A Focus on Natural Variation for Abiotic Constraints Response in the Model Species Arabidopsis thaliana
Abstract
:1. Introduction
1.1. Arabidopsis
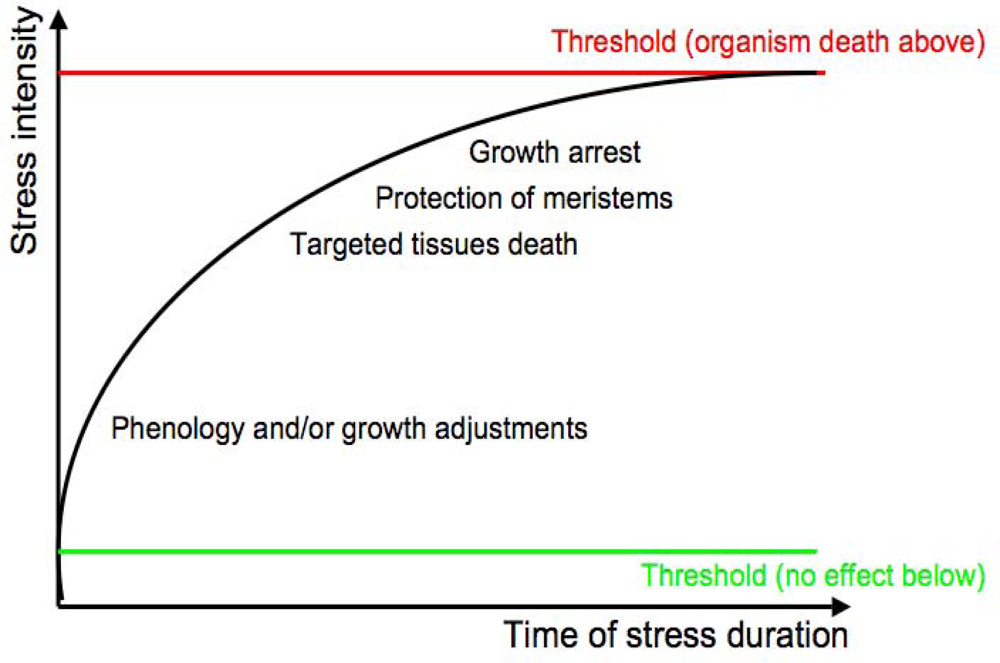
1.2. Abiotic Stress
1.3. Natural Variation
- Determine the number and position of loci controlling a trait;
- Estimate the effects and the mode of action of individual QTL;
- Detect the epistatic interactions among QTLs;
- Detect QTL by environment interactions (QTLs affecting plasticity);
- Detect putative pleiotropic effects of QTLs on various traits in the same mapping population.
2. Salt, Drought and Osmotic Potential
- Drought escape, that allows plant to reproduce and leave an offspring before the environment becomes dry, probably as a life strategy (for review, see Chaves [26]).
- Dehydration avoidance refers to growth adjustment in order to avoid internal physiological perturbations. Under drought, plants close their stomata to save water and lengthen their roots to reach more water. For most plants, dehydration avoidance is achieved primarily through the regulation of stomatal conductance in order to maintain the internal water status of the plant [27]. However, as stomata are also used in respiration, plants under drought stress need to find a balance in order to maintain a residual carbon assimilation and subsequent growth. This can be measured as the Water Use Efficiency (WUE), the amount of carbon gained per unit water consumed [28].
- Dehydration tolerance that varies with the genotype is the ability to endure tissue dehydration, sometimes through the accumulation of metabolites, in order to save the whole organism.
3. Light
4. Temperature
5. Mineral Nutrition
6. Metals
7. Carbon Dioxide
8. Other Abiotic Stress
9. Conclusions
- The mapping population should be large enough to maximize the recombination events,
- The studied trait should be easy to screen and show variation among the population lines,
- Molecular markers should be sufficiently dense on the genome, particularly in the region of interest.
Acknowledgments
Abbreviations:
| ABA | Abscisic Acid |
| Al | Aluminum |
| BC | Back-Cross |
| BRZ | Brassinazole |
| BSA | Bulk-Segregant Analysis |
| bZIP | Basic region/leucine ZIPper |
| CBF | C-repeat Binding Factor |
| Cd | Cadmium |
| CDPK | Calcium-Dependant Protein Kinase |
| Col | Columbia |
| COR | COld Regulated |
| CRY | Cryptochrom |
| DRE | Dehydration-Responsive Element |
| FACE | Free Air Carbon dioxide Enrichment |
| FR | Far-Red light |
| GA | Gibberellin |
| GSH | Glutathione |
| HKT | High affinity K+ Transporter |
| K | Potassium |
| LD | Linkage Disequilibrium |
| N | Nitrogen |
| NIL | Nearly Isogenic Line |
| P | Phosphorous |
| PAE | Phosphate Acquisition Efficiency |
| PHY | Phytochrom |
| QTL | Quantitative Trait Locus |
| QTN | Quantitative Trait Nucleotide |
| R | Red light |
| RIL | Recombinant Inbred Line |
| ROS | Reactive Oxygen Species |
| SNP | Single Nucleotide Polymorphism |
| SOD | Super Oxyde Dismutase |
| UV | Ultraviolet |
| WUE | Water Use Efficency |
References and Notes
- The Arabidopsis Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar]
- Borevitz, JO; Hazen, SP; Michael, TP; Morris, GP; Baxter, IR; Hu, TT; Chen, H; Werner, JD; Nordborg, M; Salt, DE; Kay, SA; Chory, J; Weigel, D; Jones, JD; Ecker, JR. Genome-wide patterns of single-feature polymorphism in Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA 2007, 104, 12057–12062. [Google Scholar]
- Clark, RM; Schweikert, G; Toomajian, C; Ossowski, S; Zeller, G; Shinn, P; Warthmann, N; Hu, TT; Fu, G; Hinds, DA; Chen, H; Frazer, KA; Huson, DH; Scholkopf, B; Nordborg, M; Ratsch, G; Ecker, JR; Weigel, D. Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science 2007, 317, 338–342. [Google Scholar]
- Koornneef, M; Alonso-Blanco, C; Vreugdenhil, D. Naturally occurring genetic variation in Arabidopsis thaliana. Annu. Rev. Plant Biol 2004, 55, 141–172. [Google Scholar]
- Nordborg, M; Hu, TT; Ishino, Y; Jhaveri, J; Toomajian, C; Zheng, H; Bakker, E; Calabrese, P; Gladstone, J; Goyal, R; Jakobsson, M; Kim, S; Morozov, Y; Padhukasahasram, B; Plagnol, V; Rosenberg, NA; Shah, C; Wall, JD; Wang, J; Zhao, K; Kalbfleisch, T; Schulz, V; Kreitman, M; Bergelson, J. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol 2005, 3, e196. [Google Scholar]
- Ostrowski, MF; David, J; Santoni, S; McKhann, H; Reboud, X; Le Corre, V; Camilleri, C; Brunel, D; Bouchez, D; Faure, B; Bataillon, T. Evidence for a large-scale population structure among accessions of Arabidopsis thaliana: Possible causes and consequences for the distribution of linkage disequilibrium. Mol. Ecol 2006, 15, 1507–1517. [Google Scholar]
- Pico, FX; Mendez-Vigo, B; Martinez-Zapater, JM; Alonso-Blanco, C. Natural genetic variation of Arabidopsis thaliana is geographically structured in the Iberian Peninsula. Genetics 2008, 180, 1009–1021. [Google Scholar]
- Schmid, KJ; Ramos-Onsins, S; Ringys-Beckstein, H; Weisshaar, B; Mitchell-Olds, T. A multilocus sequence survey in Arabidopsis thaliana reveals a genome-wide departure from a neutral model of DNA sequence polymorphism. Genetics 2005, 169, 1601–1615. [Google Scholar]
- Schmid, KJ; Torjek, O; Meyer, R; Schmuths, H; Hoffmann, MH; Altmann, T. Evidence for a large-scale population structure of Arabidopsis thaliana from genome-wide single nucleotide polymorphism markers. Theor. Appl. Genet 2006, 112, 1104–1114. [Google Scholar]
- Beck, JB; Schmuths, H; Schaal, BA. Native range genetic variation in Arabidopsis thaliana is strongly geographically structured and reflects Pleistocene glacial dynamics. Mol. Ecol 2008, 17, 902–915. [Google Scholar]
- Boyer, JS. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar]
- Xiong, L; Zhu, J-K. Abiotic stress signal transduction in plants: Molecular and genetic perspectives. Physiol. Plant 2001, 112, 152–166. [Google Scholar]
- Viswanathan, C; Zhu, JK. Molecular genetic analysis of cold-regulated gene transcription. Philos. Trans. R. Soc. Lond. B Biol. Sci 2002, 357, 877–886. [Google Scholar]
- Laloi, C; Przybyla, D; Apel, K. A genetic approach towards elucidating the biological activity of different reactive oxygen species in Arabidopsis thaliana. J. Exp. Bot 2006, 57, 1719–1724. [Google Scholar]
- Grene, R. Oxidative stress and acclimation mechanisms in plants. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2002. [Google Scholar]
- Bonhert, HJ; Sheveleva, E. Plant stress adaptations—Making metabolism move. Curr. Opin. Plant Biol 1998, 1, 267–274. [Google Scholar]
- McAinsh, MR; Brownlee, C; Hetherington, AM. Calcium ions as second messengers in guard cell signal transduction. Physiol. Plant 1997, 100, 16–29. [Google Scholar]
- Xiong, L; Zhu, J-K. Salt tolerance. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2002. [Google Scholar]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci 2006, 11, 15–19. [Google Scholar]
- van Volkenburgh, E. Leaf expension—An integrating plant behaviour. Plant Cell Environ 1999, 22, 1463–1473. [Google Scholar]
- Gilmour, SJ; Hajela, RK; Thomashow, MF. Cold acclimation in Arabidopsis thaliana. Plant Physiol 1988, 87, 745–750. [Google Scholar]
- Mackay, TF. The genetic architecture of quantitative traits. Annu. Rev. Genet 2001, 35, 303–339. [Google Scholar]
- Peters, JL; Cnudde, F; Gerats, T. Forward genetics and map-based cloning approaches. Trends Plant Sci 2003, 8, 484–491. [Google Scholar]
- Borevitz, JO; Chory, J. Genomics tools for QTL analysis and gene discovery. Curr. Opin. Plant Biol 2004, 7, 132–136. [Google Scholar]
- Borevitz, JO; Nordborg, M. The impact of genomics on the study of natural variation in Arabidopsis. Plant Physiol 2003, 132, 718–725. [Google Scholar]
- Chaves, MM; Maroco, JP; Pereira, JS. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol 2003, 30, 239–264. [Google Scholar]
- Ludlow, M. Strategies of response to water stress. In Structural and Functional Responses to Environmental Stresses; SPB Academic: The Hague, The Netherlands, 1989; pp. 269–281. [Google Scholar]
- Tambussi, EA; Bort, J; Araus, JL. Wate use efficiency in C3 cereals under mediterranean conditions: A review of physiological aspects. Ann. Appl. Biol 2007, 150, 307–321. [Google Scholar]
- Pigliucci, M; Whitton, J; Schlichting, CD. Reaction norms of Arabidopsis. I. Plasticity of characters and correlations across water, nutrient and light gradients. J. Evol. Biol 1995, 8, 421–438. [Google Scholar]
- Meyre, D; Leonardi, A; Brisson, G; Vartanian, N. Drought-adaptive mechanisms involved in the escape/tolerance strategies of Arabidopsis Landsberg erecta and Columbia ecotypes and their F1 reciprocal progeny. J. Plant Physiol 2001, 158, 1145–1152. [Google Scholar]
- McKay, JK; Richards, JH; Mitchell-Olds, T. Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Mol. Ecol 2003, 12, 1137–1151. [Google Scholar]
- Farquhar, GD; O’Leary, MH; Berry, JA. On the relationship between carbon isotop discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol 1982, 9, 121–137. [Google Scholar]
- Granier, C; Aguirrezabal, L; Chenu, K; Cookson, SJ; Dauzat, M; Hamard, P; Thioux, JJ; Rolland, G; Bouchier-Combaud, S; Lebaudy, A; Muller, B; Simonneau, T; Tardieu, F. PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytol 2006, 169, 623–635. [Google Scholar]
- Bouchabke, O; Chang, F; Simon, M; Voisin, R; Pelletier, G; Durand-Tardif, M. Natural variation in Arabidopsis thaliana as a tool for highlighting differential drought responses. PLoS ONE 2008, 3, e1705. [Google Scholar]
- Hausmann, NJ; Juenger, TE; Sen, S; Stowe, KA; Dawson, TE; Simms, EL. Quantitative trait loci affecting delta C-13 and response to differential water availibility in Arabidopsis thaliana. Evolution 2005, 59, 81–96. [Google Scholar]
- Ungerer, MC; Halldorsdottir, SS; Purugganan, MA; Mackay, TFC. Genotype-environment interactions at quantitative trait loci affecting inflorescence development in Arabidopsis thaliana. Genetics 2003, 165, 353–365. [Google Scholar]
- Juenger, TE; McKay, JK; Hausmann, N; Keurentjes, JJB; Sen, S; Stowe, KA; Dawson, TE; Simms, EL; Richards, JH. Identification and characterization of QTL underlying whole-plant physiology in Arabidopsis thaliana: Delta C-13, stomatal conductance and transpiration efficiency. Plant Cell Environ 2005, 28, 697–708. [Google Scholar]
- McKay, JK; Richards, JH; Nemali, KS; Sen, S; Mitchell-Olds, T; Boles, S; Stahl, EA; Wayne, T; Juenger, TE. Genetics of drought adaptation in Arabidopsis thaliana II. QTL analysis of a new mapping population, Kas-1 × Tsu-1. Evolution 2008, 62, 3014–3026. [Google Scholar]
- Masle, J; Gilmore, SR; Farquhar, GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 2005, 436, 866–870. [Google Scholar]
- Deak, KI; Malamy, J. Osmotic regulation of root system architecture. Plant J 2005, 43, 17–28. [Google Scholar]
- Fitz Gerald, JN; Lehti-Shiu, MD; Ingram, PA; Deak, KI; Biesiada, T; Malamy, JE. Identification of quantitative trait loci that regulate Arabidopsis root system size and plasticity. Genetics 2006, 172, 485–498. [Google Scholar]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol 2005, 167, 645–663. [Google Scholar]
- Amzallag, GN; Lerner, HR; Poljakoff-Mayber, A. Induction of increased salt tolerance in sorghum bicolor by NaCl pretreatment. J. Exp. Bot 1990, 41, 29–34. [Google Scholar]
- Quesada, V; Garcia-Martinez, S; Piqueras, P; Ponce, MR; Micol, JL. Genetic architecture of NaCl tolerance in Arabidopsis. Plant Physiol 2002, 130, 951–963. [Google Scholar]
- Liu, J; Ishitani, M; Halfter, U; Kim, CS; Zhu, JK. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Nat. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar]
- Rus, A; Baxter, I; Muthukumar, B; Gustin, J; Lahner, B; Yakubova, E; Salt, DE. Natural variants of AtHKT1 enhance Na(+) accumulation in two wild populations of Arabidopsis. PLoS Genet 2006, 2, e210. [Google Scholar]
- Berthomieu, P; Conejero, G; Nublat, A; Brackenbury, WJ; Lambert, C; Savio, C; Uozumi, N; Oiki, S; Yamada, K; Cellier, F; Gosti, F; Simonneau, T; Essah, PA; Tester, M; Very, AA; Sentenac, H; Casse, F. Functional analysis of AtHKT1 in Arabidopsis shows that Na(+) recirculation by the phloem is crucial for salt tolerance. Embo. J 2003, 22, 2004–2014. [Google Scholar]
- Gong, JM; Waner, DA; Horie, T; Li, SL; Horie, R; Abid, KB; Schroeder, JI. Microarray-based rapid cloning of an ion accumulation deletion mutant in Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA 2004, 101, 15404–15409. [Google Scholar]
- Uozumi, N; Kim, EJ; Rubio, F; Yamaguchi, T; Muto, S; Tsuboi, A; Bakker, EP; Nakamura, T; Schroeder, JI. The Arabidopsis HKT1 gene homolog mediates inward Na(+) currents in xenopus laevis oocytes and Na(+) uptake in Saccharomyces cerevisiae. Plant Physiol 2000, 122, 1249–1259. [Google Scholar]
- Rus, A; Lee, BH; Munoz-Mayor, A; Sharkhuu, A; Miura, K; Zhu, JK; Bressan, RA; Hasegawa, PM. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta. Plant Physiol 2004, 136, 2500–2511. [Google Scholar]
- Essah, PA; Davenport, R; Tester, M. Sodium influx and accumulation in Arabidopsis. Plant Physiol 2003, 133, 307–318. [Google Scholar]
- Komeda, Y. Genetic regulation of time to flower in Arabidopsis thaliana. Annu. Rev. Plant Biol 2004, 55, 521–535. [Google Scholar]
- Roux, F; Touzet, P; Cuguen, J; Le Corre, V. How to be early flowering: An evolutionary perspective. Trends Plant Sci 2006, 11, 375–381. [Google Scholar]
- Schepens, I; Duek, P; Fankhauser, C. Phytochrome-mediated light signalling in Arabidopsis. Curr. Opin. Plant Biol 2004, 7, 564–569. [Google Scholar]
- Botto, JF; Smith, H. Differential genetic variation in adaptive strategies to a common environmental signal in Arabidopsis accessions: Phytochrome-mediated shade avoidance. Plant Cell Environ 2002, 25, 53–63. [Google Scholar]
- Poulson, ME; Boeger, MR; Donahue, RA. Response of photosynthesis to high light and drought for Arabidopsis thaliana grown under a UV-B enhanced light regime. Photosynth. Res 2006, 90, 79–90. [Google Scholar]
- Filiault, DL; Wessinger, CA; Dinneny, JR; Lutes, J; Borevitz, JO; Weigel, D; Chory, J; Maloof, JN. Amino acid polymorphisms in Arabidopsis phytochrome B cause differential responses to light. Proc. Nat. Acad. Sci. USA 2008, 105, 3157–3162. [Google Scholar]
- Abarca, D; Roldan, M; Martin, M; Sabater, B. Arabidopsis thaliana ecotype Cvi shows an increased tolerance to photo-oxidative stress and contains a new chloroplastic copper/zinc superoxide dismutase isoenzyme. J. Exp. Bot 2001, 52, 1417–1425. [Google Scholar]
- Maloof, JN; Borevitz, JO; Dabi, T; Lutes, J; Nehring, RB; Redfern, JL; Trainer, GT; Wilson, JM; Asami, T; Berry, CC; Weigel, D; Chory, J. Natural variation in light sensitivity of Arabidopsis. Nat. Genet 2001, 29, 441–446. [Google Scholar]
- Bae, G; Choi, G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol 2008, 59, 281–311. [Google Scholar]
- Botto, JF; Alonso-Blanco, C; Garzaron, I; Sanchez, RA; Casal, JJ. The Cape Verde Islands allele of cryptochrome 2 enhances cotyledon unfolding in the absence of blue light in Arabidopsis. Plant Physiol 2003, 133, 1547–1556. [Google Scholar]
- Magliano, TM; Botto, JF; Godoy, AV; Symonds, VV; Lloyd, AM; Casal, JJ. New Arabidopsis recombinant inbred lines (Landsberg erecta × Nossen) reveal natural variation in phytochrome-mediated responses. Plant Physiol 2005, 138, 1126–1135. [Google Scholar]
- Torabinejad, J; Caldwell, MM. Inheritance of UV-B tolerance in seven ecotypes of Arabidopsis thaliana L. Heynh. and their F1 hybrids. J. Hered 2000, 91, 228–233. [Google Scholar]
- Cooley, NM; Higgins, JT; Holmes, MG; Attridge, TH. Ecotypic differences in responses of Arabidopsis thaliana L. to elevated polychromatic UV-A and UV-B + A radiation in the natural environment: A positive correlation between UV-B + A inhibition and growth rate. J. Photochem. Photobiol. B 2001, 60, 143–150. [Google Scholar]
- Balasubramanian, S; Sureshkumar, S; Lempe, J; Weigel, D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet 2006, 2, e106. [Google Scholar]
- Loudet, O; Michael, TP; Burger, BT; Le Mette, C; Mockler, TC; Weigel, D; Chory, J. A zinc knuckle protein that negatively controls morning-specific growth in Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA 2008, 105, 17193–17198. [Google Scholar]
- El-Assal, SED; Alonso-Blanco, C; Peeters, AJ; Raz, V; Koornneef, M. A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat. Genet 2001, 29, 435–440. [Google Scholar]
- Olsen, KM; Halldorsdottir, SS; Stinchcombe, JR; Weinig, C; Schmitt, J; Purugganan, MD. Linkage disequilibrium mapping of Arabidopsis CRY2 flowering time alleles. Genetics 2004, 167, 1361–1369. [Google Scholar]
- Werner, JD; Borevitz, JO; Warthmann, N; Trainer, GT; Ecker, JR; Chory, J; Weigel, D. Quantitative trait locus mapping and DNA array hybridization identify an FLM deletion as a cause for natural flowering-time variation. Proc. Nat. Acad. Sci. USA 2005, 102, 2460–2465. [Google Scholar]
- Guo, H; Yang, H; Mockler, TC; Lin, C. Regulation of flowering time by Arabidopsis photoreceptors. Science 1998, 279, 1360–1363. [Google Scholar]
- Wolyn, DJ; Borevitz, WO; Loudet, O; Schwartz, C; Maloof, J; Ecker, JR; Berry, CC; Chory, J. Light-response quantitative trait loci identified with composite interval and eXtreme array mapping in Arabidopsis thaliana. Genetics 2004, 167, 907–917. [Google Scholar]
- Franklin, KA. Shade avoidance. New Phytol 2008, 179, 930–944. [Google Scholar]
- Chory, J; Li, J. Gibberellins, brassinosteroids and light-regulated development. Plant Cell Environ 1997, 20, 801–806. [Google Scholar]
- Borevitz, JO; Maloof, JN; Lutes, J; Dabi, T; Redfern, JL; Trainer, GT; Werner, JD; Asami, T; Berry, CC; Weigel, D; Chory, J. Quantitative trait loci controlling light and hormone response in two accessions of Arabidopsis thaliana. Genetics 2002, 160, 683–696. [Google Scholar]
- Thomashow, MF. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol 1999, 50, 571–599. [Google Scholar]
- Levitt, J. Chilling, freezing and high temperature stress. In Responses of Plants to Environmental Stresses; Academic: New York, USA, 1980; pp. 3–56. [Google Scholar]
- Lugan, R; Niogret, MF; Kervazo, L; Larher, FR; Kopka, J; Bouchereau, A. Metabolome and water status phenotyping of Arabidopsis under abiotic stress cues reveals new insight into ESK1 function. Plant Cell Environ 2009, 32, 95–108. [Google Scholar]
- Xin, Z; Browse, J. Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Nat. Acad. Sci. USA 1998, 95, 7799–7804. [Google Scholar]
- Bouchabke-Coussa, O; Quashie, ML; Seoane-Redondo, J; Fortabat, MN; Gery, C; Yu, A; Linderme, D; Trouverie, J; Granier, F; Teoule, E; Durand-Tardif, M. ESKIMO1 is a key gene involved in water economy as well as cold acclimation and salt tolerance. BMC Plant Biol 2008, 8, 125. [Google Scholar]
- Smallwood, M; Bowles, DJ. Plants in a cold climate. Philos. Trans. R. Soc. Lond. B Biol. Sci 2002, 357, 831–847. [Google Scholar]
- Le, MQ; Engelsberger, WR; Hincha, DK. Natural genetic variation in acclimation capacity at sub-zero temperatures after cold acclimation at 4 degrees C in different Arabidopsis thaliana accessions. Cryobiology 2008, 57, 104–112. [Google Scholar]
- Soitamo, AJ; Piippo, M; Allahverdiyeva, Y; Battchikova, N; Aro, EM. Light has a specific role in modulating Arabidopsis gene expression at low temperature. BMC Plant Biol 2008, 8, 13. [Google Scholar]
- Rohde, P; Hincha, DK; Heyer, AG. Heterosis in the freezing tolerance of crosses between two Arabidopsis thaliana accessions (Columbia-0 and C24) that show differences in non-acclimated and acclimated freezing tolerance. Plant J 2004, 38, 790–799. [Google Scholar]
- Hasdai, M; Weiss, B; Levi, A; Samach, A; Porat, R. Differential responses of Arabidopsis ecotypes to cold, chilling and freezing temperatures. Ann. Appl. Biol 2006, 148, 113–120. [Google Scholar]
- Zhen, Y; Ungerer, MC. Clinal variation in freezing tolerance among natural accessions of Arabidopsis thaliana. New Phytol 2008, 177, 419–427. [Google Scholar]
- McKhann, HI; Gery, C; Berard, A; Leveque, S; Zuther, E; Hincha, DK; de Mita, S; Brunel, D; Teoule, E. Natural variation in CBF gene sequence, gene expression and freezing tolerance in the Versailles core collection of Arabidopsis thaliana. BMC Plant Biol 2008, 8, 105. [Google Scholar]
- Hannah, MA; Wiese, D; Freund, S; Fiehn, O; Heyer, AG; Hincha, DK. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol 2006, 142, 98–112. [Google Scholar]
- Swindell, WR; Huebner, M; Weber, AP. Plastic and adaptive gene expression patterns associated with temperature stress in Arabidopsis thaliana. Heredity 2007, 99, 143–150. [Google Scholar]
- Lee, YP; Fleming, AJ; Korner, C; Meins, F, Jr. Differential expression of the CBF pathway and cell cycle-related genes in Arabidopsis accessions in response to chronic low-temperature exposure. Plant Biol. (Stuttg) 2009, 11, 273–283. [Google Scholar]
- Lempe, J; Balasubramanian, S; Sureshkumar, S; Singh, A; Schmid, M; Weigel, D. Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genet 2005, 1, 109–118. [Google Scholar]
- Stinchcombe, JR; Dorn, LA; Schmitt, J. Flowering time plasticity in Arabidopsis thaliana: A reanalysis of Westerman & Lawrence (1970). J. Evol. Biol 2004, 17, 197–207. [Google Scholar]
- Alonso-Blanco, C; Gomez-Mena, C; Llorente, F; Koornneef, M; Salinas, J; Martinez-Zapater, JM. Genetic and molecular analyses of natural variation indicate CBF2 as a candidate gene for underlying a freezing tolerance quantitative trait locus in Arabidopsis. Plant Physiol 2005, 139, 1304–1312. [Google Scholar]
- Zarka, DG; Vogel, JT; Cook, D; Thomashow, MF. Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol 2003, 133, 910–918. [Google Scholar]
- Novillo, F; Alonso, JM; Ecker, JR; Salinas, J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc. Nat. Acad. Sci. USA 2004, 101, 3985–3990. [Google Scholar]
- Lahner, B; Gong, J; Mahmoudian, M; Smith, EL; Abid, KB; Rogers, EE; Guerinot, ML; Harper, JF; Ward, JM; McIntyre, L; Schroeder, JI; Salt, DE. Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat. Biotechnol 2003, 21, 1215–1221. [Google Scholar]
- Salt, DE. Update on plant ionomics. Plant Physiol 2004, 136, 2451–2456. [Google Scholar]
- Nord, EA; Lynch, JP. Delayed reproduction in Arabidopsis thaliana improves fitness in soil with suboptimal phosphorus availability. Plant Cell Environ 2008, 31, 1432–1441. [Google Scholar]
- Jovanovic, M; Lefebvre, V; Laporte, P; Gonzalez-Rizzo, S; Lelandais-Briére, C; Frugier, F; Hartmann, C; Crespi, M. How the environment regulates root architecture in dicots. Adv. Botanical Res 2007, 46, 35–74. [Google Scholar]
- Raghothama, KG. Phosphate acquisition. Annu. Rev. Plant Physiol 1999, 50, 665–693. [Google Scholar]
- Lopez-Bucio, J; Cruz-Ramirez, A; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol 2003, 6, 280–287. [Google Scholar]
- Torrey, JG. Root hormones and plant growth. Annu. Rev. Plant Physiol 1976, 27, 435–459. [Google Scholar]
- Lopez-Bucio, J; Hernandez-Abreu, E; Sanchez-Calderon, L; Nieto-Jacobo, MF; Simpson, J; Herrera-Estrella, L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 2002, 129, 244–256. [Google Scholar]
- Holford, ICR. Soil phosphorus: Its measurements and its uptake by plants. Aust. J. Soil Res 1997, 35, 227–239. [Google Scholar]
- Lopez-Bucio, J; Nieto-Jacobo, MF; Ramirez-Rodriguez, VV; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci 2000, 160, 1–13. [Google Scholar]
- Masclaux-Daubresse, C; Reisdorf-Cren, M; Orsel, M. Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol (Stuttg) 2008, 10(Suppl 1), 23–36. [Google Scholar]
- Scheible, WR; Morcuende, R; Czechowski, T; Fritz, C; Osuna, D; Palacios-Rojas, N; Schindelasch, D; Thimm, O; Udvardi, MK; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 2004, 136, 2483–2499. [Google Scholar]
- Narang, RA; Bruene, A; Altmann, T. Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol 2000, 124, 1786–1799. [Google Scholar]
- Reymond, M; Svistoonoff, S; Loudet, O; Nussaume, L; Desnos, T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant Cell Environ 2006, 29, 115–125. [Google Scholar]
- Pigliucci, M; Schlichting, CD; Whitton, J. Reaction norms of Arabidopsis. II. Response to stress and unordered environmental variation. Funct. Ecol 1995, 9, 537–547. [Google Scholar]
- Pigliucci, M; Schlichting, CD. Reaction norms of Arabidopsis (Brassicaceae). III. Response to nutrients in 26 populations from a worldwide collection. Amer. J. Bot 1995, 82, 1117–1125. [Google Scholar]
- Fricke, W; Flowers, TJ. Control of leaf cell elongation in barley. Generation rates of osmotic pressure and turgor, and growth-associated water potential gradients. Planta 1998, 206, 53–65. [Google Scholar]
- Loudet, O; Chaillou, S; Krapp, A; Daniel-Vedele, F. Quantitative trait loci analysis of water and anion contents in interaction with nitrogen availability in Arabidopsis thaliana. Genetics 2003, 163, 711–722. [Google Scholar]
- Loudet, O; Chaillou, S; Merigout, P; Talbotec, J; Daniel-Vedele, F. Quantitative trait loci analysis of nitrogen use efficiency in Arabidopsis. Plant Physiol 2003, 131, 345–358. [Google Scholar]
- Svistoonoff, S; Creff, A; Reymond, M; Sigoillot-Claude, C; Ricaud, L; Blanchet, A; Nussaume, L; Desnos, T. Root tip contact with low-phosphate media reprograms plant root architecture. Nat. Genet 2007, 39, 792–796. [Google Scholar]
- Sanchez-Calderon, L; Lopez-Bucio, J; Chacon-Lopez, A; Cruz-Ramirez, A; Nieto-Jacobo, F; Dubrovsky, JG; Herrera-Estrella, L. Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 2005, 46, 174–184. [Google Scholar]
- Loudet, O; Saliba-Colombani, V; Camilleri, C; Calenge, F; Gaudon, V; Koprivova, A; North, KA; Kopriva, S; Daniel-Vedele, F. Natural variation for sulfate content in Arabidopsis thaliana is highly controlled by APR2. Nat. Genet 2007, 39, 896–900. [Google Scholar]
- Shorrocks, VM. The occurnece and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar]
- Zeng, C; Han, Y; Shi, L; Peng, L; Wang, Y; Xu, F; Meng, J. Genetic analysis of the physiological responses to low boron stress in Arabidopsis thaliana. Plant Cell Environ 2008, 31, 112–122. [Google Scholar]
- Tejada-Jimenez, M; Llamas, A; Sanz-Luque, E; Galvan, A; Fernandez, E. A high-affinity molybdate transporter in eukaryotes. Proc. Nat. Acad. Sci USA 2007, 104, 20126–20130. [Google Scholar]
- Schwarz, G; Mendel, RR. Molybdenum cofactor biosynthesis and molybdenum enzymes. Annu. Rev. Plant Biol 2006, 57, 623–647. [Google Scholar]
- Jander, G; Norris, SR; Rounsley, SD; Bush, DF; Levin, IM; Last, RL. Arabidopsis map-based cloning in the post-genome era. Plant Physiol 2002, 129, 440–450. [Google Scholar]
- Wood, S; Sebastian, K; Scherr, S. Soil resource condition. In Pilot Analysis of Global Ecosystems: Agroecosystems; International Food Policy Research Institute and the World Resources Institute: Washington, DC, USA, 2000; pp. 45–54. [Google Scholar]
- Smith, KS; Balistrieri, LS; Smith, SM; Severson, RC. Distribution and mobility of molybdenum in the terrestrial environment. In Molybdenum in Agriculture; Gupta, U, Ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Baxter, I; Muthukumar, B; Park, HC; Buchner, P; Lahner, B; Danku, J; Zhao, K; Lee, J; Hawkesford, MJ; Guerinot, ML; Salt, DE. Variation in molybdenum content across broadly distributed populations of Arabidopsis thaliana is controlled by a mitochondrial molybdenum transporter (MOT1). PLoS Genet 2008, 4, e1000004. [Google Scholar]
- Tomatsu, H; Takano, J; Takahashi, H; Watanabe-Takahashi, A; Shibagaki, N; Fujiwara, T. An Arabidopsis thaliana high-affinity molybdate transporter required for efficient uptake of molybdate from soil. Proc. Nat. Acad. Sci. USA 2007, 104, 18807–18812. [Google Scholar]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar]
- Hoekenga, OA; Vision, TJ; Shaff, JE; Monforte, AJ; Lee, GP; Howell, SH; Kochian, LV. Identification and characterization of aluminum tolerance loci in Arabidopsis (Landsberg erecta × Columbia) by quantitative trait locus mapping. A physiologically simple but genetically complex trait. Plant Physiol 2003, 132, 936–948. [Google Scholar]
- Kobayashi, Y; Hoekenga, OA; Itoh, H; Nakashima, M; Saito, S; Shaff, JE; Maron, LG; Pineros, MA; Kochian, LV; Koyama, H. Characterization of AtALMT1 expression in aluminum-inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis. Plant Physiol 2007, 145, 843–852. [Google Scholar]
- Schutzendubel, A; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot 2002, 53, 1351–1365. [Google Scholar]
- Courbot, M; Willems, G; Motte, P; Arvidsson, S; Roosens, N; Saumitou-Laprade, P; Verbruggen, N. A major QTL for Cd tolerance in Arabidopsis halleri Co-localizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiol 2007, 144, 1052–1065. [Google Scholar]
- Kochian, LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol 1995, 46, 237–260. [Google Scholar]
- Taylor, GJ. Current views of aluminum stress response: The physiological basis of tolerance. Curr. Topics Plant Biochem. Physiol 1991, 6, 57–93. [Google Scholar]
- Ma, JF; Ryan, PR; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 2001, 6, 273–278. [Google Scholar]
- Yamamoto, Y; Hachiya, A; Matsumoto, H. Oxidative damage to membranes by a combination of aluminum and iron in suspension cultured tobacco cells. Plant Cell Physiol 1997, 38, 1333–1339. [Google Scholar]
- Degenhardt, J; Larsen, PB; Howell, SH; Kochian, LV. Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum–induced increase in rhizosphere pH. Plant Physiol 1998, 117, 19–27. [Google Scholar]
- Delhaize, E; Hebb, DM; Ryan, PR. Expression of a Pseudomonas aeruginosa citrate synthase gene in tobacco is not associated with either enhanced citrate accumulation or efflux. Plant Physiol 2001, 125, 2059–2067. [Google Scholar]
- Ikka, T; Kobayashi, Y; Iuchi, S; Sakurai, N; Shibata, D; Kobayashi, M; Koyama, H. Natural variation of Arabidopsis thaliana reveals that aluminum resistance and proton resistance are controlled by different genetic factors. Theor. Appl. Genet 2007, 115, 709–719. [Google Scholar]
- Kobayashi, Y; Koyama, H. QTL analysis of Al tolerance in recombinant inbred lines of Arabidopsis thaliana. Plant Cell Physiol 2002, 43, 1526–1533. [Google Scholar]
- Takita, E; Koyama, H; Hara, T. Organic acid in aluminum-phosphate utilizing cell of carrot (Daucus carota L.). Plant Cell Physiol 1999, 40, 489–495. [Google Scholar]
- Larsen, PB; Degenhardt, J; Tai, CY; Stenzler, LM; Howell, SH; Kochian, LV. Aluminum-resistant Arabidopsis mutants that exhibit altered patterns of aluminum accumulation and organic acid release from roots. Plant Physiol 1998, 117, 9–18. [Google Scholar]
- Larsen, PB; Tai, CY; Kochian, LV; Howell, SH. Arabidopsis mutants with increased sensitivity to aluminum. Plant Physiol 1996, 110, 743–751. [Google Scholar]
- Ezaki, B; Gardner, RC; Ezaki, Y; Matsumoto, H. Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol 2000, 122, 657–665. [Google Scholar]
- Hoekenga, OA; Maron, LG; Pineros, MA; Cancado, GM; Shaff, J; Kobayashi, Y; Ryan, PR; Dong, B; Delhaize, E; Sasaki, T; Matsumoto, H; Yamamoto, Y; Koyama, H; Kochian, LV. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Nat. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar]
- Prentice, IC; Farquhar, GD; Fasham, M; Goulden, M; Jaramillo, V; Kheshgi, H; Quere, C; Scholes, R; Wallace, D. The carbon cycle and atmospheric CO2. In Climate Change 2001: The Scientific Basis Contribution of Working Group 1 to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Houghton, JT, Ding, Y, Griggs, DJ, Noguer, M, van der Linden, PJ, Dai, X, Maskell, K, Johnson, CA, Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 183–237. [Google Scholar]
- Ainsworth, EA; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ 2007, 30, 258–270. [Google Scholar]
- Long, SP; Ainsworth, EA; Rogers, A; Ort, DR. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol 2004, 55, 591–628. [Google Scholar]
- Li, P; Ainsworth, EA; Leakey, AD; Ulanov, A; Lozovaya, V; Ort, DR; Bohnert, HJ. Arabidopsis transcript and metabolite profiles: Ecotype-specific responses to open-air elevated [CO2]. Plant Cell Environ 2008, 31, 1673–1687. [Google Scholar]
- Zhang, J; Lechowicz, MJ. Responses to CO2 enrichment by two genotypes of Arabidopsis thaliana differing in their sensitivity to nutrient availability. Ann. Bot 1995, 75, 491–499. [Google Scholar]
- Li, PH; Sioson, A; Mane, SP; Ulanov, A; Grothaus, G; Heath, LS; Murali, TM; Bohnert, HJ; Grene, R. Response diversity of Arabidopsis thaliana ecotypes in elevated CO2 in the field. Plant Mol. Biol 2006, 62, 593–609. [Google Scholar]
- Miyazaki, S; Fredricksen, M; Hollis, KC; Poroyko, V; Shelpley, D; Galbraith, DW; Long, SP; Bohnert, HJ. Transcript expression profiles of Arabidopsis thaliana grown under controlled conditions and open-air elevated concentrations of CO2 and of O3. Field Crops Res 2004, 90, 47–59. [Google Scholar]
- Tonsor, SJ; Scheiner, SM. Plastic trait integration across a CO2 gradient in Arabidopsis thaliana. Am. Nat 2007, 169, E119–E140. [Google Scholar]
- Cross, JM; von Korff, M; Altmann, T; Bartzetko, L; Sulpice, R; Gibon, Y; Palacios, N; Stitt, M. Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol 2006, 142, 1574–1588. [Google Scholar]
- Pigliucci, M; Kolodynska, A. Phenotypic plasticity and integration in response to flooded conditions in natural accessions of Arabidopsis thaliana (L.) Heynh (Brassicaceae). Ann. Bot. (Lond) 2002, 90, 199–207. [Google Scholar]
- Buckler, EST; Thornsberry, JM. Plant molecular diversity and applications to genomics. Curr. Opin. Plant Biol 2002, 5, 107–111. [Google Scholar]
- Yu, J; Buckler, ES. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol 2006, 17, 155–160. [Google Scholar]
- Zhao, K; Aranzana, MJ; Kim, S; Lister, C; Shindo, C; Tang, C; Toomajian, C; Zheng, H; Dean, C; Marjoram, P; Nordborg, M. An Arabidopsis example of association mapping in structured samples. PLoS Genet 2007, 3, e4. [Google Scholar]
- Aranzana, MJ; Kim, S; Zhao, K; Bakker, E; Horton, M; Jakob, K; Lister, C; Molitor, J; Shindo, C; Tang, C; Toomajian, C; Traw, B; Zheng, H; Bergelson, J; Dean, C; Marjoram, P; Nordborg, M. Genome-wide association mapping in Arabidopsis identifies previously known flowering time and pathogen resistance genes. PLoS Genet 2005, 1, e60. [Google Scholar]
- Fu, J; Keurentjes, JJ; Bouwmeester, H; America, T; Verstappen, FW; Ward, JL; Beale, MH; de Vos, RC; Dijkstra, M; Scheltema, RA; Johannes, F; Koornneef, M; Vreugdenhil, D; Breitling, R; Jansen, RC. System-wide molecular evidence for phenotypic buffering in Arabidopsis. Nat. Genet 2009, 41, 166–167. [Google Scholar]

| Accessions 1 | Stress | Trait | Reference |
|---|---|---|---|
| Light, Radiations | |||
| 4 | 100, 80, 60 and 40% of sun light | Biometric and fitness parameters | [29,109] |
| 157 | Far-red light (3 red/far-red rates) | Flowering time, hypocotyl length | [55] |
| 141 | White, blue, red, far-red and dark | Hypocotyl length | [59] |
| 52 | Far-red pulses | Hypocotyl length, angle between cotyledons | [62] |
| 7 | UV-B | Biometric traits on vegetative (2) and reproductive (4) apparatus. | [63] |
| 7 | UV-A, UV-B + A | 9 biometric traits on vegetative (6), reproductive (2) and root apparatus (1) and 3 derived parameters. | [64] |
| Temperature | |||
| 21 | 15, 20 and 25 °C | Flowering time (day of flowering, total leaf number at flowering), height at flowering | [91] |
| 9 | Cold and freezing acclimation | Electrolyte leakage, LT50, genes and metabolites expression | [87] |
| 4 | Cold and freezing acclimation | Electrolyte leakage, LT50, leaf sugar content | [81] |
| 71 | Cold acclimation, sub-zero temperatures | Tissue damage index | [85] |
| 50 | Freezing tolerance (with acclimation) | Tissue damage index, CBF and COR genes expression | [86] |
| 12 | 6 °C, 14 °C and freezing | five biometric traits, flowering time, electrolyte leakage, anthocyanin and chlorophyll contents | [84] |
| 10 | Cold (4 °C), heat (38 °C) | Gene expression | [88] |
| 150 | 16 °C | Flowering time (day of flowering, total leaf number at flowering) | [90] |
| 52 | 25 °C | Flowering time (total leaf number at flowering) | [65] |
| 23 | 10 °C chronic exposure | Root elongation rate, candidate genes expression | [89] |
| Nutrient | |||
| 4
36 | 0, 2, 4 or 6 N:P:K fertilizer doses | Biometric and fitness parameters | [29,109,110] |
| 36, then 5 contrasted | Phosphate depletion (hydroxylapatite or 2,5 μM KH2PO4) | Root morphology parameters, phosphate uptake kinetics | [107] |
| 6 | Phosphate depletion (5 μM NaH2PO4) | Root morphology parameters | [108] |
| CO2 | |||
| 3 | CO2 “enrichment” (550 ppm) | Gene expression, metabolite profile | [149] |
| 35 | CO2 low, standard and enriched (250 to 710 ppm) | Biometric, developmental and metabolic parameters | [151] |
| 24 | Reduce CO2 assimilation (Short days, low light, excess nitrate) | Rosette weight, enzymes and metabolites profile | [152] |
| Root anoxie | |||
| 47 | Soil saturated with water (waterlogging) | Biometric parameters | [153] |
| Heavy metal | |||
| 260 | Al3+ 1 mM or pH 4.7 | Relative root length | [137] |
| Oxydative | |||
| 11 | Atrazine (0.25, 0.5 μM) | Metabolic and root morphology parameters, ROS accumulation | Ramel et al., in prep. |
| Osmotic (including drought, salt) | |||
| 4 | 5, 10, 15 or 20 mL of water in 2 inches pot | Biometric and fitness parameters | [29] |
| 39 | No stress | WUE (delta13C) | [31] |
| 9 | Monitored drought stress | Projected leaves area, Transpiration rate | [33] |
| 24 | Monitored mild drought stress | Total Leaf Area, Relative Water Content, Electrolyte Leackage, Cut Rosette Water Loss | [34] |
| 102 | 250 mM NaCl in vitro | Germination, Fresh weight, dry weight | [44] |
| 12 | 100 mM NaCl in pots | Survival | [46] |
| Trait | QTL | Gene | Function | Approach | aR2% | bPlant number | cResolution (kb) | dCandidate gene and early or late evidence | Identification of QTN | Population | Functional proof | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Photoperiod responsive flowering time | ED1 | CRY2 | Cryptochrome | Positional cloning | 28–56 | 1822 | 45 | Yes (late) | Amino acid substitution | Ler × Cvi | Transgenic complementation | [67] |
| Light responsive flowering time and hypocotyls elongation | - | PHYC | Phytochrome | Positional cloning | - | 140 | 1000 | Yes (early) | Amino acid substitution | Fr-2 × Col-0 | Quantative complementation | [65] |
| Light responsive hypocotyls elongation | LIGHT2 | PHYB | Phytochrome | Association mapping | 18–22 | 140 accessio ns | NA | Yes (early) | Amino acid substitution | Ler × Cvi | Transformation | [57] |
| Light responsive hypocotyls elongation | LIGHT5 | TZP | Zinc Knucke | Positional cloning | 40 | 4600 | 7 | Yes (late) | Premature stop codon | Bay-0 × Sha | Transgenic complementation | [66] |
| Sulfate content | SOC.1 | APR2 | 5’-Phosphosulfate reductase | Positional cloning | 48 | 411 | 4000 | Yes (early) | Amino acid substitution | Bay-0 × Sha | Transgenic and quantitative complementation | [116] |
| Molybdate content | - | MOT1 | Mo transporter | Positional cloning | - | 18 | 172 | Yes (early) | 53-bp deletion in promoter | Ler × Col-0 | Heterologous system (yeast) | [125] |
| Molybdate content | - | MOT1 | Mo transporter | BSA-Positional cloning | - | 200 | 346 | Yes (early) | 53- bp deletion in promoter | Ler × Col-0 | Quantitative complementation | [124] |
| Na+ accumulation | - | AtHK T1 | Na+ transporter | BSA-Positional cloning | - | 60 | 2000 | Yes (early) | Deletion in upstream of AtHKT1 | Ts-1, Tsu-1 × Col-0 | Quantitative complementation | [46] |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lefebvre, V.; Kiani, S.P.; Durand-Tardif, M. A Focus on Natural Variation for Abiotic Constraints Response in the Model Species Arabidopsis thaliana. Int. J. Mol. Sci. 2009, 10, 3547-3582. https://doi.org/10.3390/ijms10083547
Lefebvre V, Kiani SP, Durand-Tardif M. A Focus on Natural Variation for Abiotic Constraints Response in the Model Species Arabidopsis thaliana. International Journal of Molecular Sciences. 2009; 10(8):3547-3582. https://doi.org/10.3390/ijms10083547
Chicago/Turabian StyleLefebvre, Valérie, Seifollah Poormohammad Kiani, and Mylène Durand-Tardif. 2009. "A Focus on Natural Variation for Abiotic Constraints Response in the Model Species Arabidopsis thaliana" International Journal of Molecular Sciences 10, no. 8: 3547-3582. https://doi.org/10.3390/ijms10083547





