int. Ed 2003, 42, 4331–4334. [Google Scholar]
- Ohmori, O; Kawano, M; Fujita, M. Crystal-to-crystal guest exchange of large organic molecules within a 3D coordination network. J. Am. Chem. Soc 2004, 126, 16292–16293. [Google Scholar]
- Barea, E; Navarro, JAR; Salas, JM; Masciocchi, N; Galli, S; Sironi, A. Mineralomimetic sodalite- and muscovite-type coordination frameworks. Dynamic crystal-to-crystal interconversion processes sensitive to ion pair recognition. J. Am. Chem. Soc 2004, 126, 3014–3015. [Google Scholar]
- Dybtsev, DN; Chun, H; Kim, K. Rigid and flexible: A highly porous metal-organic framework with unusual guest-dependent dynamic behavior. Angew. Chem. Int. Ed 2004, 43, 5033–5036. [Google Scholar]
- Hu, C; Englert, U. Crystal-to-crystal transformation from a chain polymer to a two-dimensional network at low temperatures. Angew. Chem. Int. Ed 2005, 44, 2281–2283. [Google Scholar]
- Kitagawa, S; Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev 2005, 34, 109–119. [Google Scholar]
- Uemura, K; Matsuda, R; Kitagawa, S. Flexible microporous coordination polymers. J. Solid State Chem 2005, 178, 2420–2429. [Google Scholar]
- Bradshaw, D; Claridge, JB; Cussen, EJ; Prior, TJ; Rosseinsky, MJ. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res 2005, 38, 273–282. [Google Scholar]
- Wu, C-D; Lin, W. Highly porous, homochiral metal-organic frameworks: Solvent-exchange-induced single-crystal to single-crystal transformations. Angew. Chem. Int. Ed 2005, 44, 1958–1961. [Google Scholar]
- Chen, C-L; Goforth, AM; Smith, MD; Su, C-Y; zur Loye, H-C. [Co2(ppca)2(H2O)(V4O12)0.5]: A framework material exhibiting reversible shrinkage and expansion through a single-crystal-to-single- crystal transformation involving a change in the cobalt coordination environment. Angew. Chem. Int. Ed 2005, 44, 6673–6677. [Google Scholar]
- Zhang, J-P; Lin, Y-Y; Zhang, W-X; Chen, X-M. Temperature- or guest-induced drastic single-crystal-to-single-crystal transformations of a nanoporous coordination polymer. J. Am. Chem. Soc 2005, 127, 14162–14163. [Google Scholar]
- Maji, TK; Mostafa, G; Matsuda, R; Kitagawa, S. Guest-induced asymmetry in a metal-organic porous solid with reversible single-crystal-to-single-crystal structural transformation. J. Am. Chem. Soc 2005, 127, 17152–17153. [Google Scholar]
- Culp, JT; Smith, MR; Bittner, E; Bockrath, B. Hysteresis in the physisorption of CO2 and N2 in a flexible pillared layer nickel cyanide. J. Am. Chem. Soc 2008, 130, 12427–12434. [Google Scholar]
- Horike, S; Shimomura, S; Kitagawa, S. Soft porous crystals. Nat. Chem 2009, 1, 695–704. [Google Scholar]
- Férey, G; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev 2009, 38, 1380–1399. [Google Scholar]
- Spencer, EC; Angel, RJ; Ross, NL; Hanson, BE; Howard, JAK. Pressure-induced cooperative bond rearrangement in a zinc imidazolate framework: A high-pressure single-crystal X-ray diffraction study. J. Am. Chem. Soc 2009, 131, 4022–4026. [Google Scholar]
- Korčok, JL; Katz, MJ; Leznoff, DB. Impact of metallophilicity on “colossal” positive and negative thermal expansion in a series of isostructural dicyanometallate coordination polymers. J. Am. Chem. Soc 2009, 131, 4866–4871. [Google Scholar]
- Li, D; Kaneko, K. Hydrogen bond-regulated microporous nature of copper complex-assembled microcrystals. Chem. Phys. Lett 2001, 335, 50–56. [Google Scholar]
- Kitaura, R; Seki, K; Akiyama, G; Kitagawa, S. Porous coordination-polymer crystals with gated channels specific for supercritical gases. Angew. Chem. Int. Ed 2003, 42, 428–431. [Google Scholar]
- Fletcher, AJ; Cussen, EJ; Bradshaw, D; Rosseinsky, MJ; Thomas, KM. Adsorption of gases and vapors on nanoporous Ni2(4,4′-bipyridine)3(NO3)4 metal-organic framework materials templated with methanol and ethanol: Structural effects in adsorption kinetics. J. Am. Chem. Soc 2004, 126, 9750–9759. [Google Scholar]
- Mukherjee, PS; Lopez, N; Arif, AM; Cervantes-Lee, F; Noveron, JC. Single-crystal to single-crystal phase transitions of bis(N-phenylisonicotinamide)silver(I) nitrate reveal cooperativity properties in porous molecular materials. Chem. Commun 2007, 1433–1435. [Google Scholar]
- Kondo, A; Noguchi, H; Carlucci, L; Proserpio, DM; Ciani, G; Kajiro, H; Ohba, T; Kanoh, H; Kaneko, K. Double-step gas sorption of a two-dimensional metal-organic framework. J. Am. Chem. Soc 2007, 129, 12362–12363. [Google Scholar]
- Uemura, K; Yamasaki, Y; Komagawa, Y; Tanaka, K; Kita, H. Two-step adsorption/desorption on a jungle-gym-type porous coordination polymer. Angew. Chem. Int. Ed 2007, 46, 6662–6665. [Google Scholar]
- Zhang, J-P; Chen, X-M. Exceptional framework flexibility and sorption behavior of a multifunctional porous cuprous triazolate framework. J. Am. Chem. Soc 2008, 130, 6010–6017. [Google Scholar]
- Ma, S; Sun, D; Yuan, D; Wang, X-S; Zhou, H-C. Preparation and gas adsorption studies of three mesh-adjustable molecular sieves with a common structure. J. Am. Chem. Soc 2009, 131, 6445–6451. [Google Scholar]
- Soldatov, DV; Ripmeester, JA. Studies in Surface Science and Catalysis: Nanoporous Materials III; Flexible Metal-Organic Frameworks with Isomerizing Building Unit; Elsevier: Oxford, UK, 2002; Volume 141, pp. 353–362. [Google Scholar]
- Dewa, T; Endo, K; Aoyama, Y. Dynamic aspects of lattice inclusion complexation involving a phase change. Equilibrium, kinetics, and energetics of guest-binding to a hydrogen-bonded flexible organic network. J. Am. Chem. Soc 1998, 120, 8933–8940. [Google Scholar]
- Sawaki, T; Aoyama, Y. Immobilization of a soluble metal complex in an organic network. Remarkable catalytic performance of a porous dialkoxyzirconium polyphenoxide as a functional organic zeolite analogue. J. Am. Chem. Soc 1999, 121, 4793–4798. [Google Scholar]
- Endo, K; Ezuhara, T; Koyanagi, M; Masuda, H; Aoyama, Y. Functional self-assembly of hydrogen-bonded networks. Construction of aromatic stacks and columns and cavity-size control via flexible intercalation of 1D chains having orthogonal aromatic substituents. J. Am. Chem. Soc 1997, 119, 499–505. [Google Scholar]
- Soldatov, DV. Encyclopedia of Supramolecular Chemistry 2: Soft and Smart Materials; Taylor & Francis: London, UK, 2004; Volume 2, pp. 1302–1306. [Google Scholar]
- Gorbatchuk, VV; Tsifarkin, AG; Antipin, IS; Solomonov, BN; Konovalov, AI; Seidel, J; Baitalov, F. Thermodynamic comparison of molecular recognition of vaporous guests by solid calixarene and diol hosts. J. Chem. Soc. Perkin Trans. 2 2000, 2287–2294. [Google Scholar]
- Soldatov, DV. Soft supramolecular materials. J. Incl. Phenom. Macrocycl. Chem 2004, 48, 3–9. [Google Scholar]
- Gorbatchuk, VV; Savelyeva, LS; Ziganshin, MA; Antipin, LS; Sidoror, VA. Molecular recognition of organic guest vapor by solid adamantylcalix[4]arene. Russ. Chem. Bull., Int. Ed 2004, 53, 60–65. [Google Scholar]
- IUPAC Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem 1985, 57, 603–619.
- Kondo, M; Yoshitomi, T; Seki, K; Matsuzaka, H; Kitagawa, S. Three-dimensional framework with channeling cavities for small molecules: {[M2(4,4′-bpy)3(NO3)4]·xH2O}n (M = Co, Ni, Zn). Angew. Chem., Int. Ed. Engl 1997, 36, 1725–1727. [Google Scholar]
- Li, H; Eddaoudi, M; Groy, TL; Yaghi, OM. Establishing microporosity in open metal-organic frameworks: Gas sorption isotherms for Zn(bdc) (bdc = 1,4-benzenedicarboxylate). J. Am. Chem. Soc 1998, 120, 8571–8572. [Google Scholar]
- Li, H; Eddaoudi, M; O’Keeffe, M; Yaghi, OM. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Kondo, M; Okubo, T; Asami, A; Noro, S-I; Yoshitomi, T; Kitagawa, S; Ishii, T; Matsuzaka, H; Seki, K. Rational synthesis of stable channel-like cavities with methane gas adsorption properties: [{Cu2(pzdc)2(L)}n] (pzdc = pyrazine-2,3-dicarboxylate; L = a pillar ligand). Angew. Chem. Int. Ed 1999, 38, 140–143. [Google Scholar]
- Chui, SS-Y; Lo, SM-F; Charmant, JPH; Orpen, AG; Williams, D. A chemically functionalizable nanopoous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar]
- Noro, S-I; Kitagawa, S; Kondo, M; Seki, K. A new, methane adsorbent, porous coordination polymer [{CuSiF6(4,4′-bipyridine)2}n]. Angew. Chem. Int. Ed 2000, 39, 2081–2084. [Google Scholar]
- Seki, K; Takamizawa, S; Mori, W. Characterization of microporus copper(II) dicarboxylates (fumarate, terephthalate and trans-1,4-cyclohexanedicarboxylate) by gas adsorption. Chem. Lett 2001, 122–123. [Google Scholar]
- Eddaoudi, M; Moler, DB; Li, H; Chen, B; Reineke, TM; O’Keeffe, M; Yaghi, OM. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res 2001, 34, 319–330. [Google Scholar]
- Seki, K. Design of an adsorbent with an ideal pore structure for methane adsorption using metal complexes. Chem. Commun 2001, 1496–1497. [Google Scholar]
- Fletcher, AJ; Cussen, EJ; Prior, TJ; Rosseinsky, MJ; Kepert, CJ; Thomas, KM. Adsorption dynamics of gases and vapors on the nanoporous metal organic framework material Ni2(4,4′-bipyridine)3(NO3)4: Guest modification of host sorption behavior. J. Am. Chem. Soc 2001, 123, 10001–10011. [Google Scholar]
- Mori, W. Syntheses and magnetic susceptibilities of copper(II) dicarboxylate complexes having gas occlusion properties. 30th Annual meeting of chemical society of Japan, Higashiosaka, Japan, April, 1974; 4D05, p. 424.
- Mori, W. Magnetic property of copper phthalate. 26th Annual Meeting of Chemical Society of Japan, Tokyo, Japan, April 1972; 2D26, p. 365.
- Mori, W; Kobayasih, TC; Kurobe, J; Amaya, K; Narumi, Y; Kumada, T; Kindo, K; Katori, HA; Goto, T; Miura, N; Takamizawa, S; Ayama, H; Yamaguchi, K. Magnetic properties of oxygen physisorbed in Cu-trans-1,4-cyclohexanedicarboxylic acid. Mol. Cryst. Liq. Cryst 1997, 306, 1–7. [Google Scholar]
- Mori, W; Inoue, F; Yoshida, K; Takamizawa SKishita, M. Synthesis of new adsobent copper(II) terephthalate. Chem. Lett 1997, 1219–1220. [Google Scholar]
- Onishi, S; Ohmori, T; Ohkubo, T; Noguchi, H; Di, L; Hanzawa, Y; Kanoh, H; Kaneko, K. Hydrogen-bond change-associated gas adsorption in inorganic-organic hybrid microporous crystals. Appl. Surf. Sci 2002, 196, 81–88. [Google Scholar]
- Noguchi, H; Kondo, A; Hattori, Y; Kajiro, H; Kanoh, H; Kaneko, K. Evaluation of an effective gas storage amount of latent nanoporous Cu-based metal-organic framework. J. Phys. Chem. C 2007, 111, 248–254. [Google Scholar]
- Noguchi, H; Kondoh, A; Hattori, Y; Kanoh, H; Kajiro, H; Kaneko, K. Clathrate-formation mediated adsorption of methane on Cu-complex crystals. J. Phys. Chem. B 2005, 109, 13851–13853. [Google Scholar]
- Blake, AJ; Hill, SJ; Hubberstey, P; Li, WS. Rectangular grid two-dimensional sheets of copper(II) bridged by both co-ordinated and hydrogen bonded 4,4′-bipyridine (4,4′-bipy) in [Cu(μ-4,4′-bipy)(H2O)2(FBF3)2]·4,4′-bipy. J. Chem. Soc. Dalton Trans 1997, 913–914. [Google Scholar]
- Cheng, Y; Kondo, A; Noguchi, H; Kajiro, H; Urita, K; Ohba, T; Kaneko, K; Kanoh, H. Reversible structural change of Cu-MOF on exposure to water and its CO2 adsorptivity. Langmuir 2009, 25, 4510–4513. [Google Scholar]
- Kondo, A; Noguchi, H; Ohnishi, S; Kajiro, H; Tohdoh, A; Hattori, Y; Xu, W-C; Tanaka, H; Kanoh, H; Kaneko, K. Novel expansion/shrinkage modulation of 2D layered MOF triggered by clathrate formation with CO2 molecules. Nano Lett 2006, 6, 2581–2584. [Google Scholar]
- Rosenthal, MR. The myth of the non-coordinating anion. J. Chem. Educ 1973, 50, 331–335. [Google Scholar]
- Byington, AR; Bull, WE. Trifluoromethanesulfonato complexes of nickel and cobalt. Inorg. Chim. Acta 1977, 21, 239–244. [Google Scholar]
- Carlucci, L; Ciani, G; Proserpio, DM; Rizzato, S. Interlinked molecular squares with [Cu(2,2′-bipy)]2+ corners generating a three-dimensional network of unprecedented topological type. Chem. Commun 2001, 1198–1199. [Google Scholar]
- Bertelli, M; Carlucci, L; Ciani, G; Proserpio, DM; Sironi, A. Structural studies of molecular-based nanoporous materials. Novel networks of silver(I) cations assembled with the polydentate N-donor bases hexamethylenetetramine and 1,3,5-triazine. J. Mater. Chem 1997, 7, 1271–1276. [Google Scholar]
- Wu, HP; Janiak, C; Rheinwald, G; Lang, H. 5,5′-Dicyano-2,2′-bipyridine silver complexes: Discrete units or co-ordination polymers through a chelating and/or bridging metal-ligand interaction. J. Chem. Soc. Dalton Trans 1999, 183–190. [Google Scholar]
- Dong, Y-B; Smith, MD; zur Loye, H-C. Metal-containing ligands for mixed-metal polymers: Novel Cu(II)-Ag(I) Mixed-Metal Coordination Polymers Generated from [Cu(2-methylpyrazine- 5-carboxylate)2(H2O)]·3H2O and Silver(I) Salts. Inorg. Chem 2000, 39, 1943–1949. [Google Scholar]
- Blake, AJ; Champness, NR; Cooke, PA; Nicolson, JEB; Wilson, C. Multi-modal bridging ligands; effects of ligand functionality, anion and crystallisation solvent in silver(I) co-ordination polymers. J. Chem. Soc. Dalton Trans 2000, 3811–3819. [Google Scholar]
- Alberti, G; Murcia-Mascarós, S; Vivani, R. Pillared derivatives of γ-zirconium phosphate containing nonrigid alkyl chain pillars. J. Am. Chem. Soc 1998, 120, 9291–9295. [Google Scholar]
- Alberti, G; Brunet, E; Dionigi, C; Juanes, O; Mata, MJDL; Rodríguez-Ubis, JC; Vivani, R. Shaping solid-state supramolecular cavities: Chemically induced accordionlike movement of γ-zirconium phosphate containing polyethylenoxide pillars. Angew. Chem. Int. Ed 1999, 38, 3351–3353. [Google Scholar]
- Tambach, TJ; Bolhuis, PG; Smit, B. A molecular mechanism of hysteresis in clay swelling. Angew. Chem., Int. Ed 2004, 43, 2649–2652. [Google Scholar]
- Ohba, T; Inaguma, Y; Kondo, A; Kanoh, H; Nugichi, H; Gubbins, KE; Kajiro, H; Kaneko, K. GCMC simulations of dynamic structural change of Cu–organic crystals with nadsorption. J. Exp. Nanosci 2006, 1, 91–95. [Google Scholar]
- Maspoch, D; Ruiz-Molina, D; Wurst, K; Domingo, N; Cavallini, M; Biscarini, F; Tejada, J; Rovira, C; Veciana, J. A nanoporous molecular magnet with reversible solvent-induced mechanical and magnetic properties. Nat. Mater 2003, 2, 190–195. [Google Scholar]
- Choi, HJ; Suh, MP. Dynamic and redox active pillared bilayer open framework: Single-crystal-to- single-crystal transformations upon guest removal, guest exchange, and framework oxidation. J. Am. Chem. Soc 2004, 126, 15844–15851. [Google Scholar]
- Kondo, A; Chinen, A; Kajiro, H; Nakagawa, T; Kato, K; Takata, M; Hattori, Y; Okino, F; Ohba, T; Kaneko, K; Kanoh, H. Metal-ion-dependent gas sorptivity of elastic layer-structured MOFs. Chem. Eur. J 2009, 15, 7549–7553. [Google Scholar]
- Venkataraman, D; Lee, S; Moore, JS; Zhang, P; Hirsch, KA; Gardner, GB; Covey, AC; Prentice, CL. Coordination networks based on multitopic ligands and silver(I) salts: A study of network connectivity and topology as a function of counterion. Chem. Mater 1996, 8, 2030–2040. [Google Scholar]
- Munakata, M; Wu, LP; Kuroda-Sowa, T; Maekawa, M; Moriwaki, K; Kitagawa, S. Two types of new polymeric copper(I) complexes of pyrazinecarboxamide having channel and helical structures. Inorg. Chem 1997, 36, 5416–5418. [Google Scholar]
- Khlobystov, AN; Blake, AJ; Champness, NR; Lemenovskii, DA; Majouga, AG; Zyk, NV; Schröder, M. Supramolecular design of one-dimensional coordination polymers based on silver(I) complexes of aromatic nitrogen-donor ligands. Coord. Chem. Rev 2001, 222, 155–192. [Google Scholar]
- Suh, MP; Ko, JW; Choi, HJ. A metal-organic bilayer open framework with a dynamic component: Single-crystal-to-single-crystal transformations. J. Am. Chem. Soc 2002, 124, 10976–10977. [Google Scholar]
- Jung, O-S; Kim, YJ; Lee, Y-A; Park, K-M; Lee, SS. Subtle role of polyatomic anions in molecular construction: Structures and properties of AgX bearing 2,4′-thiobis(pyridine) (X− = NO3−, BF4−, ClO4−, PF6−, CF3CO2−, and CF3SO3−). Inorg. Chem 2003, 42, 844–850. [Google Scholar]
- Maji, TK; Matsuda, R; Kitagawa, S. A flexible interpenetrating coordination framework with a bimodal porous functionality. Nat. Mater 2007, 6, 142–148. [Google Scholar]
- Dong, Y-B; Jiang, Y-Y; Li, J; Ma, J-P; Liu, F-L; Tang, B; Huang, R-Q; Batten, SR. Temperature-dependent synthesis of metal-organic frameworks based on a flexible tetradentate ligand with bidirectional coordination donors. J. Am. Chem. Soc 2007, 129, 4520–4521. [Google Scholar]
- Cussen, EJ; Claridge, JB; Rosseinsky, MJ; Kepert, CJ. Flexible sorption and transformation behavior in a microporous metal-organic framework. J. Am. Chem. Soc 2002, 124, 9574–9581. [Google Scholar]
- Noro, S-I; Kitaura, R; Kondo, M; Kitagawa, S; Ishii, T; Matsuzaka, H; Yamashita, M. Framework engineering by anions and porous functionalities of Cu(II)/4,4′-bpy coordination polymers. J. Am. Chem. Soc 2002, 124, 2568–2583. [Google Scholar]
- Huang, Y-Q; Ding, B; Song, H-B; Zhao, B; Ren, P; Cheng, P; Wang, H-G; Liao, D-Z; Yan, S-P. A novel 3D porous metal–organic framework based on trinuclear cadmium clusters as a promising luminescent material exhibiting tunable emissions between UV and visible wavelengths. Chem. Commun 2006, 47, 4906–4908. [Google Scholar]
- Hobza, P; Zahradnik, R. Intermolecular Complexes: The Role of van der Waals Systems in Physical Chemistry and in the Biodisciplines; Elsevier: Oxford, UK, 1988. [Google Scholar]
- Dincá, M; Long, JR. Hydrogen storage in microporous metal-organic frameworks with exposed metal sites. Angew. Chem. Int. Ed 2008, 47, 6766–6779. [Google Scholar]
- Vitillo, JG; Regli, L; Chavan, S; Ricchiardi, G; Spoto, G; Dietzel, PDC; Bordiga, S; Zecchina, A. Role of exposed metal sites in hydrogen storage in MOFs. J. Am. Chem. Soc 2008, 130, 8386–8396. [Google Scholar]
- Nouar, F; Eckert, J; Eubank, JF; Forster, P; Eddaoudi, M. Zeolite-like metal-organic frameworks (ZMOFs) as hydrogen storage platform: Lithium and magnesium ion-exchange and H2-(rho-ZMOF) interaction studies. J. Am. Chem. Soc 2009, 131, 2864–2870. [Google Scholar]
- Hamon, L; Serre, C; Devic, T; Loiseau, T; Millange, F; Férey, G; Weireld, GD. Comparative study of hydrogen sulfide adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal-Organic Frameworks at Room Temperature. J. Am. Chem. Soc 2009, 131, 8775–8777. [Google Scholar]
- Dincá, M; Long, JR. High-enthalpy hydrogen adsorption in cation-exchanged variants of the microporous metal-organic framework Mn3[(Mn4Cl)3(BTT)8(CH3OH)10]2. J. Am. Chem. Soc 2007, 129, 11172–11176. [Google Scholar]
- Caskey, SR; Wong-Foy, AG; Matzger, AJ. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc 2008, 130, 10870–10871. [Google Scholar]
- Zhou, W; Wu, H; Yildirim, T. Enhanced H2 adsorption in isostructural metal-organic frameworks with open metal sites: Strong dependence of the binding strength on metal ions. J. Am. Chem. Soc 2008, 130, 15268–15269. [Google Scholar]
- Wu, H; Zhou, W; Yildirim, T. High-capacity methane storage in metal-organic frameworks M(dhtp): The important role of open metal sites. J. Am. Chem. Soc 2009, 131, 4995–5000. [Google Scholar]
- Serre, C; Millange, F; Thouvenot, C; Noguès, M; Marsolier, G; Louër, D; Férey, G. Very large breathing effect in the first nanoporous cromium(III)-based solids: MIL-53 or Cr(III)(OH)·{O2C-C6H4-CO2}·{HO2C-C6H4-CO2H}x·H2Oy. J. Am. Chem. Soc 2002, 124, 13519–13526. [Google Scholar]
- Férey, G; Latroche, M; Serre, C; Millange, F; Loiseau, T; Percheron-Guégan, A. Hydrogen adsorption in the nanoporous metal-benzenedicarboxylate M(OH)(O2C-C6H4-CO2) (M = Al3+, Cr3+), MIL-53. Chem. Commun 2003, 2976–2977. [Google Scholar]
- Bourrelly, S; Llewellyn, PL; Serre, C; Millange, F; Loiseau, T; Férey, G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc 2005, 127, 13519–13521. [Google Scholar]
- Ramsahye, NA; Maurin, G; Bourrelly, S; Llewellyn, PL; Devic, T; Serre, C; Loiseau, T; Férey, G. Adsorption of CO2 in metal organic frameworks of different metal centres: Grand canonical monte carlo simulations compared to experiments. Adsorption 2007, 13, 461–467. [Google Scholar]
- Buckingham, AD; Disch, RL; Dunmur, DA. Quadrupole moments of some simple molecules. J. Am. Chem. Soc 1968, 90, 3104–3107. [Google Scholar]
- Wang, K; Do, DD. Characterizing the micropore size distribution of activated carbon using equilibrium data of many adsorbates at various temperatures. Langmuir 1997, 13, 6226–6233. [Google Scholar]
- Bojan, MJ; Steele, WA. Interactions of diatomic molecules with graphite. Langmuir 1987, 3, 1123–1127. [Google Scholar]
- Garciía-Pérez, E; Gascón, J; Morales-Flórez, V; Castillo, JM; Kapteijn, F; Calero, S. Identification of adsorption sites in Cu-btc by experimentation and molecular simulation. Langmuir 2009, 25, 1725–1731. [Google Scholar]
- Jiang, Y; Huang, J; Kasumaj, B; Jeschke, G; Hunger, M; Mallat, T; Baiker, A. Adsorption-desorption induced structural changes of Cu-MOF evidenced by solid state NMR and EPR spectroscopy. J. Am. Chem. Soc 2009, 131, 2058–2059. [Google Scholar]
- Kaneko, K; Shimizu, K; Suzuki, T. Intrapore field-dependent micropore filling of supercritical N2 in slit-shaped micropores. J. Chem. Phys 1992, 97, 8705–8711. [Google Scholar]
- Yaghi, OM; O’Keeffe, M; Ockwig, NW; Chae, HK; Eddaoudi, M; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar]
- Lin, X; Jia, J; Zhao, X; Thomas, KM; Blake, AJ; Walker, GS; Champness, NR; Hubberstey, P; Schröder, M. High H2 adsorption by coordination-framework materials. Angew. Chem. Int. Ed 2006, 45, 7358–7364. [Google Scholar]
- Collins, DJ; Zhou, H-C. Hydrogen storage in metal-organic frameworks. J. Mater. Chem 2007, 17, 3154–3160. [Google Scholar]
- Wang, B; Côte, AP; Furukawa, H; O’Keeffe, M; Yaghi, OM. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 2008, 453, 207–211. [Google Scholar]
- Wang, X-S; Ma, S; Rauch, K; Simmons, JM; Yuan, D; Wang, X; Yildirim, T; Cole, WC; López, JJ; Meijere, AD; Zhou, H-C. Metal-organic frameworks based on double-bond-coupled di-isophthalate linkers with high hydrogen and methane uptakes. Chem. Mater 2008, 20, 3145–3152. [Google Scholar]
- Cavka, JH; Jakobsen, S; Olsbye, U; Guillou, N; Lamberti, C; Bordiga, S; Lillerud, KP. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc 2008, 130, 13850–13851. [Google Scholar]
- Banerjee, R; Furukawa, H; Britt, D; Knobler, C; O’Keeffe, M; Yaghi, OM. Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties. J. Am. Chem. Soc 2009, 131, 3875–3877. [Google Scholar]
- Yang, S; Lin, X; Dailly, A; Blake, AJ; Hubberstey, P; Champness, NR; Schröder, M. Enhancement of H2 adsorption in coordination framework materials by use of ligand curvature. Chem. Eur. J 2009, 15, 4829–4835. [Google Scholar]
- Steel, PJ. Aromatic nitrogen heterocycles as bridging ligands; a survey. Coord. Chem. Rev 1990, 106, 227–265. [Google Scholar]
- Biradha, K; Fujita, M. A ‘three-in-one’ crystal of coordination networks. Chem. Commun 2002, 8, 1866–1867. [Google Scholar]
- Biradha, K; Sarkar, M; Rajput, L. Crystal engineering of coordination polymers using 4,4- bipyridine as a bond between transition metal atoms. Chem. Commun 2006, 38, 4169–4179. [Google Scholar]
- Noro, S-I; Kitagawa, S; Akutagawa, T; Nakamura, T. Coordination polymers constructed from transition metal ions and organic N-containing heterocyclic ligands: Crystal structures and microporous properties. Prog. Polym. Sci 2009, 34, 240–279. [Google Scholar]
- Moulton, B; Zaworotko, MJ. From molecules to crystal engineering: Supramolecular isomerism and polymorphism in network solids. Chem. Rev 2001, 101, 1629–1658. [Google Scholar]
- Haynes, JS; Rettig, SJ; Sams, JR; Thompson, RC; Trotter, J. Structure and magnetic exchange in poly-bis(pyrazine)bis(methanesulfonato-o)-copper(II). One-dimensional exchange in a two-dimensional polymer. Can. J. Chem 1987, 65, 420–426. [Google Scholar]
- Real, JA; De Munno, G; Muñoz, MC; Julve, M. Crystal structure and magnetic properties of bis(isothiocyanato)bis(pyrazine)iron polymer, a 2D sheetlike polymer. Inorg. Chem 1991, 30, 2701–2704. [Google Scholar]
- Tong, ML; Chen, XM; Yu, XL; Mak, TCW. A novel two-dimensional rectangular network. Synthesis and structure of {[Cu(4,4′-bpy)(pyz)(H2O)2][PF6]2}n (4,4′-bpy = 4,4′-bipyridine, pyz = pyrazine). J. Chem. Soc. Dalton Trans 1998, 2, 5–6. [Google Scholar]
- Kondo, M; Shimamura, M; Noro, S-I; Minakoshi, S; Asami, A; Seki, K; Kitagawa, S. Microporous materials constructed from the interpenetrated coordination networks. Structures and methane adsorption properties. Chem. Mater 2000, 12, 1288–1299. [Google Scholar]
- Biradha, K; Hongo, Y; Fujita, M. Crystal-to-crystal sliding of 2D coordination layers triggered by guest exchange. Angew. Chem. Int. Ed 2002, 41, 3395–3398. [Google Scholar]
- Zaman, MB; Udachin, K; Ripmeester, JA; Smith, MD; zur Loye, H-C. Synthesis and characterization of diverse coordination polymers. Linear and zigzag chains involving their structural transformation via intermolecular hydrogen-bonded, interpenetrating ladders polycatenane, and noninterpenetrating square grid from long, rigid N,N′, -bidentate ligands: 1,4- bis[(X-pyridyl)ethynyl]benzene (X = 3 and 4). Inorg. Chem 2005, 44, 5047–5059. [Google Scholar]
- Carlucci, L; Ciani, G; Proserpio, DM. Three-dimensional architectures of intertwined planar coordination polymers: The first case of interpenetration involving two different bidimensional polymeric motifs. New J. Chem 1998, 22, 1319–1321. [Google Scholar]
- Maji, TK; Ohba, M; Kitagawa, S. Transformation from a 2D stacked layer to 3D interpenetrated framework by changing the spacer functionality: Synthesis, structure, adsorption, and magnetic properties. Inorg. Chem 2005, 44, 9225–9231. [Google Scholar]
- Du, M; Jiang, X-J; Zhao, X-J. Direction of unusual mixed-ligand metal–organic frameworks: A new type of 3-D polythreading involving 1-D and 2-D structural motifs and a 2-fold interpenetrating porous network. Chem. Commun 2005, 11, 5521–5523. [Google Scholar]
- Domasevitch, KV; Gural’skiy, IYA; Solntsev, PV; Rusanov, EB; Krautscheid, H; Howard, JA; Chernega, AN. 4,4-Bipyridazine: A new twist for the synthesis of coordination polymers. Dalton Trans 2007, 29, 3140–3148. [Google Scholar]
- Real, JA; André, E; Muñoz, MC; Julve, M; Granier, T; Bousseksou, A; Varret, F. Spin crossover in a catenane supramolecular system. Science 1995, 268, 265–267. [Google Scholar]
- Zhou, J-S; Cai, J; Wang, L; Ng, S-W. Reversible and selective amine interactions of [Cd(μ2- N,O-p-NH2C6H4SO3)2(H2O)2]n. Dalton Trans 2004, 9, 1493–1497. [Google Scholar]
- Uemura, K; Kitagawa, S; Fukui, K; Saito, K. A contrivance for a dynamic porous framework: Cooperative guest adsorption based on square grids connected by amide-amide hydrogen bonds. J. Am. Chem. Soc 2004, 126, 3817–3828. [Google Scholar]
- Takaoka, K; Kawano, M; Tominaga, M; Fujita, M. In situ observation of a reversible single-crystal- to-single-crystal apical-ligand-exchange reaction in a hydrogen-bonded 2D coordination network. Angew. Chem. Int. Ed 2005, 44, 2151–2154. [Google Scholar]
- Custelcean, R; Gorbunova, MG. A metal-organic framework functionalized with free carboxylic acid sites and its selective binding of a Cl(H2O)4− cluster. J. Am. Chem. Soc 2005, 127, 16362–16363. [Google Scholar]
- Davidson, GJE; Loeb, SJ. Channels and cavities lined with interlocked components: Metal-based polyrotaxanes that utilize pyridinium axles and crown ether wheels as ligands. Angew. Chem. Int. Ed 2003, 42, 74–77. [Google Scholar]
- Pschirer, NG; Ciurtin, DM; Smith, MD; Bunz, UHF; zur Loye, H-C. Noninterpenetrating square-grid coordination polymers with dimensions of 25 × 25 Å2 prepared by using N,N′-type ligands: The first chiral square-grid coordination polymer. Angew. Chem. Int. Ed 2002, 41, 583–585. [Google Scholar]
- Hagrman, D; Hammond, RP; Haushalter, R; Zubieta, J. Organic/inorganic composite materials: Hydrothermal syntheses and structures of the one-, two-, and three-dimensional copper(II) sulfate-organodiamine phases [Cu(H2O)3(4,4′-bipyridine)(SO4)]·2H2O, [Cu(bpe)2][Cu(bpe)(H2O)2(SO4)2]·2H2O, and [Cu(bpe)(H2O)(SO4)] (bpe = trans-1,2-Bis(4- pyridyl)ethylene). Chem. Mater 1998, 10, 2091–2100. [Google Scholar]
- Withersby, MA; Blake, AJ; Champness, NR; Cooke, PA; Hubberstey, P; Realf, AL; Teat, SJ; Schröder, M. Engineering of co-ordination polymers of trans-4,4′-azobis(pyridine) and trans-1,2-bis(pyridin-4-yl)ethene: A range of interpenetrated network motifs. J. Chem. Soc., Dalton Trans 2000, 3261–3268. [Google Scholar]
- Zaworotko, MJ. Superstructural diversity in two dimensions: Crystal engineering of laminated solids. Chem. Commun 2001, 1–9. [Google Scholar]
- Evans, OR; Lin, W. Crystal engineering of NLO materials based on metal-organic coordination networks. Acc. Chem. Res 2002, 35, 511–522. [Google Scholar]
- Pothiraja, R; Sathiyendiran, M; Butcher, RJ; Murugavel, R. Cobalt and manganese nets via their wires: Facile transformation in metal-diorganophosphates. Inorg. Chem 2004, 43, 7585–7587. [Google Scholar]
- Pothiraja, R; Sathiyendiran, M; Butcher, RJ; Murugavel, R. Non-interpenetrating transition metal diorganophosphate 2-dimensional rectangular grids from their 1-dimensional wires: Structural transformations under mild conditions. Inorg. Chem 2005, 44, 6314–6323. [Google Scholar]
- Lu, J; Paliwala, T; Lim, SC; Yu, C; Niu, T; Jacobson, AJ. Coordination polymers of Co(NCS)2 with pyrazine and 4,4′-bipyridine: Syntheses and structures. Inorg. Chem 1997, 36, 923–929. [Google Scholar]
- Reichert, WM; Holbrey, JD; Vigour, KB; Morgan, TD; Broker, GA; Rogers, RD. Approaches to crystallization from ionic liquids: Complex solvents–complex results, or, a strategy for controlled formation of new supramolecular architectures? Chem. Commun 2006, 46, 4767–4779. [Google Scholar]
- Lu, J; Li, H-F; Xiao, F-X; Cao, R. A new lamellar solid trapping water clusters and intercalated organosulfonate guests. Inorg. Chem. Commun 2007, 10, 614–617. [Google Scholar]
- Felloni, M; Blake, AJ; Champness, NR; Hubberstey, P; Wilson, C; Schröder, M. Supramolecular interactions in 4,4′-bipyridine cobalt(II) nitrate networks. J. Supramol. Chem 2002, 2, 163–174. [Google Scholar]
- Fu, R; Hu, S; Wu, X. Syntheses, structures, and properties of five coordination polymers containing fluorescent whitener. Inorg. Chem 2007, 46, 9630–9640. [Google Scholar]
- Biradha, K; Domasevitch, KV; Hogg, C; Moulton, B; Power, KN; Zaworotko, MJ. Interpenetrating covalent and noncovalent nets in the crystal structures of [M(4,4′-Bipyridine)2(NO3)2]3C10H8 (M = Co, Ni). Cryst. Eng 1999, 2, 37–45. [Google Scholar]
- Moulton, B; Rather, EB; Zaworotko, MJ. Interpenetration of covalent and noncovalent networks in the crystal structures of {[M(4,4′-bipyridine)2(NO3)2]·2p-nitroaniline}n where M=Co, 1, Ni, 2, Zn, 3. Cryst. Eng 2001, 4, 309–317. [Google Scholar]
- Biradha, K; Mondai, A; Moulton, B; Zaworotko, MJ. Coexisting covalent and non-covalent planar networks in the crystal structures of {[M(bipy)2(NO3)2]·arene} (M = Ni, 1; Co, 2; arene = chlorobenzene, o-dichlorobenzene, benzene, nitrobenzene, toluene or anisole). J. Chem. Soc. Dalton Trans 2000, 3837–3844. [Google Scholar]
- Biradha, K; Domasevitch, KV; Moulton, B; Seward, C; Zaworotko, MJ. Covalent and noncovalent interpenetrating planar networks in the crystal structure of {[Ni(4,4′-bipyridine)2(NO3)2·2pyrene}n. Chem. Commun 1999, 1327–1328. [Google Scholar]
- Zhang, Y; Jianmin, L; Nishiura, M; Imamoto, T. Spectral and structural properties of 2D network complex [Ni(4,4′-bipyridine)2(NCS)2]n. J. Mol. Struct 2000, 519, 219–224. [Google Scholar]
- Zhang, Y; Jianmin, L; Wei, D; Nishihara, M; Imamoto, T. The most effective packing of layers: Synthesis and structure of [Ni(4,4′-bipyridine)2(NCS)2]n. Chem. Lett 1999, 195–196. [Google Scholar]
- Jiang, Y-C; Lai, Y-C; Wang, S-L; Lii, K-H. [Ni(4,4′-bpy)2(H2PO4)2]·C4H9OH·H2O: A novel metal phosphate that exhibits interpenetration of 2D net into 3D framework. Inorg. Chem 2001, 40, 5320–5321. [Google Scholar]
- Kanoh, H; Kondo, A; Noguchi, H; Kajiro, H; Tohdoh, A; Hattori, Y; Xu, W-C; Inoue, M; Sugiura, T; Morita, K; Tanaka, H; Ohba, T; Kaneko, K. Elastic layer-structured metal organic frameworks (ELMs). J. Colloid Interface Sci 2009, 334, 1–7. [Google Scholar]
- Du, M; Chen, S-T; Bu, X-H; Ribas, J. Crystal structure and properties of a Cu(II) coordination polymer with 2-D grid-like host architecture for the inclusion of organic guest molecule. Inorg. Chem. Commun 2002, 5, 1003–1006. [Google Scholar]
- Jiana, Z; Zhao-Jia, L; Ye-Yana, Q; Yi-Hanga, W; Yaoa, K; Jian-Kaia, C; Yuan-Gena, Y. Syntheses and characterizations of two copper coordination polymers constructed by 4,4′-bipyridine. Chin. J. Struct. Chem 2004, 23, 1366–1370. [Google Scholar]
- Naumov, P; Jovanovski, G; Hanna, JV; Razak, IA; Chantrapromma, S; Fun, H-K; Ng, SW. Diaquabis(4,4′-bipyridine)copper(II) di(o-sulfobenzimidate) dichloromethane solvate, a two-dimensional Cu4(4,4′-C5H4NC5H4N)4 rhombic grid clathrating guest dichloromethane. Inorg. Chem. Commun 2001, 4, 766–768. [Google Scholar]
- Thuéry, P. Uranyl citrate dimers as guests in a copper-bipyridine framework: A novel heterometallic inorganic–organic hybrid compound. CrystEngComm 2007, 9, 358–360. [Google Scholar]
- Williams, PAM; Ferrer, EG; Baran, EJ. Characterization of a novel Cu(II)/4,4′-bipyridine coordination polymer containing square grids. Z. Anorg. Allg. Chem 2002, 628, 2044–2048. [Google Scholar]
- Niu, C; Wu, B; Zhang, H; Li, Z; Hou, H. Chiral metal-organic and supramolecular interpenetrating 3-D frameworks constructed by one angular ligand and 4,4′-dipyridine. Inorg. Chem. Commun 2008, 11, 377–380. [Google Scholar]
- Tong, M-L; Ye, B-H; Cai, J-W; Chen, X-M; Ng, SW. Clathration of two-dimensional coordination polymers: Synthesis and structures of [M(4,4′-bpy)2(H2O)2](ClO4)2(2,4′-bpy)2·H2O and [Cu(4,4′-bpy)2(H2O)2](ClO4)4·(4,4′-H2bpy) (M = CdII, ZnII and bpy = bipyridine). Inorg. Chem 1998, 37, 2645–2650. [Google Scholar]
- Tong, M-L; Zheng, S-L; Chen, X-M. Synthesis and structures of two-dimensional coordination polymers constructed by metal salts and 4,4′-bipyridine. Polyhedron 2000, 19, 1809–1814. [Google Scholar]
- Lian, Z-X; Cai, J; Chen, C-H. A series of metal-organic frameworks constructed with arenesulfonates and 4,4′-bipy ligands. Polyhedron 2007, 26, 2647–2654. [Google Scholar]
- Mi, L; Hou, H; Song, Z; Han, H; Fan, Y. Polymeric zinc ferrocenyl sulfonate as a molecular aspirator for the removal of toxic metal ions. Chem. Eur. J 2008, 14, 1814–1821. [Google Scholar]
- McManus, GJ; Perry, JJ; Perry, M; Wagner, BD; Zaworotko, MJ. Exciplex fluorescence as a diagnostic probe of structure in coordination polymers of Zn2+ and 4,4′-bipyridine containing intercalated pyrene and enclathrated aromatic solvent guests. J. Am. Chem. Soc 2007, 129, 9094–9101. [Google Scholar]
- Gable, RW; Hoskins, BF; Robson, R. A new type of interpenetration involving enmeshed independent square grid sheets. The structure of diaquabis-(4,4′-bipyridine)zinc hexafluorosilicate. J. Chem. Soc. Chem. Commun 1990, 1677–1678. [Google Scholar]
- Ohmori, O; Fujita, M. Heterogeneous catalysis of a coordination network: Cyanosilylation of imines catalyzed by a Cd(II)-(4,4′-bipyridine) square grid complex. Chem. Commun 2004, 10, 1586–1587. [Google Scholar]
- Aoyagi, M; Biradha, K; Fujita, M. Formation of two, one, and zero-dimensional coordination assemblies from Cd(II) ion and 4,4′-bipyridine. Bull. Chem. Soc. Jpn 2000, 73, 1369–1373. [Google Scholar]
- Fu, Z-Y; Lin, P; Du, W-X; Chen, L; Cui, C-P; Zhang, W-J; Wu, X-T. Two new coordination host frameworks for the inclusion of 4-amino-benzophenone guest molecules. Polyhedron 2001, 20, 1925–1931. [Google Scholar]
- Huang, SD; Lewandowski, BJ; Lro, CC; Shan, Y. [Cd(4,4′-bipy)2(NO3)2](2-nitroaniline)2, a novel two-dimensional lattice inclusioncompound. Acta. Cryst 1999, C55, 2016–2018. [Google Scholar]
- Liu, C-M; Xiong, R-G; You, X-Z; Chen, W. A two-dimensional square network inclusion compound incorporating guest molecules through both hydrogen bonding and nonionic electrostatic attraction. Crystal structure of [Cd(4,4′-bpy)2(H2O)2]·(ClO4)2·1.5(4,4′-bpy)·(C6H4NO3Cl)·H2O. Acta Chem. Scand 1998, 52, 1353–1358. [Google Scholar]
- Huang, SD; Xiong, R-G. Molecular recognition of organic chromosphores by coordination polymers: Design and construction of nonlinear optical supramolecular assemblies. Polyhedron 1997, 16, 3929–3939. [Google Scholar]
- Robson, R; Abrahams, BF; Batten, SR; Gable, RW; Hoskins, BF; Liu, J. Crystal Engineering of Novel Materials Composed of Infinite Two- and Three-Dimensional Frameworks. In Supramolecular Architecture; American Chemical Society: Washington, DC, USA, 1992; pp. 256–273. [Google Scholar]
- Liu, C-M; Xiong, R-G; You, X-Z; Chen, W; Lo, K-M. Molecular recognition of an organic molecule through a two dimensional square network inclusion complex. Synthesis and crystal structure of [Cd(4,4′-bpy)2(H2O)2](BF4)2·2(4,4′-bpy)·(C6H6N2O2)·2H2O. J. Coord. Chem 1998, 46, 211–220. [Google Scholar]
- Yang, E-C; Dai, P-X; Wang, X-G; Ding, B; Zhao, X-J. Three novel mixed-ligand cadmium(II) sulfonates with aza-aromatic skeltons as co-ligands. Z. Anorg. Allg. Chem 2007, 633, 615–620. [Google Scholar]
- Liang, FP; Chen, ZL; Hu, RX; Liang, H; Zhou, ZH. Infinite three-dimensional coordination polymers: Synthesis and structures of [Cd(4,4′-bpy)2(H2O)2]n·(pic)2n, [Zn(4,4′-bpy)2(H2O)2]n·(pic)2n(H2O)2n, and [Zn(4,4′-bpy)2(H2O)2]n·(4,4′-bpy)n(H2O)n(pic)2n. Chin. Chem. Lett 2000, 11, 369–372. [Google Scholar]
- Liang, F-P; Chen, Z-L; Hu, RX; Liang, H; Yu, KB; Zhou, ZH. Syntheses and crystal structures of the complexes formed by zinc and 4,4′-bipyridin. Chin. J. Inorg. Chem 2001, 17, 699–703. [Google Scholar]
- Liang, F-P; Chen, Z-L; Hu, RX; Liang, H; Yu, KB; Zhou, ZH. Syntheses and crystal structures of the complexes of transition metal with 4,4′-bipyridine. Acta Chim. Sinica 2001, 59, 405–412. [Google Scholar]
- Kwon, YJ. Infinite framework material composed of 4,4′-bipyridine: Shapeselective clathration of aromatic guests. J. Korean Fiber Soc 1996, 33, 980–984. [Google Scholar]
- Kitagawa, S; Kondo, M. Functional micropore chemistry of crystalline metal complex-assembled compounds. Bull. Chem. Soc. Jpn 1998, 71, 1739–1753. [Google Scholar]
- Fletcher, AJ; Thomas, KM; Rosseinsky, MJ. Flexibility in metal-organic framework materials: Impact on sorption properties. J. Solid State Chem 2005, 178, 2491–2510. [Google Scholar]
- Hirsch, KA; Venkataraman, D; Wilson, SR; Moore, JS; Lee, S. Crystallization of 4,4′-biphenyldicarbonitrile with silver(I) salts: A change in topology concomitant with a change in counterion leading to a ninefold diamondoid network. J. Chem. Soc. Chem. Commun 1995, 2199–2200. [Google Scholar]
- Hagrman, PJ; Hagrman, D; Zubieta, J. Organic-inorganic hybrid materials: From “simple” coordination polymers to organodiamine-templated molybdenum oxides. Angew. Chem. Int. Ed 1999, 38, 2638–2684. [Google Scholar]
- Maekawa, M; Nabei, A; Tominaga, T; Sugimoto, K; Minematsu, T; Okubo, T; Kuroda-Sowa, T; Munakata, M; Kitagawa, S. A unique chair-shaped hexanuclear Cu(I) metallamacrocyclic C2H4 adduct encapsulating a BF4− anion. Dalton Trans 2009, 415–417. [Google Scholar]
- Kim, H-J; Lee, J-H; Lee, M. Stimuli-responsive gels from reversible coordination polymers. Angew. Chem. Int. Ed 2005, 44, 5810–5814. [Google Scholar]
- Kobayashi, K; Asakawa, Y; Kikuchi, Y; Toi, H; Aoyama, Y. CH-π interaction as an important driving force of host-guest complexation in apolar organic media. Binding of monools and acetylated compounds to resorcinol cyclic tetramer as studied by proton NMR and circular dichroism spectroscopy. J. Am. Chem. Soc 1993, 115, 2648–2654. [Google Scholar]
- Janiak, C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans 2000, 21, 3885–3896. [Google Scholar]
- Tuna, F; Hamblin, J; Jackson, A; Clarkson, G; Alcock, NW; Hannon, MJ. Metallo-supramolecular libraries: Triangles, polymers and double-helicates assembled by copper(I) coordination to directly linked bis-pyridylimine ligands. Dalton Trans 2003, 2141–2148. [Google Scholar]
- Black, CA; Hanton, LR; Spicer, MD. A coordination polymer strategy for anion encapsulation: Anion-π interactions in (4,4) nets formed from Ag(I) salts and a flexible pyrimidine ligand. Chem. Commun 2007, 30, 3171–3173. [Google Scholar]
- Uemura, K; Kumamoto, Y; Kitagawa, S. Zipped-up chain-type coordination polymers: Unsymmetrical amide-containing ligands inducing beta-sheet or helical structures. Chem. Eur. J 2008, 14, 9565–9576. [Google Scholar]
- Shimoni, L; Camell, HL; Clusker, JP; Coombs, MM. Intermolecular effects in crystals of 11-(trifluoromethy1)-15,16-dihydrocyclopenta[α]phenanthren-17-one. J. Am. Chem. Soc 1994, 116, 8162–8168. [Google Scholar]
- Soldatov, DV; Ripmeester, JA; Shergina, SI; Sokolov, IE; Zanina, AS; Gromilov, SA; Dyadin, YA. α- and β-Bis(1,1,1-trifluoro-5,5-dimethyl-5-methoxyacetylacetonato)copper(II): Transforming the dense polymorph into a versatile new microporous framework. J. Am. Chem. Soc 1999, 121, 4179–4188. [Google Scholar]
- Adams, H; Cockroft, SL; Guardigli, C; Hunter, CA; Lawson, KR; Perkins, J; Spey, SE; Urch, CJ; Ford, R. Experimental measurement of noncovalent interactions between halogens and aromatic rings. ChemBioChem 2004, 5, 657–665. [Google Scholar]
- Hof, F; Scofield, DM; Schweizer, WB; Diederich, F. A weak attractive interaction between organic fluorine and an amide group. Angew. Chem. Int. Ed 2004, 43, 5056–5059. [Google Scholar]
- Pan, L; Olson, DH; Ciemnolonski, LR; Heddy, R; Li, J. Separation of hydrocarbons with a microporous metal-organic framework. Angew. Chem. Int. Ed 2006, 45, 616–619. [Google Scholar]
- Sato, S; Iida, J; Suzuki, K; Kawano, M; Ozeki, T; Fujita, M. Fluorous nanodroplets structurally confined in an organopalladium sphere. Science 2006, 313, 1273–1276. [Google Scholar]
- Yang, C; Wang, X; Omary, MA. Crystallographic observation of dynamic gas adsorption sites and thermal expansion in a breathable fluorous metal-organic framework. Angew. Chem. Int. Ed 2009, 48, 2500–2505. [Google Scholar]
- Biradha, K; Fujita, M. Co-ordination polymers containing square grids of dimension 15 × 15 Å. J. Chem. Soc. Dalton Trans 2000, 3805–3810. [Google Scholar]
- Zhao, X; Xiao, B; Fletcher, AJ; Thomas, KM; Bradshaw, D; Rosseinsky, MJ. Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks. Science 2004, 306, 1012–1015. [Google Scholar]
- Uemura, K; Saito, K; Kitagawa, S; Kita, H. Hydrogen-bonded porous coordination polymers: Structural transformation, sorption properties, and particle size from kinetic studies. J. Am. Chem. Soc 2006, 128, 16122–16130. [Google Scholar]
- Tanaka, D; Nakagawa, K; Higuchi, M; Horike, S; Kubota, Y; Kobayashi, TC; Takata, M; Kitagawa, S. Kinetic gate-opening process in a flexible porous coordination polymer. Angew. Chem. Int. Ed 2008, 47, 3914–3918. [Google Scholar]
- Wei, Q; Nieuwenhuyzen, M; Meunier, F; Hardacre, C; James, SL. Guest sorption and desorption in the metal-organic framework [Co(ina)2] (ina = isonicotinate)-evidence of intermediate phases during desorption. Dalton Trans 2004, 1807–1811. [Google Scholar]
- Lee, EY; Jang, SY; Suh, MP. Multifunctionality and crystal dynamics of a highly stable, porous metal-organic framework [Zn4O(ntb)2]. J. Am. Chem. Soc 2005, 127, 6374–6381. [Google Scholar]
- Halder, GJ; Kepert, CJ. In situ single-crystal X-ray diffraction studies of desorption and sorption in a flexible nanoporous molecular framework material. J. Am. Chem. Soc 2005, 127, 7891–7900. [Google Scholar]
- Horike, S; Matsuda, R; Tanaka, D; Matsubara, S; Mizuno, M; Endo, K; Kitagawa, S. Dynamic motion of building blocks in porous coordination polymers. Angew. Chem. Int. Ed 2006, 45, 7226–7230. [Google Scholar]
- Gould, SL; Tranchemontagne, D; Yaghi, OM; Garcia-Garibay, MA. Amphidynamic character of crystalline MOF-5: Rotational dynamics of terephthalate phenylenes in a free-volume, sterically unhindered environment. J. Am. Chem. Soc 2008, 130, 3246–3247. [Google Scholar]
- Seo, J; Matsuda, R; Sakamoto, H; Bonneau, C; Kitagawa, S. A pillared-layer coordination polymer with a rotatable pillar acting as a molecular gate for guest molecules. J. Am. Chem. Soc 2009, 131, 12792–12800. [Google Scholar]
- Ruthven, DM; Farooq, S; Knaebel, KS. Pressure Swing Adsorption; John Wiley & Sons, Inc: New York, NY, USA, 1993. [Google Scholar]
- Kerry, FG. Industrial Gas Handbook: Gas Separation and Purification; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Koshland, DE; Némethy, G; Filmer, D. Comparison of experimental binding sata and theoretical models in proteins containing subunits. Biochemistry (Mosc) 1966, 5, 365–385. [Google Scholar]
- Perutz, MF. Regulation of oxygen affinity of hemoglobin: Influence of structure of the globin on the heme iron. Annu. Rev. Biochem 1979, 48, 327–386. [Google Scholar]
- Perutz, MF; Wilkinson, AJ; Paoli, M; Dodson, GG. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct 1998, 27, 1–34. [Google Scholar]
- Finsy, V; Ma, L; Alaerts, L; De Vos, DE; Baron, GV; Denayer, JFM. Separation of CO2/CH4 mixtures with the MIL-53(Al) metal-organic framework. Microporous Mesoporous Mater 2009, 120, 221–227. [Google Scholar]
- Horike, S; Matsuda, R; Tanaka, D; Mizuno, M; Endo, K; Kitagawa, S. Immobilization of sodiumions on the pore surface of a porous coordination polymer. J. Am. Chem. Soc 2006, 128, 4222–4223. [Google Scholar]
- Dhakshinamoorthy, A; Alvaro, M; Garcia, H. Metal organic frameworks as efficient heterogeneous catalysts for the oxidation of benzylic compounds with t-butylhydroperoxide. J. Catal 2009, 267, 1–4. [Google Scholar]
- Zhang, X; Llabrés i Xamena, FX; Corma, A. Gold(III)-metal organic framework bridges the gap between homogeneous and heterogeneous gold catalysts. J. Catal 2009, 265, 155–160. [Google Scholar]
- Hwang, YK; Hong, D-Y; Chang, J-S; Jhung, SH; Seo, Y-K; Kim, J; Vimont, A; Daturi, M; Serre, C; Férey, G. Amine grafting on coordinatively unsaturated metal centers of MOFs: Consequences for catalysis and metal encapsulation. Angew. Chem. Int. Ed 2008, 47, 4144–4148. [Google Scholar]
- Lee, J; Farha, OK; Roberts, J; Scheidt, KA; Nguyen, ST; Hupp, JT. Metal-organic framework materials as catalysts. Chem. Soc. Rev 2009, 38, 1450–1459. [Google Scholar]
- Shultz, AM; Farha, OK; Hupp, JT; Nguyen, ST. A catalytically active, permanently microporous MOF with metalloporphyrin struts. J. Am. Chem. Soc 2009, 131, 4204–4205. [Google Scholar]
- Ravon, U; Savonnet, M; Aguado, S; Domine, ME; Janneau, E; Farrusseng, D. Engineering of coordination polymers for shape selective alkylation of large aromatics and the role of defects. Microporous Mesoporous Mater 2010, 129, 319–329. [Google Scholar]
- Arai, T; Takasugi, H; Sato, T; Noguchi, H; Kanoh, H; Kaneko, K; Yanagisawa, A. Catalytic synthesis of α-hydroxy ketones using organic–inorganic hybrid polymer. Chem. Lett 2005, 34, 1590–1591. [Google Scholar]
- Arai, T; Sato, T; Noguchi, H; Kanoh, H; Kaneko, K; Yanagisawa, A. Direct α-hydroxylation of ketones catalyzed by organic–inorganic hybrid polymer. Chem. Lett 2006, 35, 1094–10095. [Google Scholar]
- Jiang, D; Mallat, T; Krumeich, F; Baiker, A. Copper-based metal-organic framework for the facile ring-opening of epoxides. J. Catal 2008, 257, 390–395. [Google Scholar]
- Mandel-Gutfreund, Y; Margalit, H; Jernigan, RL; Zhurkin, VB. A role for CH...O interactions in protein-DNA recognition. J. Mol. Biol 1998, 277, 1129–1140. [Google Scholar]
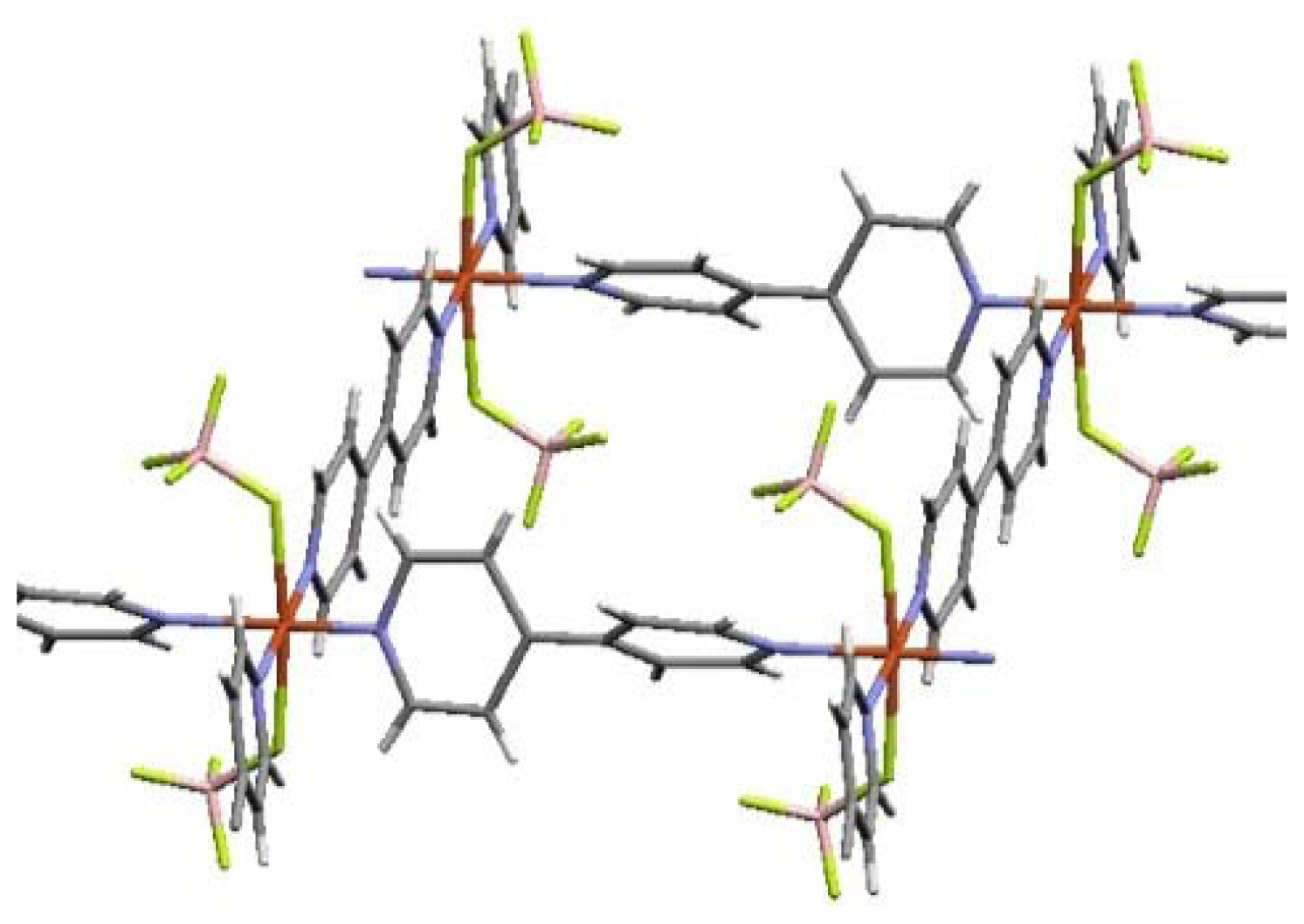



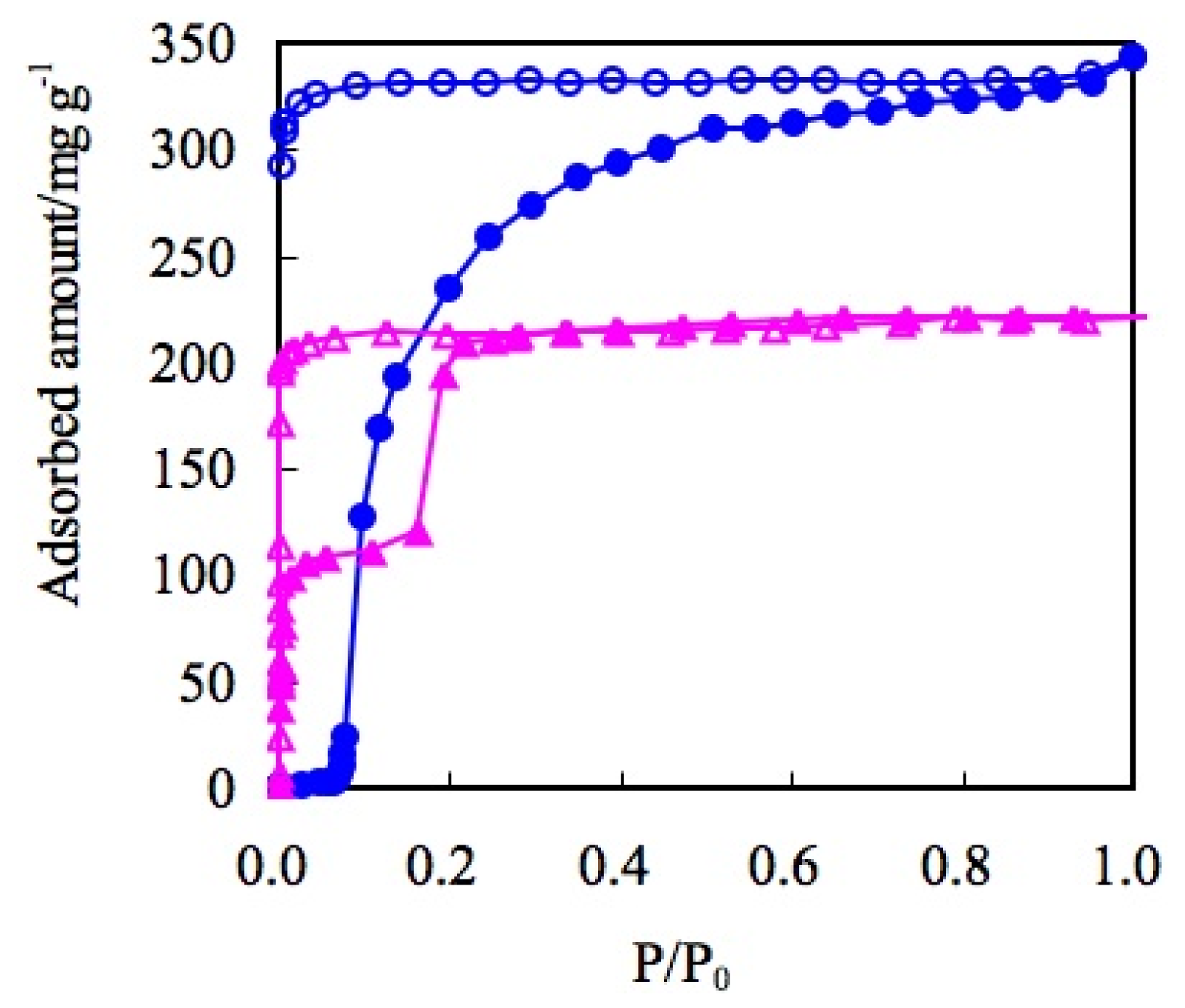
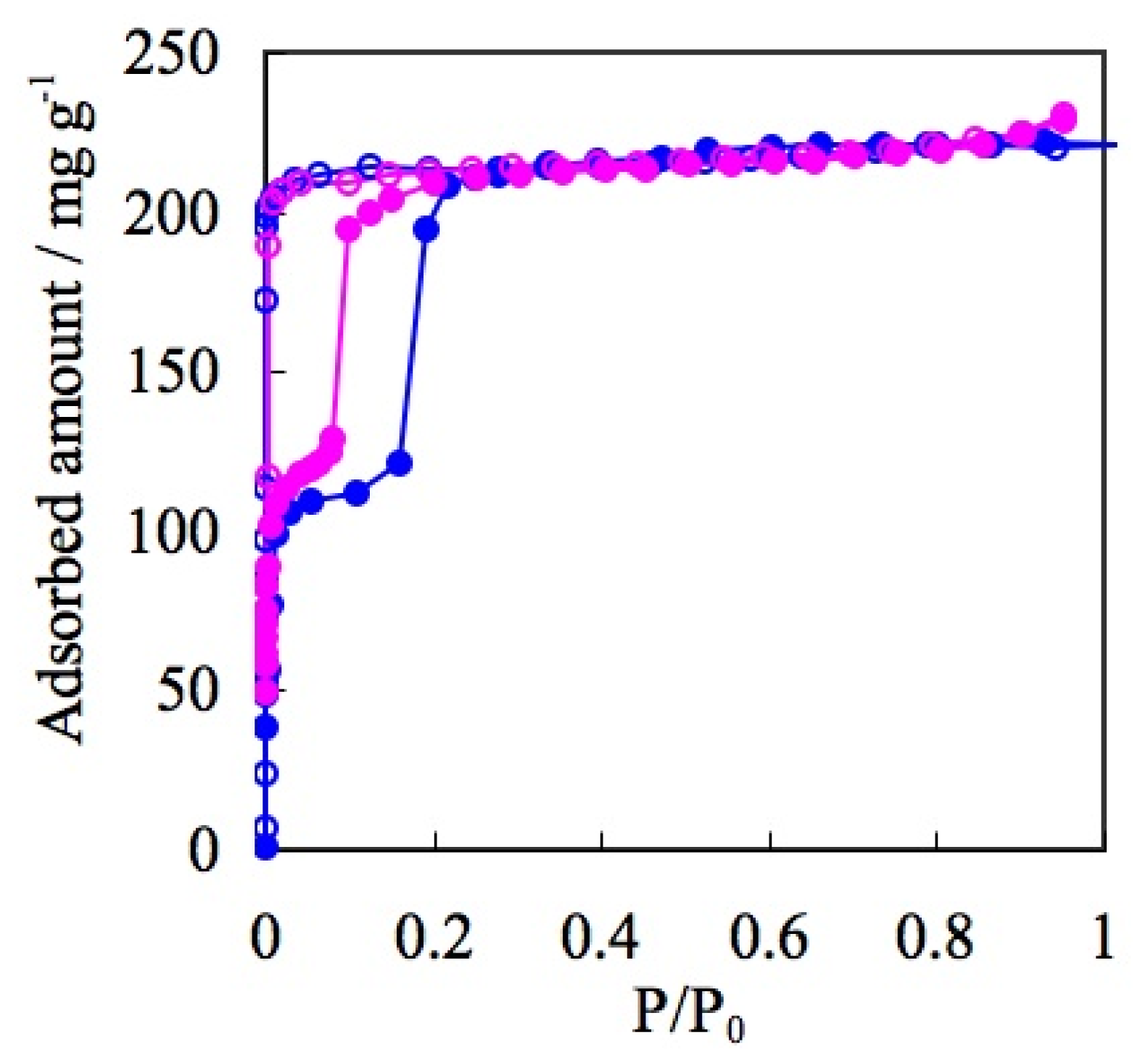
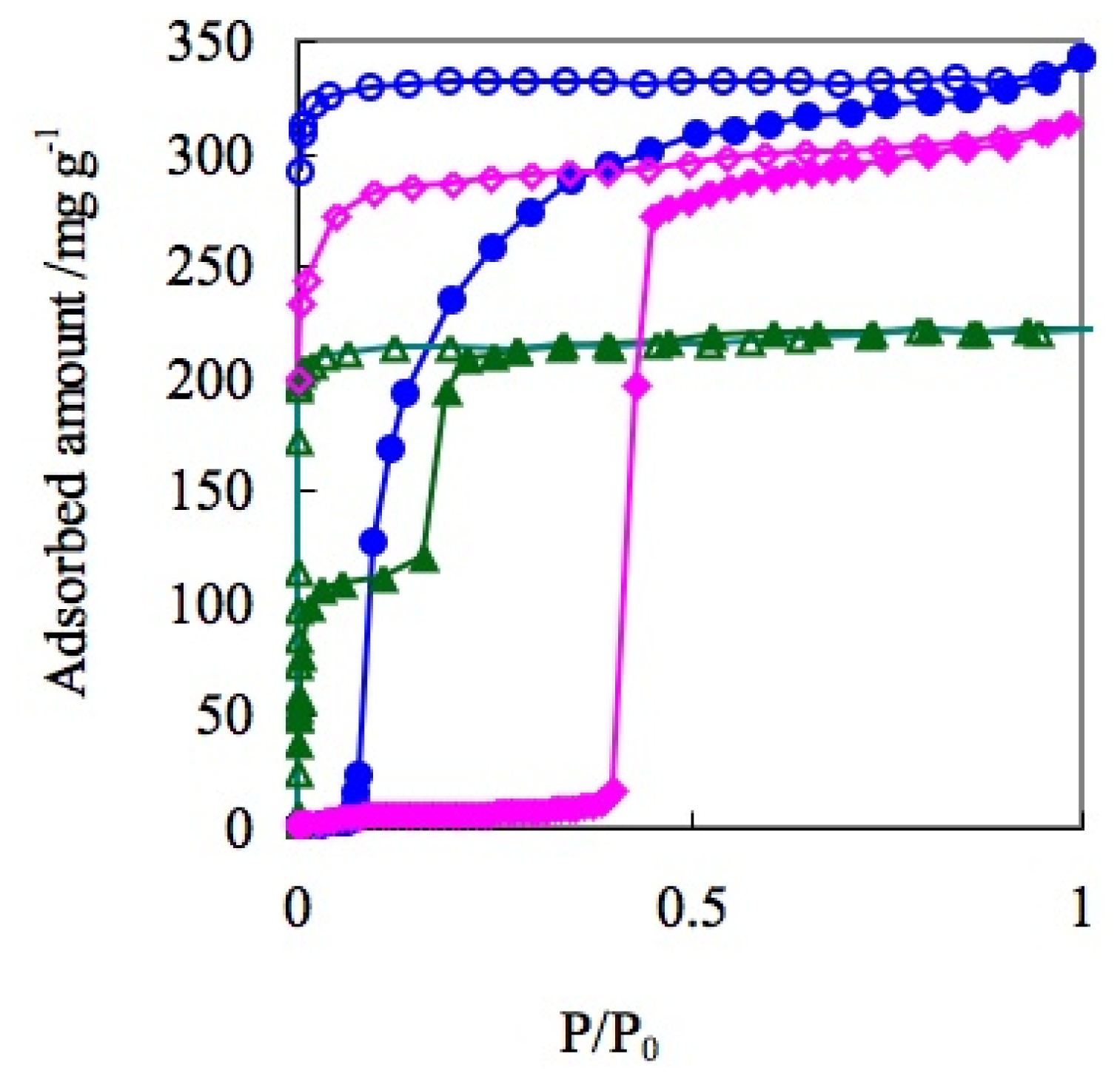
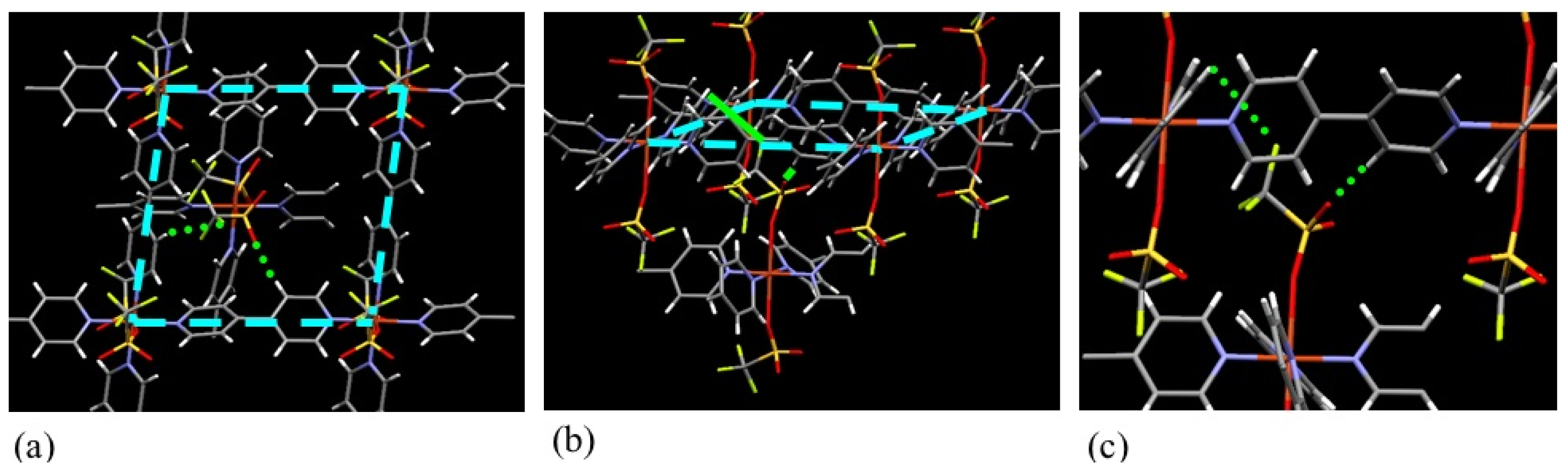

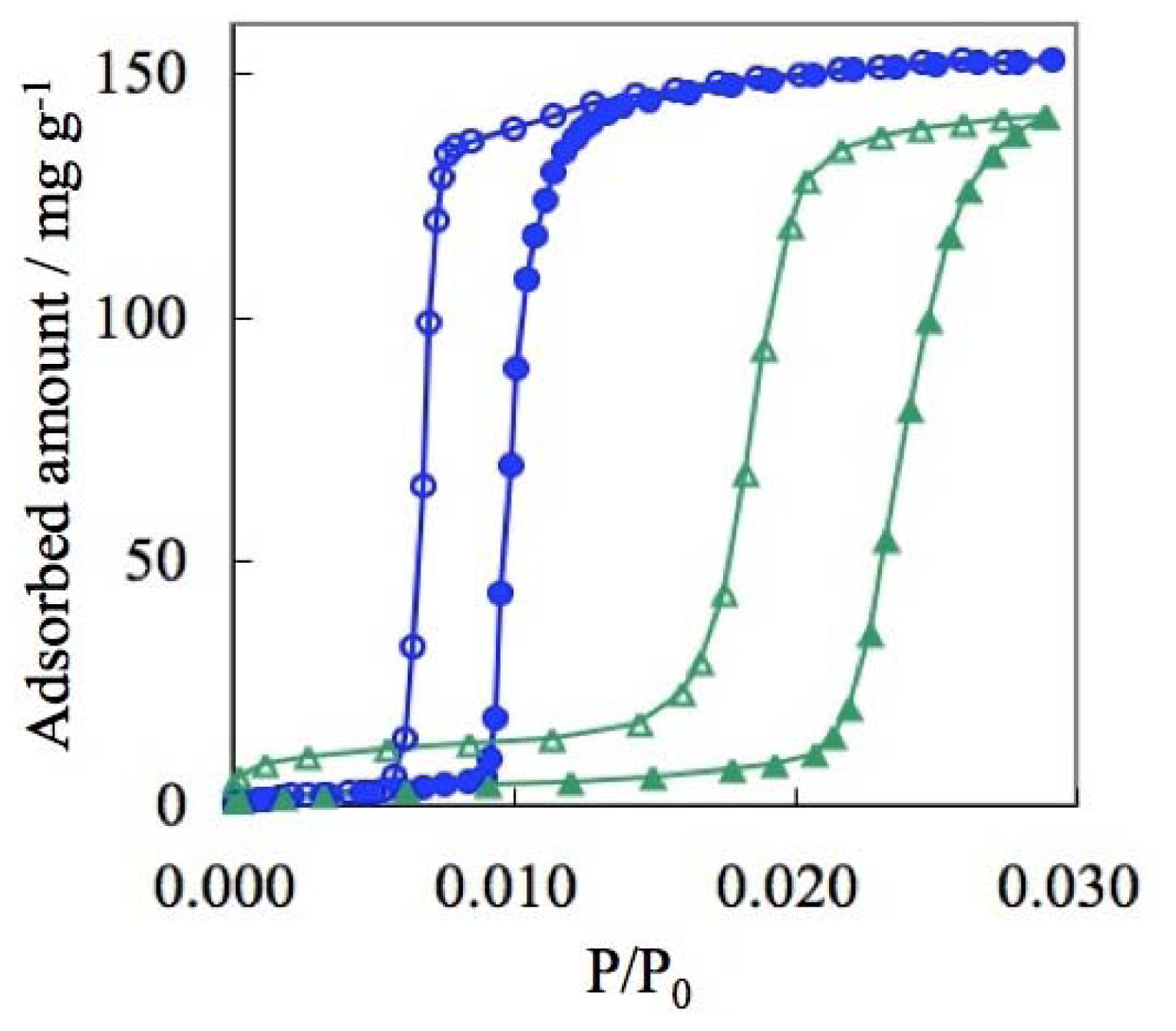
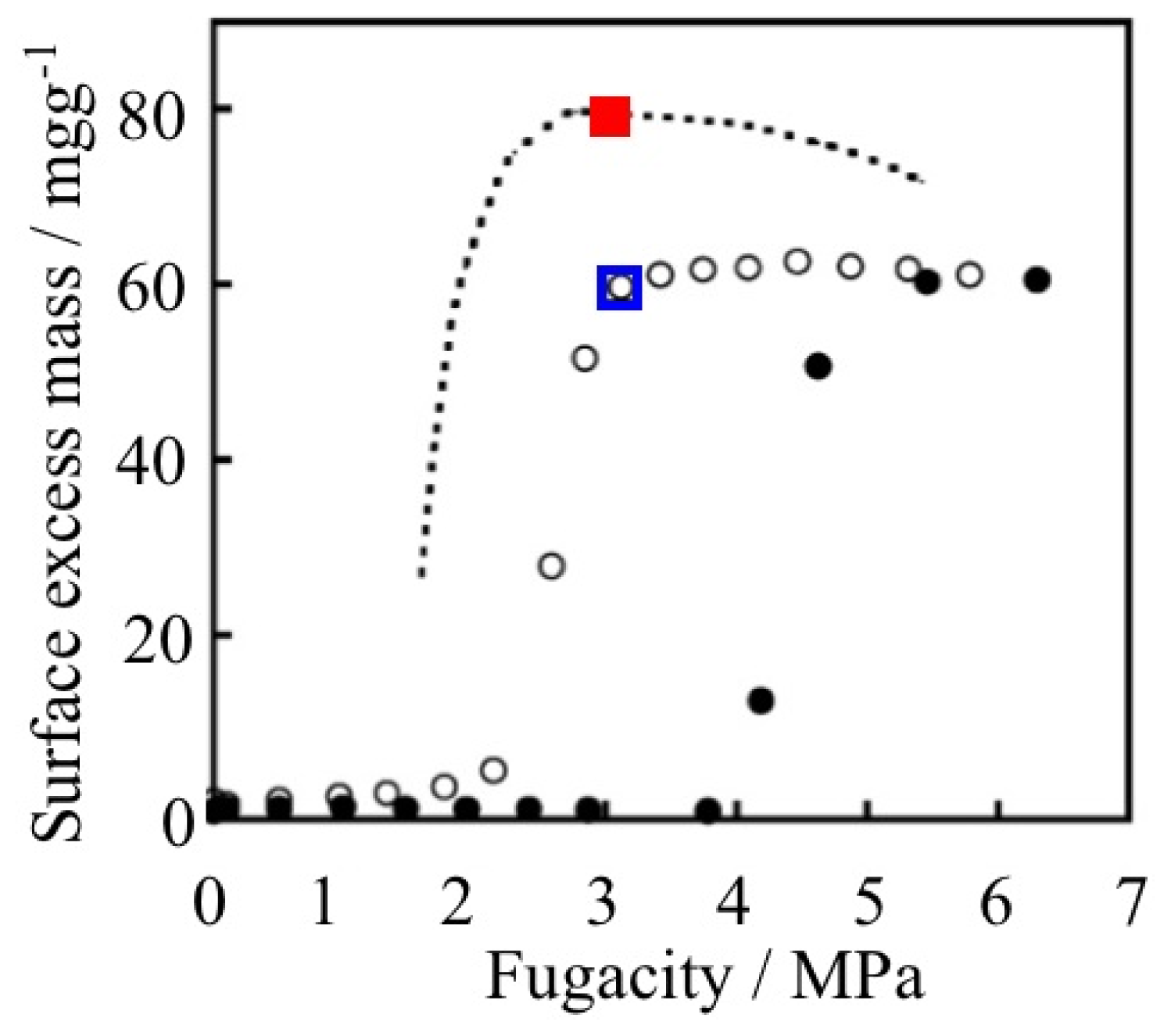




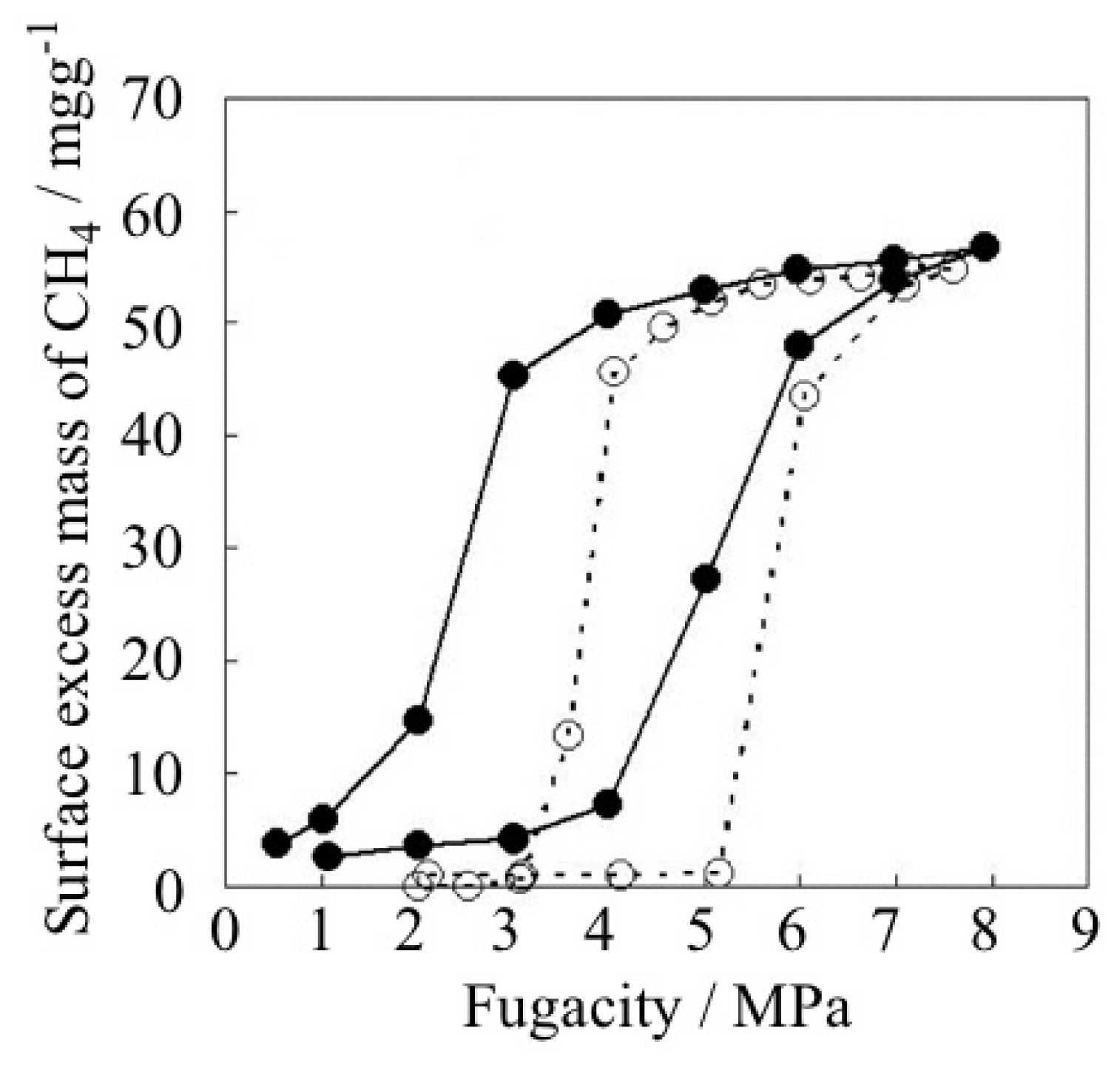
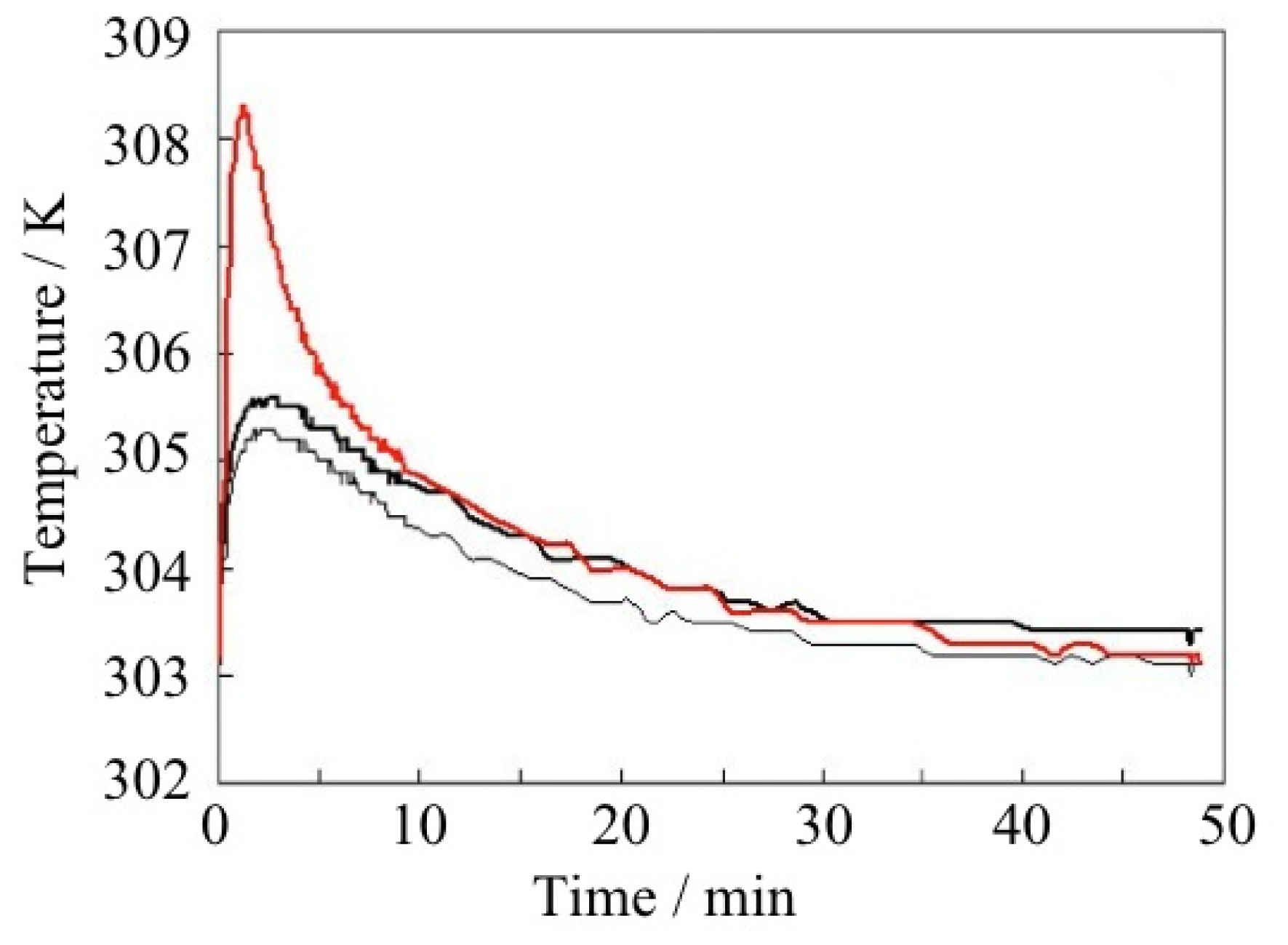
| ELM- | Counter Ion | Coordination Bond | Hydrogen Bond | Gate Type | Amount of Gas Adsorptiona/mg g−1 |
|---|---|---|---|---|---|
| 11 | BF4− | F-Met | F-H | one step | 340 |
| 12 | OTf | O-Met | S=O-H | two steps | 220 |
| 13 | CF3BF3− | F-Met | F-H | one step | 314 |
| Parameters | N2 | O2 | CO2 |
|---|---|---|---|
| Adsorbed molecular numbers per one copper atom of ELM-12 | 5.3 | 7.8 | 5.6 |
| Quadrupole moment (10−40 Cm2) | −4.9 | −1.33 | −14.9 |
| Lennard-Jones potential (e/kB/K) | 104.2 | 126.3 | 245.3 |
| ELM-12 (Cu-OTf) | ELM-22 (Co-OTf) | |
|---|---|---|
| H2 adsorption amount [mg g−1, 1 atm at 77 K] | 5.9 | 6.8 |
| surface area [m2 g−1] | 390 | 400 |
| micropore volume [mL g−1] | 0.14 | 0.15 |
| adsorption capacity [mg g−1] | 118 | 125 |
| total pore volume [mL g−1] | 0.27 | 0.28 |
| isosteric heat of adsorption [kJ mol−1] | 12.2 | 13.0 |
| Compounda | Functionb | Apical Ligand | Counter Ion | References |
|---|---|---|---|---|
| [M(bpy)2(dtbp)2]·2H2O (M = Mn, Co, Cd) | GI | H2O | dtbp | [177,178] |
| [Co(bpy)2(NCS)2]·2Et2O | GI | NCS− | NCS− | [179] |
| [Co(bpy)2(H2O)2]·3NTf2·mim | GI | H2O | NTf2 | [180] |
| [Co(bpy)2(H2O)2]·2ps·10H2O | GI | H2O | ps | [181] |
| [Co(bpy)2(H2O)2]·2NO3·2bpy·2H2O | GI | H2O | NO3− | [182] |
| [Co(bpy)2(H2O)2]·bpy·bsb | GI | H2O | bsb | [183] |
| [Co(bpy)2(OTf)2] (ELM-22) | GA | OTf | OTf | [113] |
| [M(bpy)2(NO3)2]·3np (M = Co, Ni) | GI | H2O | NO3− | [184] |
| [M(bpy)2(NO3)2](na)2 (M = Co, Ni, Zn) | GI | NO3− | NO3− | [185] |
| [M(bpy)2(NO3)2]·arenes (M = Co, Ni) | GI | NO3− | NO3− | [186] |
| [Ni(bpy)2(BF4)2] (ELM-31) | GA | BF4− | BF4− | [113] |
| [Ni(bpy)2(NO3)2]·2pyrene | GI | NO3− | NO3− | [187] |
| [Ni(bpy)2(NCS)2] | - | NCS− | NCS− | [188,189] |
| [Ni(bpy)2(H2PO4)2]·G G = n-BuOH·H2O, 2bpy·3H2O, or 2bpy·ethylene glycol·H2O | GI | H2PO4− | H2PO4− | [190] |
| [Cu(bpy)2(BF4)2] (ELM-11) | GA | BF4− | BF4− | [99] |
| [Cu(bpy)2(OTf)2] (ELM-12) | GA | OTf | OTf | [67] |
| [Cu(bpy)2(CF3BF3)2] (ELM-13) | GA | CF3BF3− | CF3BF3− | [99] |
| [Cu(bpy)2(OTf)(CF3BF3)] (ELM-12/3) | GA | CF3BF3−, OTf | CF3BF3−OTf | [191] |
| [Cu(bpy)2(H2O)2]·2ClO4·bpo·3H2O | GI | H2O | ClO4− | [192] |
| [Cu(bpy)2(H2O)2]·2ClO4·H2O | GI | H2O | ClO4− | [193] |
| [Cu(bpy)2(H2O)]·2sac·CH2Cl2 | GI | H2O | sac | [194] |
| [Cu(PF6)(bpy)2(CH3CN)]·PF6·2CH3CN | GI | PF6−, CH3CN | PF6− | [122] |
| [Cu(bpy)2(H2O)2]·PF6·BF4 | GI | H2O | PF6−·BF4− | [122] |
| [Cu(bpy)2(H2O)2]·2PF6 | GI | H2O | PF6− | [122] |
| [Cu(bpy)2(H2O)2]·(UO2·Hcit)2·7H2O | GI | H2O | UO2·Hcit | [195] |
| [Cu(bpy)2(H2O)2]·2sac·DMF | GI | H2O | sac | [196] |
| [Cu(bpy)2(H2O)2]·2PF6·2H2O·2tdp | GI | H2O | PF6− | [197] |
| [Cu(bpy)2(H2O)2]·4ClO4·H2bpy | GI | H2O | ClO4− | [198] |
| [M(bpy)2(H2O)2]·2ClO4·(2,4′-bpy)2·H2O (M = Zn, Cd) | GI | H2O | ClO4− | [198] |
| [Cu(bpy)2(NO3)2]·3paba | GI | NO3− | NO3− | [199] |
| [Cd(bpy)2(H2O)2]·2NO3·4H2O | GI | H2O | NO3− | [199] |
| [Zn(bpy)2(H2O)2]·bpy·bs | GI | H2O | bs | [200] |
| [Zn(bpy)2(fcph)2] | ME | fcph | fcph | [201] |
| [Zn(bpy)2(NO3)2]·2dcb·pyrene | GI | NO3− | NO3− | [202] |
| [Cd(bpy)2]·2NO3 | Cat | - | NO3− | [203] |
| [Cd(bpy)2(NO3)2]·2dbb | GI | NO3− | NO3− | [203] |
| [Cd(bpy)2(H2O)2]·2NO3·4H2O | Cat, GI | H2O | NO3− | [204] |
| [Cd(bpy)2]·2NO3·2dbb | GI | - | NO3− | [205] |
| [Cd(bpy)2(H2O)2]·2NO3·4H2O | GI | H2O | NO3− | [206] |
| [Cd(bpy)2(NO3)(H2O)]·NO3·2abp | GI | H2O, NO3− | NO3− | [206] |
| [Cd(bpy)2(NO3)2]·2na | GI | NO3− | NO3− | [207] |
| [Cd(bpy)2(H2O)2]·2ClO4·1.5bpy·cnp·4H2O | GI | H2O | ClO4− | [208] |
| [Cd(bpy)2(H2O)2]·bpy·2nan·2ClO4·H2O | GI | H2O | ClO4− | [209] |
| [Cd(bpy)2(ClO4)2]·2mna | GI | ClO4− | ClO4− | [209] |
| [Cd(bpy)2(H2O)2]·2PF6·2bpy·4H2O | GI | H2O | PF6− | [210] |
| [Cd(bpy)2(H2O)(OH)]·PF6 | GI | H2O, OH− | PF6−, OH− | [210] |
| [Cd(bpy)2(H2O)2]·2BF4·2bpy·nab·2H2O | GI | H2O | BF4− | [211] |
| [Cd(ans)2(bpy)2] | GI | ans | ans | [212] |
| [Cd(bpy)2(H2O)2]·2pic | GI | H2O | pic | [213] |
| [Zn(bpy)2(H2O)2]·2pic·2H2O | GI | H2O | pic | [214] |
| [Zn(bpy)2(H2O)2]·bpy·2pic·H2O | GI | H2O | pic | [215] |
| [Cd(bpy)2(NO3)2]·G; G = chlorobenzene, dbb, or p-chlorobenzene | GI | NO3− | NO3− | [216] |
| Adsorbent | Maximum amount of adsorbed gas/ml g−1a | Recovered gas amount by pressure swing (45→20 kPa)/ml g−1b |
|---|---|---|
| ELM-11 | 80 | 71 |
| C300 | 121 | 30 |
| A100 | 88 | 22 |
| 13X APG | 179 | 13 |
| A20 | 110 | 17 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kajiro, H.; Kondo, A.; Kaneko, K.; Kanoh, H. Flexible Two-Dimensional Square-Grid Coordination Polymers: Structures and Functions. Int. J. Mol. Sci. 2010, 11, 3803-3845. https://doi.org/10.3390/ijms11103803
Kajiro H, Kondo A, Kaneko K, Kanoh H. Flexible Two-Dimensional Square-Grid Coordination Polymers: Structures and Functions. International Journal of Molecular Sciences. 2010; 11(10):3803-3845. https://doi.org/10.3390/ijms11103803
Chicago/Turabian StyleKajiro, Hiroshi, Atsushi Kondo, Katsumi Kaneko, and Hirofumi Kanoh. 2010. "Flexible Two-Dimensional Square-Grid Coordination Polymers: Structures and Functions" International Journal of Molecular Sciences 11, no. 10: 3803-3845. https://doi.org/10.3390/ijms11103803





