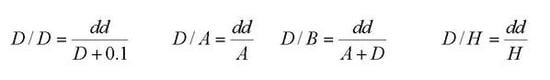QSPR Studies on Aqueous Solubilities of Drug-Like Compounds
Abstract
:1. Introduction
2. Some Different in silico Methods for Solubility Estimation
3. Predicting Solubility through Linear Regression Based QSPR-QSAR
4. The Proposal of Descriptors Based on Lipinski Rules for Modeling Aqueous Solubilities
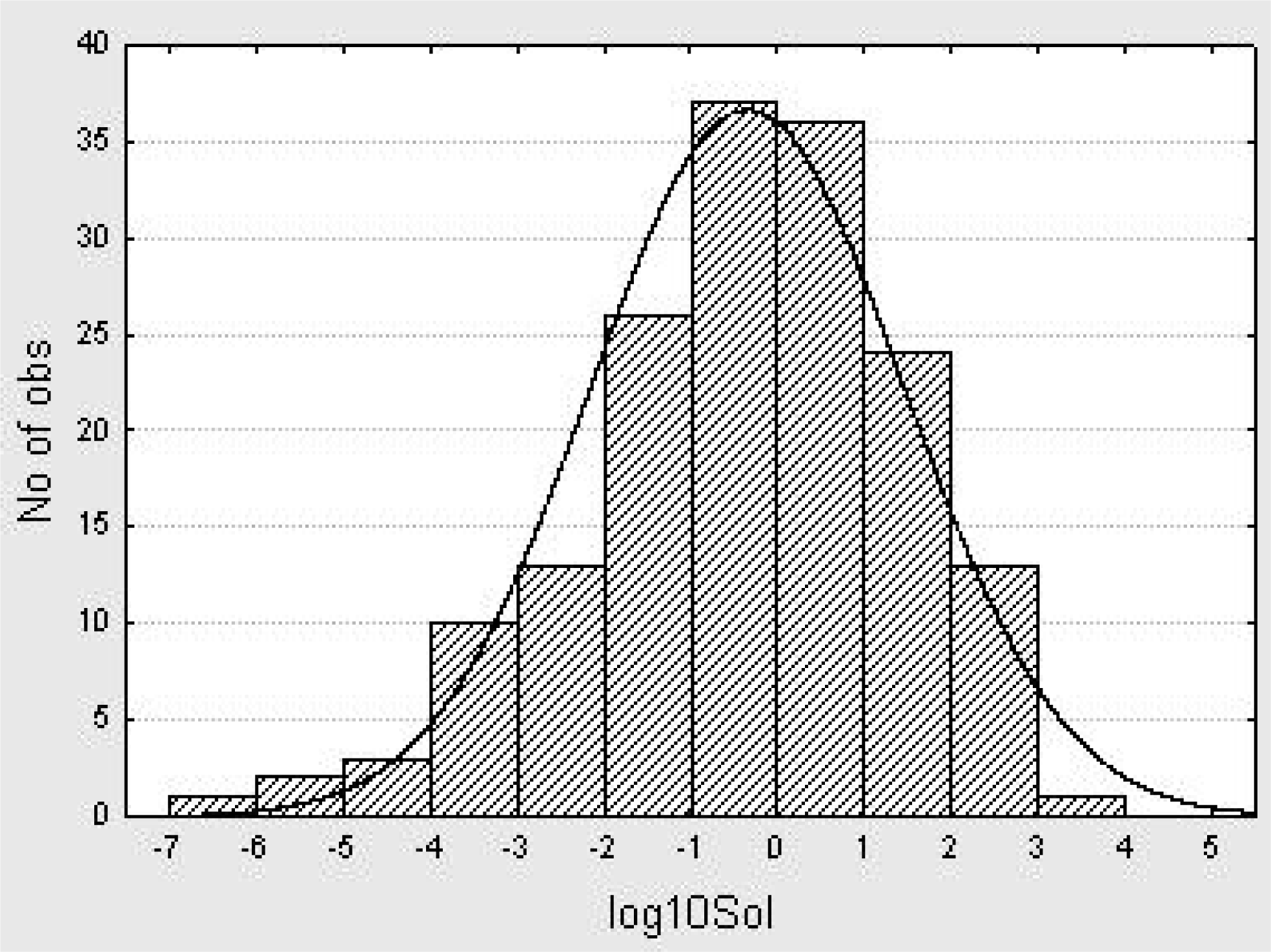
5. A QSPR Designed upon a Balanced Aqueous Solubility Data Set
4. Conclusions
Acknowledgments
References and Notes
- Balakin, KV; Savchuk, NP; Tetko, IV. In Silico approaches to prediction of aqueous and DMSO Solubility of drug-like compounds: Trends, problems and solutions. Curr. Med. Chem 2006, 13, 226–241. [Google Scholar]
- Delaney, JS. Prediction of aqueous solubility from structure. Drug Disc. Today 2005, 10, 289–295. [Google Scholar]
- Goodwin, JJ. Rationale and benefit of using high throughput solubility screens in drug discovery. Drug Disc. Today Technol 2006, 3, 67–71. [Google Scholar]
- Johnson, SR; Zheng, W. Recent progress in the computational prediction of aqueous solubility and absorption. AAPS J 2006, 8, E27–E40. [Google Scholar]
- Schneider, G; So, S. Adaptative Systems in Drug Design; Landes Bioscience: Austin, TX, USA, 2003. [Google Scholar]
- Yu, H; Adedoyin, A. ADME-Tox in drug discovery: integration of experimental and computational technologies. Drug Disc. Today 2003, 8, 852–861. [Google Scholar]
- Lipinski, CA; Lombardo, F; Dominy, DW; Feeney, PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev 2001, 46, 3–26. [Google Scholar]
- Smith, CJ; Hansch, C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem. Toxicol 2000, 38, 637–646. [Google Scholar]
- Amidon, GL; Yalkowsky, SH; Anik, ST; Valvani, SC. Solubility of nonelectrolytes in polar solvents. V. Estimation of the solubility of aliphatic monofunctional compounds in water using a molecular surface area approach. J. Phys. Chem. A 1975, 79, 2239–2246. [Google Scholar]
- Hansch, C; Bjorkroth, JP; Leo, A. Hydrophobicity and central nervous system agents: on the principle of minimal hydrophobicity in drug design. J. Pharm. Sci 1987, 76, 663–687. [Google Scholar]
- Kariv, I; Rourick, RA; Kassel, DB; Chung, TD. Improvement of “hit-to-lead” optimization by integration of in vitro HTS experimental models for early determination of pharmacokinetic properties. Comb. Chem. High Throughput Screen 2002, 5, 459–472. [Google Scholar]
- Bhattachar, SN; Deschenes, LA; Wesley, JA. Solubility: it's not just for physical chemists. Drug Disc. Today 2006, 11, 1012–1018. [Google Scholar]
- Katritzky, AR; Maran, U; Lobanov, VS; Karelson, M. Structurally diverse quantitative structure-property relationship correlations of technologically relevant physical properties. J. Chem. Inf. Model 2000, 40, 1–18. [Google Scholar]
- Klopman, G; Wang, S; Balthasar, DM. Estimation of aqueous solubility of organic molecules by the group contribution approach. Application to the study of biodegradation. J. Chem. Inf. Model 1992, 32, 474–482. [Google Scholar]
- McFarland, JW; Avdeef, A; Berger, CM; Raevsky, OA. Estimating the water solubilities of crystalline compounds from their chemical structure alone. J. Chem. Inf. Model 2001, 41, 1355–1359. [Google Scholar]
- Pogliani, L. Modeling purines and pyrimidines with the linear combination of connectivity indices–molecular connectivity “LCCI-MC” method. J. Chem. Inf. Model 1996, 36, 1082–1091. [Google Scholar]
- Yalkowsky, SH; Valvani, SC. Solubility and partitioning I: solubility of nonelectrolytes in water. J. Pharm. Sci 1980, 69, 912–922. [Google Scholar]
- Yalkowsky, SH; Valvani, SC; Roseman, TJ. Water solubility: A critique of the solvatochromic approach. J. Pharm. Sci 1983, 72, 866–870. [Google Scholar]
- Yang, G; Ran, Y; Yalkowsky, SH. Prediction of the aqueous solubility: comparison of the general solubility equation and the method using an amended solvation energy relationship. J. Pharm. Sci 2002, 91, 517–533. [Google Scholar]
- Peterson, DL; Yalkowski, SH. Comparison of two methods for predicting aqueous solubility. J. Chem. Inf. Comput. Sci 2001, 41, 1531–1534. [Google Scholar]
- Ran, Y; Yalkowsky, SH. Prediction of drug solubility by the general solubility equation (GSE). J. Chem. Inf. Comput. Sci 2001, 41, 354–357. [Google Scholar]
- Ran, Y; Jain, N; Yalkowsky, SH. Prediction of aqueous solubility of organic compounds by the general solubility equation (GSE). J. Chem. Inf. Comput. Sci 2001, 41, 1208–1207. [Google Scholar]
- Meylan, WM; Howard, PH; Boethling, RS. Improved method for estimating water solubility from octanol/water coefficient. Environ. Toxicol. Chem 1996, 15, 100–106. [Google Scholar]
- Meylan, WM; Howard, PH. Estimating log P with atom/fragments and water solubility with log P. Persp. Drug Disc. Design 2000, 19, 67–84. [Google Scholar]
- Myrdal, P; Ward, GH; Dannenfelser, RM; Mishra, DS; Yalkowsky, SH. AQUAFAC 1: Aqueous Functional group activity coefficients: Application to hydrocarbons. Chemosphere 1992, 24, 1047–1061. [Google Scholar]
- Pinsuwan, S; Myrdal, PB; Lee, YC; Yalkowsky, SH. AQUAFAC 5: Applications to alcohols and acids. Chemosphere 1997, 35, 2503–2513. [Google Scholar]
- Morris, JJ; Bruneau, PP. Prediction of physicochemical properties. In Virtual Screening for Bioactive Molecules; Bohm, HG, Schneider, G, Eds.; Wiley-VCH: Weinheim, Germany, 2000; Volume 10, pp. 33–58. [Google Scholar]
- Thompson, JD; Cramer, CJ; Truhlar, DG. Predicting aqueous solubilities from aqueous free energies of solvation and experimental or calculated vapor pressures of pure substances. J. Chem. Phys 2003, 119, 1661–1670. [Google Scholar]
- Yaws, CL; Xiang, P; Xiaoyin, L. Water solubility data for 151 hydrocarbons. Chem. Eng 1993, 100, 108–111. [Google Scholar]
- Jorgensen, WL; Duffy, EM. Prediction of drug solubility from Monte Carlo simulations. Bioorg. Med. Chem. Lett 2000, 10, 1155–1158. [Google Scholar]
- Kier, LB; Cheng, C-K; Seybold, PG. Cellular automata models of aqueous solution systems. In Reviews in Computational Chemistry; Lipkowitz, KB, Boyd, DB, Eds.; Wiley-VCH: Weinheim, Germany, 2001; Volume 17, pp. 205–254. [Google Scholar]
- Cramer, CJ; Truhlar, DG. Continuum solvation models: Classical and quantum mechanical implementations. In Reviews in Computational Chemistry; Lipkowitz, KB, Boyd, DB, Eds.; Wiley-VCH: Weinheim, Germany, 1995; Volume 6, pp. 1–72. [Google Scholar]
- Klamt, A. Prediction of aqueous solubility of drugs and pesticides with COSMO-RS. J. Comput. Chem 2002, 23, 275–281. [Google Scholar]
- Artist.
- ChemEng Software Design.
- Predict.
- Nirmalakhandan, NNP; Speece, RE. Prediction of aqueous solubility of organic chemicals based on molecular structure. 2. Application to PNAs, PCBs, PCDDs, etc. Environ. Sci. Technol 1989, 23, 708–713. [Google Scholar]
- Suzuki, T. Development of an automatic estimation system for both the partition coefficient and aqueous solubility. J. Comput.-Aided Mol. Des 1991, 5, 149–166. [Google Scholar]
- Kuhne, R; Ebert, RU; Kleint, F; Schmidt, G; Schuurmann, G. Group contribution methods to estimate water solubility of organic chemicals. Chemosphere 1995, 30, 2061–2077. [Google Scholar]
- Lee, Y; Myrdal, PB; Yalkowsky, SH. Aqueous functional group activity coefficients (AQUAFAC) 4: Applications to complex organic compounds. Chemosphere 1996, 33, 2129–2144. [Google Scholar]
- Klopman, G; Zhu, H. Estimation of aqueous solubility of organic molecules by the group contribution approach. J. Chem. Inf. Model 2001, 41, 439–445. [Google Scholar]
- Free, SM; Wilson, JW. A mathematical contribution to structure-activity studies. J. Med. Chem 1964, 7, 395–399. [Google Scholar]
- Hansch, C. p-σ-π analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc 1964, 86, 1616–1626. [Google Scholar]
- Hansch, C; Leo, A. Exploring QSAR Fundamentals and Applications in Chemistry and Biology; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Katritzky, AR; Lobanov, VS; Karelson, M. QSPR - the correlation and quantitative prediction of chemical and physical properties from structure. Chem. Soc. Rev 1995, 24, 279–287. [Google Scholar]
- Trinajstic, N. Chemical Graph Theory; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Worth, AP; Bassan, A; De Bruijn, J; Saliner, AG; Netzeva, T; Patlewicz, G; Pavan, M; Tsakovska, I; Eisenreich, S. The role of the European Chemicals Bureau in promoting the regulatory use of QSARs methods. SAR QSAR Environ. Res 2007, 18, 111–125. [Google Scholar]
- Noringer, U. In silico modelling of ADMET-a minireview of work from 2000 to 2004. SAR QSAR Environ. Res 2005, 16, 1–11. [Google Scholar]
- Martin, YC. A bioavailability score. J. Med. Chem 2005, 48, 3164–3170. [Google Scholar]
- Yoshida, F. QSAR model for drug human bioavailability. J. Med. Chem 2000, 43, 2575–2585. [Google Scholar]
- Molecular Descriptors Family Home page.
- Karelson, M. Molecular Descriptors in QSAR/QSPR; Wiley-Interscience: New York, NY, USA, 2000. [Google Scholar]
- Todeschini, R; Consonni, V. Handbook of Molecular Descriptors; Wiley VCH: Weinheim, Germany, 2000. [Google Scholar]
- Apostol, TM. Calculus; Blaisdell Publishing Co: Waltham, MA, USA, 1969. [Google Scholar]
- Malinowski, ER. Factor Analysis in Chemistry; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Leardi, R. Genetic algorithms in feature selection. In Genetic Algorithms in Molecular Modeling Principles of QSAR and Drug Design; Devillers, J, Ed.; Academic Press: London, UK, 1996; Volume 1, pp. 67–86. [Google Scholar]
- Duchowicz, PR; Castro, EA; Fernández, FM. Alternative algorithm for the search of an optimal set of descriptors in QSAR-QSPR studies. MATCH Commun. Math. Comput. Chem 2006, 55, 179–192. [Google Scholar]
- Zupan, J. Encyclopedia of Computational Chemistry; Wiley: Chichester, UK, 1998 2006.
- Vapanik, V. The Nature of Statistical Learning Theory; Springer Verlag: New York, NY, USA, 1995. [Google Scholar]
- Livingstone, DJ; Manallack, DT. Statistics using neural networks: chance effects. J. Med. Chem 1993, 36, 1295–1297. [Google Scholar]
- Tetko, IV; Luik, AI; Poda, GI. Applications of neural networks in structure-activity relationships of a small number of molecules. J. Med. Chem 1993, 36, 811–814. [Google Scholar]
- Talevi, A; Castro, EA; Bruno-Blanch, LE. New solubility models based on descriptors derived from the detour matrix. J. Arg. Chem. Soc 2006, 44, 129–141. [Google Scholar]
- Harary, F. Graph Theory; Addison-Wesley: Upper Saddle River, NJ, USA, 1969. [Google Scholar]
- Castro, EA; Tueros, M; Toropov, AA. Maximum topological distances based indices as molecular descriptors for QSPR: 2--application to aromatic hydrocarbons. Comput. Chem 2000, 24, 571–576. [Google Scholar]
- Devillers, J; Balaban, AT. Topological Indices and Related Descriptors in QSAR and QSPR; Gordon and Breach Science Publishers: New York, NY, USA, 1999. [Google Scholar]
- Firpo, M; Gavernet, L; Castro, EA; Toropov, AA. Maximum topological distances based indices as molecular descriptors for QSPR. Part 1. Application to alkyl benzenes boiling points. J. Mol. Struc-Theochem 2000, 501, 419–425. [Google Scholar]
- Lukovits, I. The detour index. Croat. Chem. Acta 1996, 69, 873–882. [Google Scholar]
- Trinajstić, N; Nikolić, S; Lučić, B. The detour matrix in chemistry. J. Chem. Inf. Model 1997, 37, 631–638. [Google Scholar]
- Milano Chemometrics and QSAR Research Group Homepage.
- Duchowicz, PR; Castro, EA; Fernández, FM; González, MP. A new search algorithm of QSPR/QSAR theories: Normal boiling points of some organic molecules. Chem. Phys. Lett 2005, 412, 376–380. [Google Scholar]
- Duchowicz, PR; Fernández, M; Caballero, J; Castro, EA; Fernández, FM. QSAR of non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg. Med. Chem 2006, 16, 5876–5889. [Google Scholar]
- Hawkins, DM; Basak, SC; Mills, D. Assessing model fit by cross validation. J. Chem. Inf. Model 2003, 43, 579–586. [Google Scholar]
- The Merck Index An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th Ed ed; Merck & Co: Rahway, NJ, USA, 2001.
- Consonni, V; Todeschini, R; Pavan, M. Structure/response correlations and similarity/diversity analysis by GETAWAY descriptors. 2. Application of the novel 3D molecular descriptors to QSAR/QSPR studies. J. Chem. Inf. Model 2002, 42, 693–705. [Google Scholar]
- Consonni, V; Todeschini, R. Rational Approaches to Drug Design; Prous Science: Barcelona, Spain, 2001; pp. 235–240. [Google Scholar]
- Viswanadhan, VN; Ghose, AK; Revankar, GR; Robins, RK. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J. Chem. Inf. Model 1989, 29, 163–172. [Google Scholar]
- Silverman, DB. Three-dimensional moments of molecular property fields. J. Chem. Inf. Model 2000, 40, 1470–1476. [Google Scholar]
- Duchowicz, PR; Talevi, A; Bruno-Blanch, LE; Castro, EA. New QSPR study for the prediction of aqueous solubility of drug-like compounds. Bioorg. Med. Chem 2008, 16, 7944–7955. [Google Scholar]
- Veber, DF; Johnson, SR; Cheng, H; Smith, BR; Ward, KW; Kopple, KD. Molecular property that influence the drug bioavailability of drug candidates. J. Med. Chem 2002, 45, 2615–2623. [Google Scholar]
- Charifson, PS; Walters, WP. Filtering databases and chemical libraries. J. Comput. Aided Mol. Des 2002, 16, 311–323. [Google Scholar]
- Monge, A; Arrault, A; Marot, C; Morin-Allory, L. Managing, profiling and analyzing a library of 2.6 million compounds gathered from 32 chemical providers. Mol. Divers 2006, 10, 339–403. [Google Scholar]
- Walters, WP; Murcko, MA. Prediction of “drug-likeness”. Adv. Drug Deliv. Rev 2002, 54, 255–271. [Google Scholar]
- Liu, K; Feng, J; Young, SS. PowerMV: A software environment for molecular viewing, descriptor generation, data analysis and hit evaluation. J. Chem. Inf. Model 2005, 45, 515–522. [Google Scholar]
- Hyperchem (Hypercube) Homepage.
- Randic, M. Resolution of ambiguities in structure-property studies by use of orthogonal descriptors. J. Chem. Inf. Model 1991, 31, 311–320. [Google Scholar]
- Golbraikh, A; Tropsha, A. Beware of q2! J. Mol. Graphics Model 2002, 20, 269–276. [Google Scholar]
- Wold, S; Eriksson, L. Chemometrics Methods in Molecular Design; VCH: Weinheim, Germany, 1995. [Google Scholar]
- Draper, NR; Smith, H. Applied Regression Analysis; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
- Antipin, IS; Arslanov, NA; Palyulin, VA; Konovalov, AI; Zefirov, NS; of Disperse Interactions. Dokl Akad Nauk SSSR 1991, 316, 925–928, (Chem. Abstr. 115, 91390)..
- Moriguchi, I; Hirono, S; Liu, Q; Nakagome, I; Matsuchita, Y. Simple method of calculating octanol/water partition coefficient. Chem. Pharm. Bull 1992, 40, 127–130. [Google Scholar]
- Yan, A; Gasteiger, J. Prediction of aqueous solubility of organic compounds based on a 3D structure representation. J. Chem. Inf. Model 2003, 43, 429–434. [Google Scholar]
- Hou, TJ; Xia, K; Zhang, W; Xu, XJ. ADME evaluation in drug discovery. 4. Prediction of aqueous solubility based on atom contribution approach. J. Chem. Inf. Model 2004, 44, 266–275. [Google Scholar]
- Huuskonen, J. Estimation of aqueous solubility for a diverse set of organic compounds based on molecular topology. J. Chem. Inf. Model 2000, 40, 773–777. [Google Scholar]


| Description | Requirements | Speed |
|---|---|---|
| Methods based on other experimental physico-chemical properties | log P, MP, etc. | Tens to hundreds compounds per day |
| Methods using 3D parameters depending on molecular stereochemistry | Optimized 3D structure, Monte Carlo, quantum chemical calculations | Tens to tens of thousands compounds per day |
| Fragmental and atom-type based methods using 1D or 2D parameters | Molecule as a smile, 2D graph | Million of compounds per day |
| Lead author | Method | Type of descriptors | Number of parameters | rms | N/d | Reference | Year |
|---|---|---|---|---|---|---|---|
| Klopman | GCM | 2D Substructures | 34 | 1.213 | 0.62 | [14] | 1992 |
| Yan | MLR | 3D Descriptors | 40 | 1.286 | 0.53 | [91] | 2003 |
| Hou | GCM | Atomic | 78 | 0.664 | 0.27 | [92] | 2004 |
| Huuskonen | MLR | Topologicals | 30 | 0.810 | 0.70 | [93] | 2000 |
| Duchowicz | MLR | Dragon | 3 | 1.202 | 7.00 | this study | 2008 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Duchowicz, P.R.; Castro, E.A. QSPR Studies on Aqueous Solubilities of Drug-Like Compounds. Int. J. Mol. Sci. 2009, 10, 2558-2577. https://doi.org/10.3390/ijms10062558
Duchowicz PR, Castro EA. QSPR Studies on Aqueous Solubilities of Drug-Like Compounds. International Journal of Molecular Sciences. 2009; 10(6):2558-2577. https://doi.org/10.3390/ijms10062558
Chicago/Turabian StyleDuchowicz, Pablo R., and Eduardo A. Castro. 2009. "QSPR Studies on Aqueous Solubilities of Drug-Like Compounds" International Journal of Molecular Sciences 10, no. 6: 2558-2577. https://doi.org/10.3390/ijms10062558