Molecular Momentum Transport at Fluid-Solid Interfaces in MEMS/NEMS: A Review
Abstract
:1. Introduction
1.1. Backgrounds
1.2. History
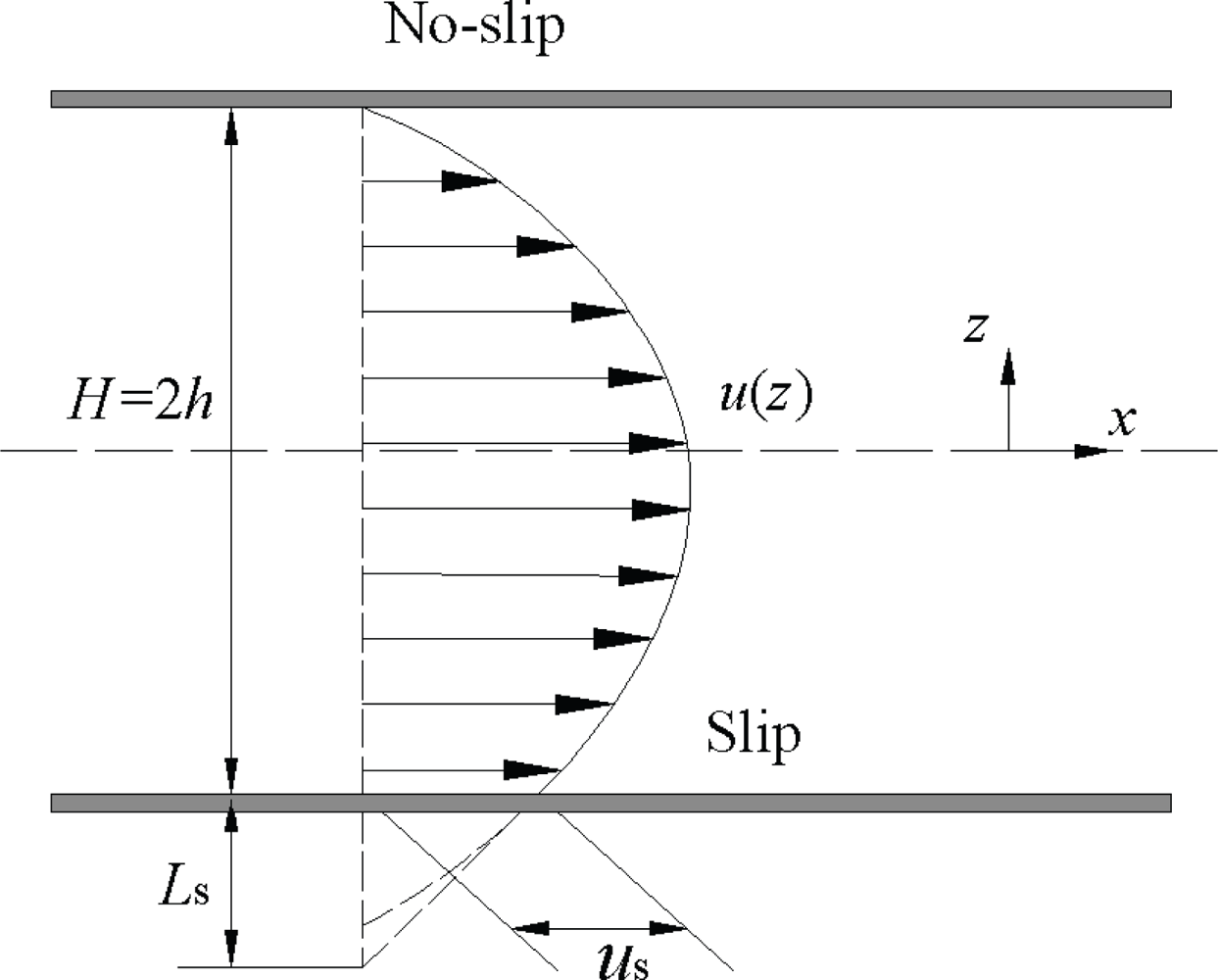
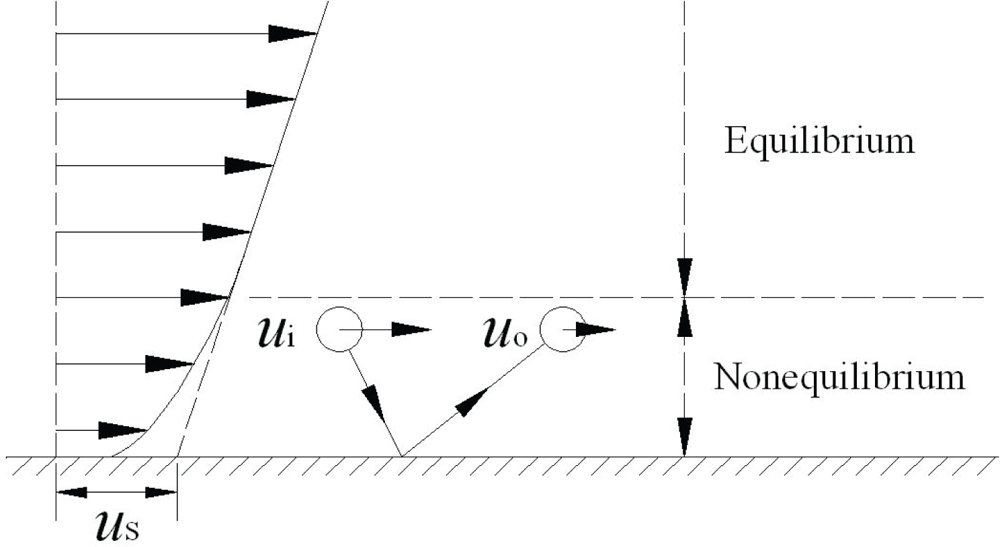
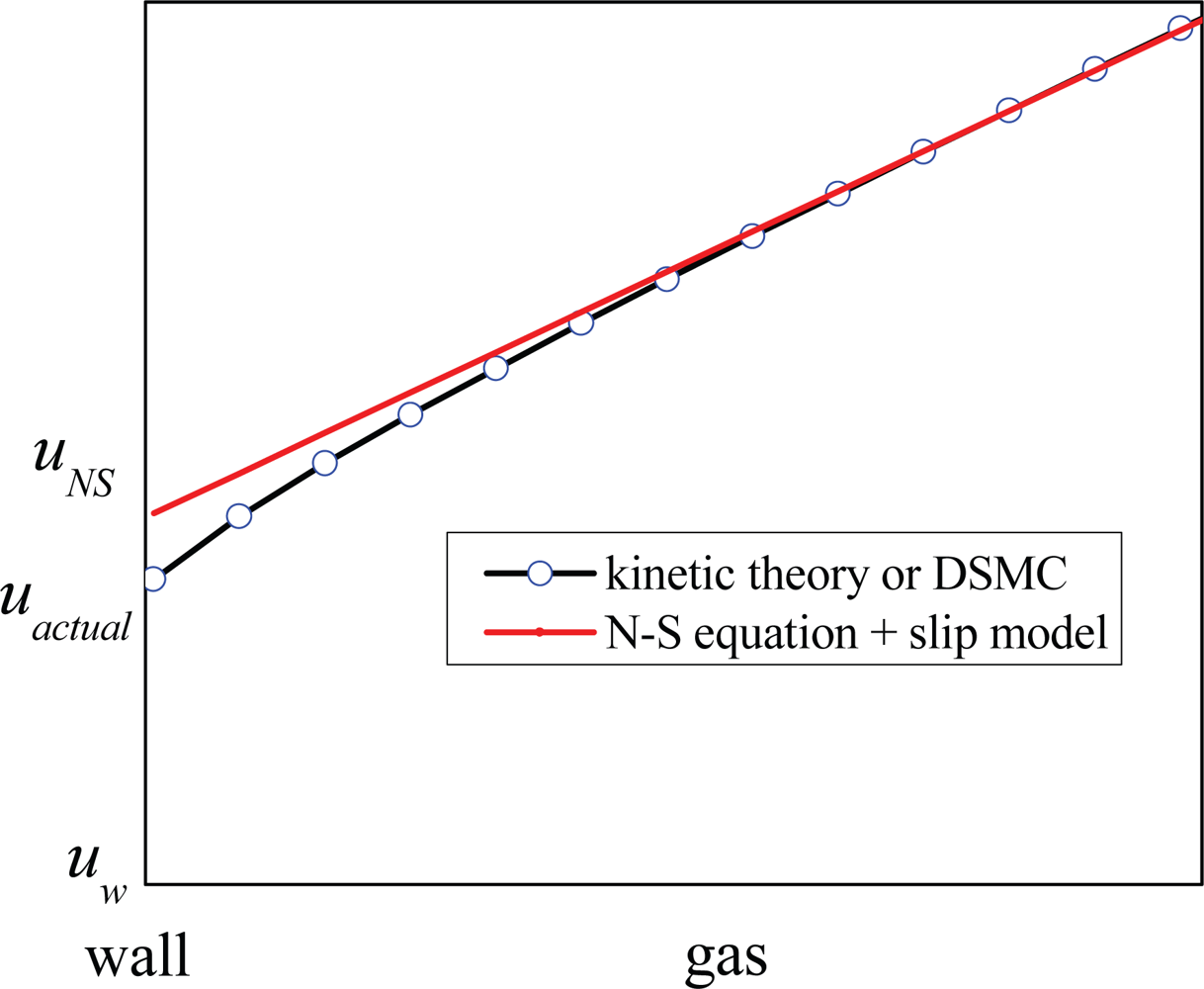
1.3. Molecular Momentum Transport and Boundary Conditions at Fluid-Solid Interfaces
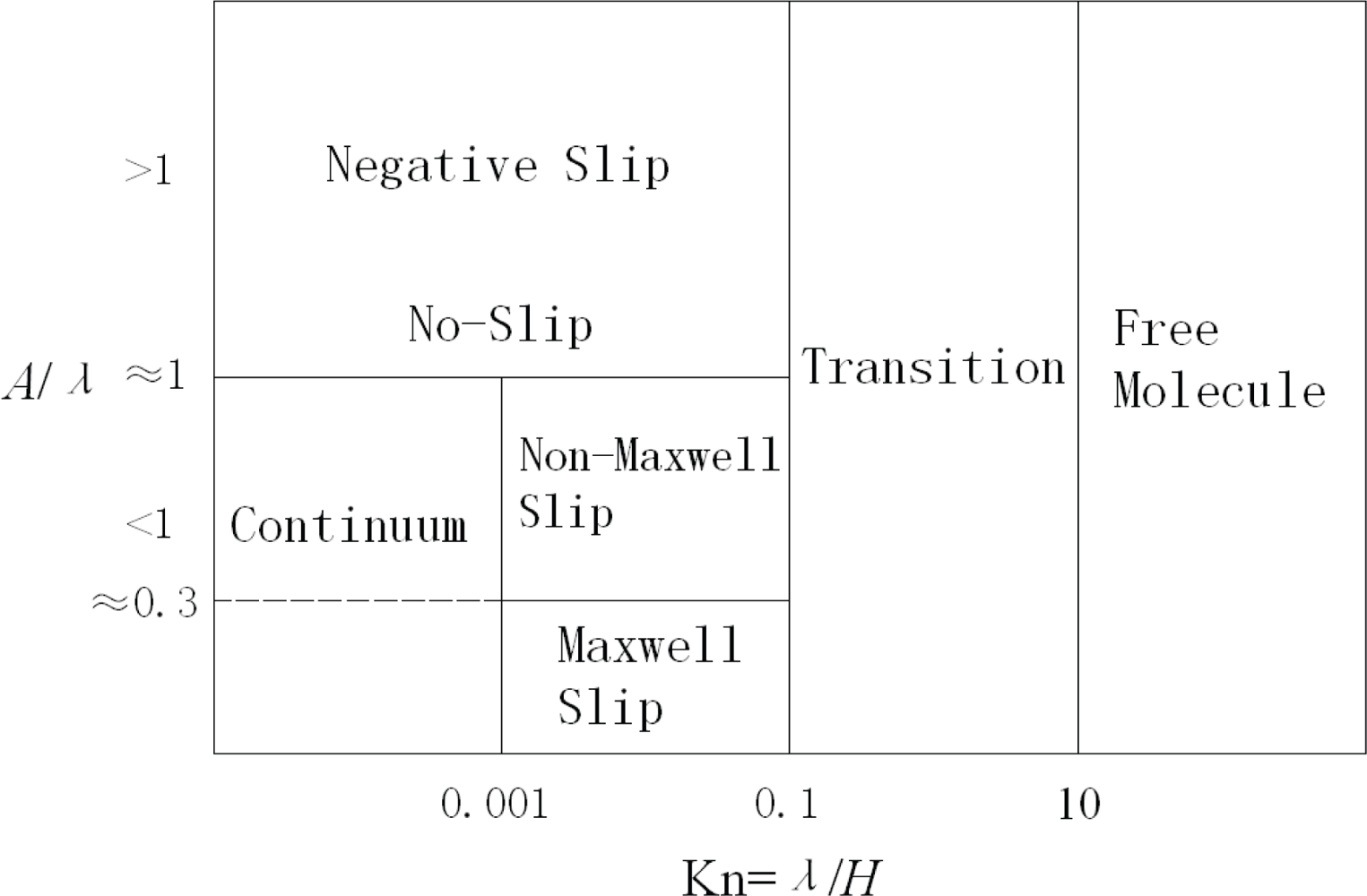
2. Gas-Solid Interfaces
| Kn < 0.001 | Continuum regime |
| 0.001< Kn < 0.1 | Slip regime |
| 0.1< Kn < 10 | Transition regime |
| Kn > 10 | Free molecular regime |
2.1. Description of Molecular Distributions
2.2. Tangential Momentum Transport
2.2.1. Slip Models
2.2.1.1. Linear Slip Models
2.2.1.2. Nonlinear Slip Models
2.2.2. TMAC
2.2.2.1. Experiments on TMAC
2.2.2.2. Simulations on TMAC
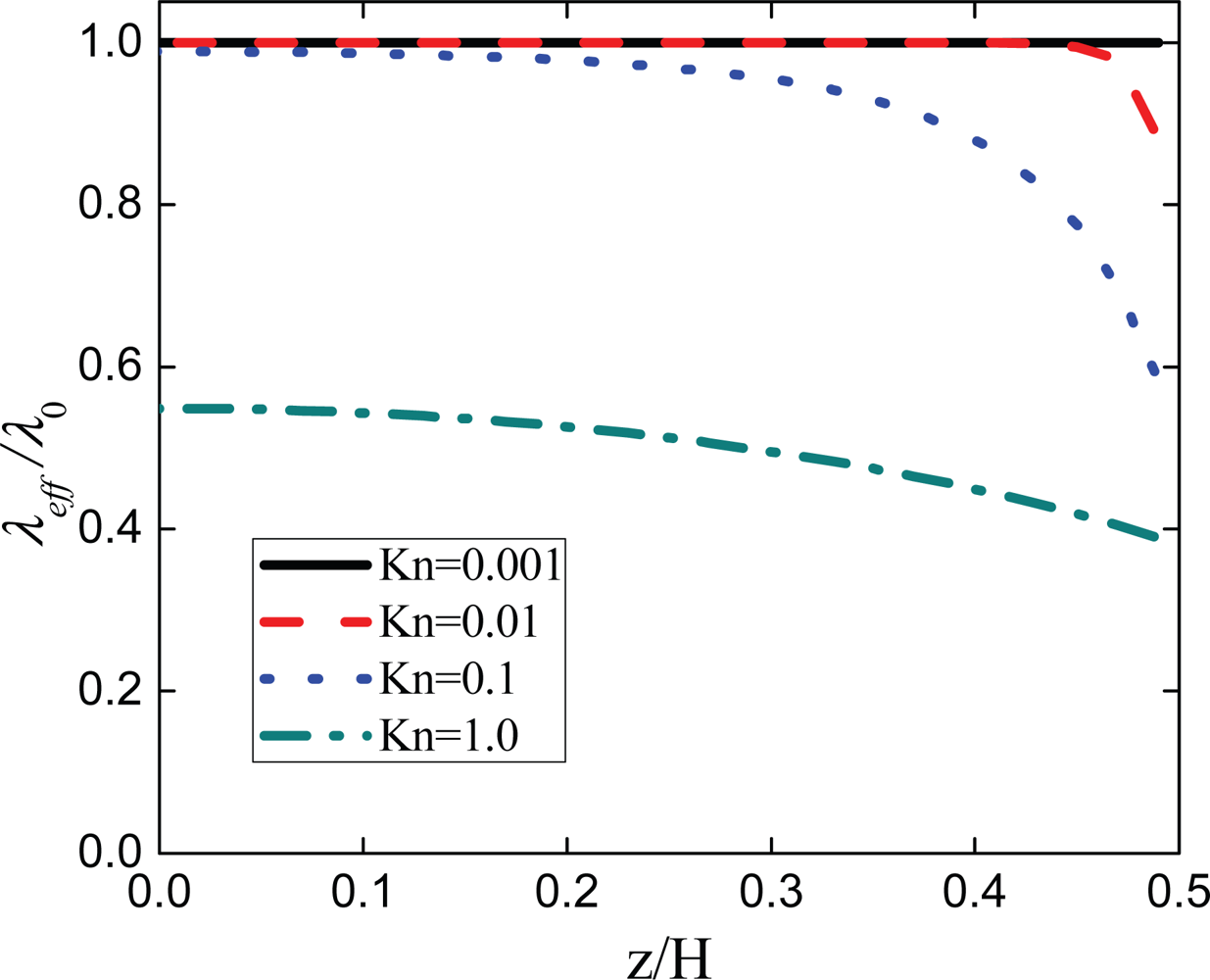
2.3. Research Insufficiency
2.3.1. Non-Maxwell Reflections
2.3.2. Normal Momentum Transport
3. Liquid-Solid Interfaces
3.1. Experimental Measurements
3.1.1. Indirect Methods
3.1.1.1. Flow Rate through Capillaries or Microchannels (FR)
3.1.1.2. Drainage Force (DF)
3.1.1.3. Other Techniques
3.1.2. Direct Methods
3.1.2.1. Micro Particle Image Velocimetry (μPIV)
3.1.2.2. Near-Field Laser Velocimetry Using Fluorescence Recovery (NFLV-FR)
3.1.2.3. Fluorescence Cross-Correlations (FCC)
3.1.2.4. Total Internal Reflection Velocimetry (TRIC)
3.2. Molecular Dynamics Simulations
3.3. Dependence on Physical Factors
3.3.1. Surface Wettability
3.3.2. Surface Roughness
3.3.3. Shear Rate
3.3.4. Nanobubbles or Gas Films
3.3.5. Other Factors
3.3.5.1. Polarity of Liquids
3.3.5.2. Viscosity
3.3.5.3. Temperature
3.3.5.4. Pressure or Pressure Gradient
3.3.5.5. Carbon Nanotubes
4. Summary and Conclusions
Acknowledgments
References
- Feynman, RP. There’s plenty of room at the bottom. In Miniaturization; Gillbert, HD, Ed.; Reinhold Publishing: New York, NY, USA, 1961; pp. 282–296. [Google Scholar]
- Craighead, HG. Nanoelectromechanical systems. Science 2000, 290, 1532–1535. [Google Scholar]
- Hsu, TR. MEMS and Microsystems: Design and Manufacture; McGraw-Hill: Boston, MA, USA, 2002. [Google Scholar]
- Lyshevski, SE. MEMS and NEMS: Systems, Devices, and Structures; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Meyyappan, M. Carbon Nanotubes: Science and Applications; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Allen, JJ. Micro Electro Mechanical System Design; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Leondes, CT. MEMS/NEMS: Handbook Techniques and Applications; Springer: New York, NY, USA, 2006. [Google Scholar]
- Rogers, B; Pennathur, S; Adams, J. Nanotechnology: Understanding Small Systems; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cao, BY; Hou, QW; Guo, ZY. Nanomotors actuated by phonon current. In MEMS: Technology, Fabrication Processes and Applications; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Gravesen, P; Branebjerg, J; Jensen, OS. Microfluidics – A review. J Micromech Microeng 1993, 3, 168–182. [Google Scholar]
- Ho, CM; Tai, YC. MEMS and its applications for flow control. J Fluids Eng 1996, 118, 437–447. [Google Scholar]
- Ho, CM; Tai, YC. Micro-electro-mechanical-systems (MEMS) and fluid flows. Annu Rev Fluid Mech 1998, 30, 579–612. [Google Scholar]
- Gad-el-Hak, M. The fluid mechanics of microdevices-the Freeman scholar lecture. J. Fluids Eng 1999, 121, 5–33. [Google Scholar]
- Giordano, N; Cheng, JT. Microfluid mechanics: Progress and opportunities. J. Phys.-Condens. Mat 2001, 13, R271–R295. [Google Scholar]
- Rostami, AA; Majumdar, AS; Saniel, N. Flow and heat transfer for gas flowing in microchannels: A review. Heat Mass Transfer 2002, 38, 339–367. [Google Scholar]
- Liou, WW; Fang, YC. Microfluid Mechanics: Principles and Modeling; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Guo, ZY; Li, ZX. Size effect on microscale single-phase flow and heat transfer. Int. J. Heat Mass Tran 2003, 46, 149–159. [Google Scholar]
- Stone, HA; Stroock, AD; Ajdari, A. Engineering flows in small devices: Microfluidics toward a lab-on-a-chip. Annu. Rev. Fluid Mech 2004, 36, 381–411. [Google Scholar]
- Squires, TM; Quake, SR. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys 2005, 77, 977–1026. [Google Scholar]
- Whitesides, GM. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar]
- Mahulikar, SP; Herwig, H; Hausner, O. Study of Gas microconvection for synthesis of rarefaction and nonrarefaction effects. J. Microelectromech. Syst 2007, 16, 1542–1556. [Google Scholar]
- Holt, JK. Methods for probing water at the nanoscale. Microfluid Nanofluid 2008, 5, 425–442. [Google Scholar]
- Nicholson, D; Bhatia, SK. Fluid transport in nanospaces. Mol. Simulat 2009, 35, 109–121. [Google Scholar]
- White, FM. Fluid Mechanics; WCB/McGraw-Hill: Boston, MA, USA, 2003. [Google Scholar]
- Kauzmann, W. Kinetic Theory of Gases; Benjamin, Inc: New York, NY, USA, 1976. [Google Scholar]
- Succi, S. The Lattice Boltzmann Equation for Fluid Mechanics and Beyond; Oxford University Press Inc: New York, NY, USA, 2001. [Google Scholar]
- Bird, GA. Molecular Gas Dynamics and the Direct Simulation of Gas Flows; Oxford University Press Inc: New York, NY, USA, 1994. [Google Scholar]
- Vinogradova, OI. Slippage of water over hydrophobic surfaces. Int. J. Miner. Process 1999, 56, 31–60. [Google Scholar]
- Granick, S; Zhu, YX; Lee, HJ. Slippery questions about complex fluids flowing past solids. Nat. Mater 2003, 2, 221–227. [Google Scholar]
- Neto, C; Evans, DR; Bonaccurso, E; Butt, HJ; Craig, VSJ. Boundary slip in Newtonian liquids: A review of experimental studies. Rep. Prog. Phys 2005, 68, 2859–2897. [Google Scholar]
- Lauga, E; Brenner, MP; Stone, HA. Microfluidics: The no-slip boundary condition. In Handbook of Experimental Fluid Dynamics; Tropea, C, Yarin, AL, Foss, JF, Eds.; Springer: New York, NY, USA, 2007; ; Chapter 19, pp. 1219–1240. [Google Scholar]
- Bocquet, L; Barrat, JL. Flow boundary conditions from nano- to micro-scales. Soft Matter 2007, 3, 685–693. [Google Scholar]
- Voronov, RS; Papavassilion, DV; Lee, LL. Review of fluid slip over superhydrophobic surfaces and its dependence on the contact angle. Ind. Eng. Chem. Res 2008, 47, 2455–2477. [Google Scholar]
- Goldstein, S. Note on the condition at the surface of contact of a fluid with a solid body. In Modern Development in Fluid Mechanics; Volume 2, Goldstein, S, Ed.; Clarendon Press: Oxford, UK, 1938. [Google Scholar]
- Goldstein, S. Fluid mechanics in first half of this century. Annu. Rev. Fluid Mech 1969, 1, 1–28. [Google Scholar]
- Navier, CLMH. Mémoire sur les lois du movement des fluides. Mémoires de l’Académie Royale des Sciences de l’Institut de France 1823, 6, 389–440. [Google Scholar]
- Stokes, GG. On the theories of the internal friction of fluids in motion, and of the equilibrium and motion of elastic solids. In Mathematical and Physical Papers by George Gabriel Stokes; Stokes, GG, Ed.; Cambridge University Press: Cambridge, UK, 1966; Volume 1, pp. 75–187. [Google Scholar]
- Poiseuille, J. Recherches expérimentales sur le mouvement des liquides dans les tubes de tréspetits diamétres. C. R. Acad. Sci 1841, 12, 112–115. [Google Scholar]
- Darcy, H. Recherches Expérimentales Relatives au Mouvement de L’eau Dans les Tuyaux; Mallet-Bachelier: Paris, France, 1857. [Google Scholar]
- von Helmholtz, H. Wissenschaftliche Abhandlungen; Barth, JA, Leipzig, GER, Eds.; 1882; Volume 1, pp. 196–222. [Google Scholar]
- Maxwell, JC. On the viscosity or internal friction of air and other gases. In The Scientific Papers of James Clerk Maxwell; Cambridge University Press: Cambridge, UK, 1890; Volume 2, pp. 1–25. [Google Scholar]
- Whetham, WCD. On the alleged slipping at the boundary of a liquid in motion. Philos. T. Roy. Soc. A 1890, 181, 559–582. [Google Scholar]
- Couette, M. Etudes sur le frottement des liquids. Annales des Chimie et des Physique 1890, 21, 433–510. [Google Scholar]
- Ladenburg, R. Uber der einfluss von wanden auf die bewegung einer kugel in einer reibenden flussigkeit. Ann. Phys 1907, 4, 447–458. [Google Scholar]
- Lamb, H. Hydrodynamics; Dover: New York, NY, USA, 1932. [Google Scholar]
- Batchelor, GK. An Introduction to Fluid Dynamics; Cambridge University Press: Cambridge, UK, 1967. [Google Scholar]
- Ciccotti, G; Hoover, WG. Molecular Dynamics Simulation of Statistical mechanics Systems; North-Holland: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Allen, MP; Tildesley, DJ. Computer Simulation of Liquids; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Haile, JM. Molecular Dynamics Simulation: Elementary Methods; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Koplik, J; Banavar, JR. Continuum deductions from molecular hydrodynamics. Annu. Rev. Fluid Mech 1995, 27, 257–292. [Google Scholar]
- Binder, K; Horbach, J; Kov, W; Paul, W; Varnik, F. Molecular dynamics simulations. J. Phys.-Condens. Mat 2004, 16, S429–S453. [Google Scholar]
- Maxwell, JC. On stresses in rarefied gases arising from inequalities of temperature. Philos. T. Roy. Soc 1879, 170, 231–256. [Google Scholar]
- Knudsen, M. Die Gesetze der Molekularströmung und der inneren Reibungsströmung der Gase durch Röhren. Ann. Phys 1909, 28, 75–130. [Google Scholar]
- Schaaf, SA; Chambre, PL. Flow of Rarefied Gases; Princeton University: New Jersey, NJ, USA, 1961. [Google Scholar]
- Muntz, EP. Rarefied-gas dynamics. Annu. Rev. Fluid Mech 1989, 21, 287–417. [Google Scholar]
- Cercignani, C. Rarefied Gas Dynamics: From Basic Concepts to Actual Calculations; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Millikan, RA. Coefficients of slip in gases and the law of reflection of molecules from the surfaces of solids and liquids. Phys. Rev 1923, 21, 217–238. [Google Scholar]
- van Dyke, KS. The coefficients of viscosity and of slip of air and of carbon dioxide by the rotating cylinder method. Phys. Rev 1923, 21, 250–265. [Google Scholar]
- Chiang, SF. Drag Forces on Rotating Cylinders at Low Pressures, , PhD Thesis, University of California, US, 1952.
- Beams, JW; Young, JL, III; Moore, JW. The production of high centrifugal fields. J. Appl. Phys 1946, 17, 886–890. [Google Scholar]
- Kuhlthau, AR. Air friction on rapidly moving surfaces. J. Appl. Phys 1949, 20, 217–223. [Google Scholar]
- Willis, DR. Comparison of kinetic theory analyses of linearized Couette flow. Phys. Fluids 1962, 5, 127–135. [Google Scholar]
- Cercignani, C; Pagani, CD. Variational approach to boundary-value problems in kinetic theory. Phys. Fluids 1966, 9, 1167–1173. [Google Scholar]
- Reddy, KC. Rarefied-gas flow in the Knudsen layer. Phys. Fluids 1968, 11, 1308–1311. [Google Scholar]
- Lan, XD; Sun, J; Li, ZX. Modified relaxation time Monte Carlo method for continuum-transition gas flows. J. Comput. Phys 2008, 227, 4763–4775. [Google Scholar]
- Karniadakis, GE; Beskok, A. Micro Flows: Fundamentals and Simulations; Springer: New York, NY, USA, 2002. [Google Scholar]
- Lockerby, DA; Reese, JM; Gallis, MA. The usefulness of higher-order constitutive relations for describing the Knudsen layer. Phys. Fluids 2005, 17, 100609. [Google Scholar] [Green Version]
- Cercignani, C. The Boltzmann Equation and Its Applications; Springer-Verlag: New York, NY, USA, 1988. [Google Scholar]
- Zhong, X; Chapman, DR; MacCormack, RW. Stabilization of the Burnett equations and application to hypersonic flows. AIAA J 1993, 31, 1036–1043. [Google Scholar]
- Balakrishnan, R. An approach to entropy consistency in second-order hydrodynamic equations. J. Fluid Mech 2004, 503, 201–245. [Google Scholar]
- Jin, S; Slemrod, M. Regularization of the Burnett equations via relaxation. J. Stat. Phys 2001, 103, 1009–1033. [Google Scholar]
- Struchtrup, H; Torrilhon, M. Regularization of Grad’s 13 moment equations: Derivation and linear analysis. Phys. Fluids 2003, 15, 2668–2680. [Google Scholar]
- Guo, ZY; Li, ZX. Size effect on single-phase channel flow and heat transfer at microscale. Int. J. Heat Fluid Flow 2003, 24, 284–298. [Google Scholar]
- Kuscer, I. Phenomenology of gas-surface accommodation. In Rarefied Gas Dynamics, Proceeding of the Ninth International Symposium; Becker, M, Fiebig, M, Eds.; DFVLR: Porz-Wahn: Germany, 1974; pp. E.1–1–21. [Google Scholar]
- Kennard, EH. Kinetic Theory of Gases: With an Introduction to Statistical Mechanics; McGraw-Hill Book Company: New York, NY/London, USA/UK, 1938. [Google Scholar]
- Cercignani, C; Lampis, M. Kinetic models for gas-surface interactions. Transp. Theory Stat. Phys 1971, 1, 101–114. [Google Scholar]
- Lord, RG. Some extensions to the Cercignani-Lampis gas-surface scattering kernel. Phys. Fluids A 1991, 3, 706–710. [Google Scholar]
- Lord, RG. Some further extensions of the Cercignani-Lampis gas-surface interaction model. Phys. Fluids 1995, 7, 1159–1161. [Google Scholar]
- Yamanishi, N; Matsumoto, Y; Shobatake, K. Multistage gas-surface interaction model for the direct simulation Monte Carlo method. Phys. Fluids 1999, 11, 3540–3552. [Google Scholar]
- Nocilla, S. The surface re-emission law in free molecule flow. In Rarefied Gas Dynamics, Proceedings of the Third International Symposium; Laurmann, JA, Ed.; Academic: Paris, France, 1962; Volume 1, p. 327. [Google Scholar]
- Hurlbut, FC; Sherman, FS. Application of the Nocilla wall reflection model to free-molecule kinetic theory. Phys. Fluids 1968, 11, 486–496. [Google Scholar]
- Collins, FG; Knox, EC. Determination of wall boundary-conditions for high-speed-ratio direct simulation Monte-Carlo calculations. J. Spacecraft Rockets 1994, 31, 965–970. [Google Scholar]
- Collins, FG; Knox, EC. Parameters of Nocilla gas-surface interaction-model from measured accommodation coefficients. AIAA J 1994, 32, 765–773. [Google Scholar]
- Wadsworth, DC; VanGilder, DB; Dogra, VK. Gas-surface interaction model evaluation for DSMC applications. Rarefied Gas Dynamics 2003, 663, 965–972. [Google Scholar]
- Burnett, D. The distribution of velocities in a slightly non-uniform gas. P. Lond. Math. Soc 1935, 39, 385–430. [Google Scholar]
- Shavaliyev, MS. Super-Burnett corrections to the stress tensor and the heat-flux in a gas of Maxwellian molecules. PMM J. Appl. Math. Mech 1993, 57, 573–576. [Google Scholar]
- Torrilhon, M; Struchtrup, H. Regularized 13-moment equations: Shock structure calculations and comparison to Burnett models. J. Fluid Mech 2004, 513, 171–198. [Google Scholar]
- Agrawal, A; Prabhu, SV. Survey on measurement of tangential momentum accommodation coefficient. J. Vac. Sci. Technol. A 2008, 26, 634–645. [Google Scholar]
- Albertoni, S; Cercignani, C; Gotusso, L. Numerical evaluation of the slip coefficient. Phys. Fluids 1963, 6, 993–996. [Google Scholar]
- Chapman, S; Cowling, TG. The Mathematical Theory of Non-uniform Gases, 3rd ed; The University Press: Cambridge, UK, 1970. [Google Scholar]
- Loyalka, SK. Approximate method in kinetic theory. Phys. Fluids 1971, 14, 2291–2294. [Google Scholar]
- Loyalka, SK; Petrellis, N; Storvick, TS. Some numerical results for BGK model - thermal creep and viscous slip problems with arbitrary accomodation at surface. Phys. Fluids 1975, 18, 1094–1099. [Google Scholar]
- Hadjiconstantinou, NG. Comment on Cercignani’s second-order slip coefficient. Phys. Fluids 2003, 15, 2352–2354. [Google Scholar]
- Maurer, J; Tabeling, P; Joseph, P; Willaime, H. Second-order slip laws in microchannels for helium and nitrogen. Phys. Fluids 2003, 15, 2613–2621. [Google Scholar]
- Dongari, N; Agrawal, A; Agrawal, A. Analytical solution of gaseous slip flow in long microchannels. Int. J. Heat Mass Tran 2007, 50, 3411–3421. [Google Scholar]
- Tang, GH; He, YL; Tao, WQ. Comparison of gas slip models with solutions of linearized Boltzmann equation and direct simulation of Monte Carlo method. Int. J. Mod. Phys. C 2007, 18, 203–216. [Google Scholar]
- Barber, RW; Emerson, DR. Challenges in modeling gas-phase flow in microchannels: From slip to transition. Heat Transfer Eng 2006, 27, 3–12. [Google Scholar]
- Mitsuya, Y. Modified reynolds equation for ultra-thin film gas lubrication using 1.5-order slip-flow model and considering surface accommodation coefficient. J. Trib 1993, 115, 289–294. [Google Scholar]
- Pan, LS; Liu, GR; Lam, KY. Determination of slip coefficient for rarefied gas flows using direct simulation Monte Carlo. J. Micromech. Microeng 1999, 9, 89–96. [Google Scholar]
- Lockerby, DA; Reese, JM; Emerson, DR; Barber, RW. Velocity boundary condition at solid walls in rarefied gas calculations. Phys. Rev. E 2004, 70, 017303. [Google Scholar] [Green Version]
- Wu, LA. slip model for rarefied gas flows at arbitrary Knudsen number. Appl. Phys. Lett 2008, 93, 253103. [Google Scholar]
- Lockerby, DA; Reese, JM; Gallis, MA. Capturing the Knudsen layer in continuum-fluid models of nonequilibrium gas flows. AIAA J 2005, 43, 1391–1393. [Google Scholar]
- Schram, PPJM. Kinetic Theory of Gases and Plasmas; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Einzel, D; Panzer, P; Liu, M. Boundary-condition for fluid-flow - curved or rough surfaces. Phys. Rev. Lett 1990, 64, 2269–2272. [Google Scholar]
- Reese, JM; Zheng, YS; Lockerby, DA. Computing the near-wall region in gas micro- and nanofluidics: Critical Knudsen layer phenomena. J. Comput. Theor. Nanos 2007, 4, 807–813. [Google Scholar]
- O’Hare, L; Lockerby, DA; Reese, JM; Emerson, DR. Near-wall effects in rarefied gas microflows: Some modern hydrodynamic approaches. Int. J. Heat Fluid Flow 2007, 28, 37–43. [Google Scholar]
- Bhatnagar, PL; Gross, EP; Krook, M. A model for collision processes in gases i: Small amplitude processes in charged and neutral onecomponent systems. Phys. Rev 1954, 94, 511–525. [Google Scholar]
- Lockerby, DA; Reese, JM. On the modelling of isothermal gas flows at the microscale. J. Fluid Mech 2008, 604, 235–261. [Google Scholar]
- Stops, DW. The mean free path of gas molecules in the transition regime. J. Phys. D-Appl. Phys 1970, 3, 685–696. [Google Scholar]
- Guo, ZL; Zheng, CG; Shi, BC. The extended second-order slip boundary condition in microscale flows. Proceedings of National Heat and Mass Transfer Conference of Engineering Thermophysics Society of China, Zhengzhou, China; 2008. [Google Scholar]
- Arlemark, EJ; Dadzie, SK; Reese, JM. An extension to the Navier-Stokes-Fourier equations by considering molecular collisions with boundaries. Proceedings of the 6th International Conference on Nanochannels, Microchannels, and Minichannels, Darmstadt, Germany; 2008; pp. 95–102. [Google Scholar]
- Arlemark, EJ; Dadzie, SK; Reese, JM. An extension to the Navier-Stokes equations to incorporate gas molecular collisions with boundaries. J Heat Transfer 2009. in Press.. [Google Scholar]
- Guo, ZL; Shi, BC; Zheng, CG. An extended Navier-Stokes formulation for gas flows in the Knudsen layer near a wall. Europhys. Lett 2007, 80, 24001. [Google Scholar]
- Ohwada, T; Sone, Y; Aoki, K. Numerical analysis of the Poiseuille and thermal transpiration flows between two parallel plates on the basis of the Boltzmann equation for hard-sphere molecules. Phys. Fluids A 1989, 1, 2042–2049. [Google Scholar]
- Ohwada, T; Sone, Y; Aoki, K. Erratum: Numerical analysis of the Poiseuille and thermal transpiration flows between two parallel plates on the basis of the Boltzmann equation for hard-sphere molecules” [phys. Fluids a [bold 1], 2042 (1989)]. Phys. Fluids A 1990, 2, 639–639. [Google Scholar]
- Dadzie, SK; Reese, JM; McInnes, CR. A continuum model of gas flows with localized density variations. Physica A 2008, 387, 6079–6094. [Google Scholar] [Green Version]
- Sun, J; Li, ZX. Effect of gas adsorption on momentum accommodation coefficients in microgas flows using molecular dynamic simulations. Mol. Phys 2008, 106, 2325–2332. [Google Scholar]
- Knechtel, ED; Pitts, WC. Experimental momentum accommodation on metal surfaces of ions near and above earth-satellite speeds. In Rarefied gas dynamics: Proceedings of the sixth International Symposium on Rarefied Gas Dynamics; Trilling, L, Wachman, HY, Eds.; Academic: New York, NY, USA, 1969; pp. 1257–1266. [Google Scholar]
- Yamamoto, K. Slightly rarefied gas flow over a smooth platinum surface. In Rarefied Gas Dynamics: 22nd International Symposium; Bartel, TJ, Gallis, MA, Eds.; 2001; pp. 339–346. [Google Scholar]
- Park, JH; Baek, SW. Investigation of influence of thermal accommodation on oscillating microflow. Int. J. Heat Mass Tran 2004, 47, 1313–1323. [Google Scholar]
- Toennies, JP. Scattering of molecular beams from surfaces. Appl. Phys 1974, 3, 91–114. [Google Scholar]
- Goodman, FO; Wachman, HY. Dynamics of Gas-Surface Scattering; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Hurlbut, FC. Gas surface interactions: Recent observations and interpretations. In Rarefied gas dynamics: Proceedings of the 20th International Symposium: 19–23, August, 1996, Bejing, China; Shen, C, Ed.; Peking University Press: Beijing, China, 1997; pp. 355–367. [Google Scholar]
- Ewart, T; Perrier, P; Graur, I; Méolans, J. Tangential momemtum accommodation in microtube. Microfluid. Nanofluid 2007, 3, 689–695. [Google Scholar]
- Doughty, RO; Schaetzle, WJ. Experimental determination of momentum accommodation coefficients at velocities up to and exceeding earth escape velocity. In Rarefied Gas Dynamics, Proceedings of the Sixth International Symposium; Trilling, L, Wachman, HY, Eds.; Academic Press: New York, NY, USA, 1969; pp. 1035–1054. [Google Scholar]
- Seidl, M; Steinheil, E. Measurement of momentum accommodation coefficients on surfaces characterized by Auger Spectroscopy, Sims and Leed. In Rarefied Gas Dynamics, Ninth International Symposium; Becker, M, Fiebig, M, Eds.; DFVLR: Porz-Wahn: Germany, 1974; pp. E 9.1–E 9.12. [Google Scholar]
- Liu, SM; Sharma, PK; Knuth, EL. Satellite drag coefficients calculated from measured distributions of reflected helium atoms. AIAA J 1979, 17, 1314–1319. [Google Scholar]
- Rettner, CT. Thermal and tangential-momentum accommodation coefficients for N-2 colliding with surfaces of relevance to disk-drive air bearings derived from molecular beam scattering. IEEE T. Magn 1998, 34, 2387–2395. [Google Scholar]
- Beams, JW; Young, JL; Moore, JW. The production of high centrifugal fields. J. Appl. Phys 1946, 17, 886–890. [Google Scholar]
- Loyalka, SK. Theory of the spinning rotor gauge in the slip regime. J. Vac. Sci. Technol. A 1996, 14, 2940–2945. [Google Scholar]
- Chang, RF; Abbott, PJ. Factors affecting the reproducibility of the accommodation coefficient of the spinning rotor gauge. J. Vac. Sci. Technol. A 2007, 25, 1567–1576. [Google Scholar]
- Comsa, G; Fremerey, JK; Lindenau, B; Messer, G; Rohl, P. Calibration of a spinning rotor gas friction gauge against a fundamental vacuum pressure standard. J. Vac. Sci. Technol 1980, 17, 642–644. [Google Scholar]
- Gabis, DH; Loyalka, SK; Storvick, TS. Measurements of the tangential momentum accommodation coefficient in the transition flow regime with a spinning rotor gauge. J. Vac. Sci. Technol. A 1996, 14, 2592–2598. [Google Scholar]
- Tekasakul, P; Bentz, JA; Tompson, RV; Loyalka, SK. The spinning rotor gauge: Measurements of viscosity, velocity slip coefficients, and tangential momentum accommodation coefficients. J. Vac. Sci. Technol. A 1996, 14, 2946–2952. [Google Scholar]
- Bentz, JA; Tompson, RV; Loyalka, SK. The spinning rotor gauge: Measurements of viscosity, velocity slip coefficients, and tangential momentum accommodation coefficients for N2 and CH4. Vacuum 1997, 48, 817–824. [Google Scholar]
- Bentz, JA; Tompson, RV; Loyalka, SK. Viscosity and velocity slip coefficients for gas mixtures: Measurements with a spinning rotor gauge. J. Vac. Sci. Technol. A 1999, 17, 235–241. [Google Scholar]
- Jousten, K. Is the effective accommodation coefficient of the spinning rotor gauge temperature dependent? J. Vac. Sci. Technol. A 2003, 21, 318–324. [Google Scholar]
- Bentz, JA; Tompson, RV; Loyalka, SK. Measurements of viscosity, velocity slip coefficients, and tangential momentum accommodation coefficients using a modified spinning rotor gauge. J. Vac. Sci. Technol. A 2001, 19, 317–324. [Google Scholar]
- Marino, L. Experiments on rarefied gas flows through tubes. Microfluid. Nanofluid 2009, 6, 109–119. [Google Scholar]
- Arkilic, EB; Breuer, KS; Schmidt, MA. Mass flow and tangential momentum accommodation in silicon micromachined channels. J. Fluid Mech 2001, 437, 29–43. [Google Scholar]
- Colin, S; Lalonde, P; Caen, R. Validation of a second-order slip flow model in rectangular microchannels. Heat Transfer Eng 2004, 25, 23–30. [Google Scholar]
- Hsieh, SS; Tsai, HH; Lin, CY; Huang, CF; Chien, CM. Gas flow in a long microchannel. Int. J. Heat Mass Tran 2004, 47, 3877–3887. [Google Scholar]
- Jang, J; Wereley, ST. Effective heights and tangential momentum accommodation coefficients of gaseous slip flows in deep reactive ion etching rectangular microchannels. J. Micromech. Microeng 2006, 16, 493–504. [Google Scholar]
- Jang, J; Wereley, ST. Gaseous slip flow analysis of a micromachined flow sensor for ultra small flow applications. J. Micromech. Microeng 2007, 17, 229–237. [Google Scholar]
- Huang, C; Gregory, JW; Sullivan, JP. Microchannel pressure measurements using molecular sensors. J. Microelectromech. Syst 2007, 16, 777–785. [Google Scholar]
- Cooper, SM; Cruden, BA; Meyyappan, M; Raju, R; Roy, S. Gas transport characteristics through a carbon nanotubule. Nano Lett 2004, 4, 377–381. [Google Scholar]
- Blanchard, D; Ligrani, P. Slip and accommodation coefficients from rarefaction and roughness in rotating microscale disk flows. Phys. Fluids 2007, 19, 063602–063612. [Google Scholar]
- Sun, Y; Barber, RW; Emerson, DR. Inverted velocity profiles in rarefied cylindrical couette gas flow and the impact of the accommodation coefficient. Phys. Fluids 2005, 17, 047102–047107. [Google Scholar]
- Stacy, LJ. A determination by the constant deflection method of the value of the coefficient of slip for rough and for smooth surfaces in air. Phys. Rev 1923, 21, 239–249. [Google Scholar]
- Agrawal, A; Prabhu, SV. Deduction of slip coefficient in slip and transition regimes from existing cylindrical couette flow data. Exp. Therm. Fluid Sci 2008, 32, 991–996. [Google Scholar]
- Maali, A; Bhushan, B. Slip-length measurement of confined air flow using dynamic atomic force microscopy. Phys Rev E 2008, 78, 027302.1–027302.4. [Google Scholar]
- Suetin, PE; Porodnov, BT; Chernjak, VG; Borisov, SF. Poiseuille flow at arbitrary Knudsen numbers and tangential momentum accommodation. J. Fluid Mech 1973, 60, 581–592. [Google Scholar]
- Porodnov, BT; Suetin, PE; Borisov, SF; Akinshin, VD. Experimental investigation of rarefied gas flow in different channels. J. Fluid Mech 1974, 64, 417–438. [Google Scholar]
- Shields, FD. An acoustical method for determining the thermal and momentum accommodation of gases on solids. J. Chem. Phys 1975, 62, 1248–1252. [Google Scholar]
- Shields, FD. More on the acoustical method of measuring energy and tangential momentum accommodation coefficients. J. Chem. Phys 1980, 72, 3767–3772. [Google Scholar]
- Shields, FD. Energy and momentum accommodation coefficients on platinum and silver. J. Chem. Phys 1983, 78, 3329–3333. [Google Scholar]
- Gronych, T; Ullman, R; Peksa, L; Repa, P. Measurements of the relative momentum accommodation coefficient for different gases with a viscosity vacuum gauge. Vacuum 2004, 73, 275–279. [Google Scholar]
- Bremner, JGM. The thermal accommodation coefficients of gases. I. An investigation of the effect of flashing. P. Roy. Soc. Lond. A 1950, 201, 305–320. [Google Scholar]
- Ulmanella, U; Ho, CM. Molecular effects on boundary condition in micro/nanoliquid flows. Phys. Fluids 2008, 20, 101512. [Google Scholar]
- Thomas, LB; Lord, RG. Comparative measurement of tangential momentum and thermal accommodation on polished and roughened steelspheres. In Proceedings of the Eighth International Symposium on Rarefied Gas Dynamics; Karamcheti, R, Ed.; Academic: New York, NY, USA, 1974; pp. 405–412. [Google Scholar]
- Veijola, T; Kuisma, H; Lahdenpera, J. The influence of gas-surface interaction on gas-film damping in a silicon accelerometer. Sensor. Actuat. A-Phys 1998, 66, 83–92. [Google Scholar]
- Sazhin, OV; Borisov, SF; Sharipov, F. Accommodation coefficient of tangential momentum on atomically clean and contaminated surfaces. J. Vac. Sci. Technol. A 2001, 19, 2499–2503. [Google Scholar]
- Sazhin, OV; Borisov, SF; Sharipov, F. Erratum: Accommodation coefficient of tangential momentum on atomically clean and contaminated surfaces (vol a 19, pg 2499, 2001). J. Vac. Sci. Technol. A 2002, 20, 957–957. [Google Scholar]
- Jang, JS; Zhao, YB; Wereley, ST. Pressure distributions and tmac measurements in near unity aspect ratio, anodically bonded microchannels. MEMS-03: IEEE the Sixteenth Annual International Conference on Micro Electro Mechanical Systems, Kyoto, Japan, Jan. 19–23; 2003; pp. 287–290. [Google Scholar]
- Ewart, T; Perrier, P; Graur, IA; Méolans, JG. Mass flow rate measurements in a microchannel, from hydrodynamic to near free molecular regimes. J. Fluid Mech 2007, 584, 337–356. [Google Scholar]
- Ewart, T; Graur, IA; Perrier, P; Meolans, JG. Mass flow rate measurements: From hydrodynamic to free molecular regime. Proceedings of the 6th International Conference on Nanochannels, Microchannels, and Minichannels, Darmstadt, Germany; 2008; pp. 65–73. [Google Scholar]
- Gad-el-Hak, M. Gas and liquid transport at the microscale. Heat Transfer Eng 2006, 27, 13–29. [Google Scholar]
- Wang, M; Li, ZX. Nonideal gas flow and heat transfer in micro- and nanochannels using the direct simulation Monte Carlo method. Phys. Rev. E 2003, 68, 046704. [Google Scholar]
- Wang, M; Lan, XD; Li, ZX. Analyses of gas flows in micro- and nanochannels. Int. J. Heat Mass Tran 2008, 51, 3630–3641. [Google Scholar]
- Chen, S; Doolen, GD. Lattice Boltzmann method for fluid flows. Annu. Rev. Fluid Mech 1998, 30, 329–364. [Google Scholar]
- Tang, GH; Tao, WQ; He, YL. Lattice Boltzmann method for gaseous microflows using kinetic theory boundary conditions. Phys. Fluids 2005, 17, 058101. [Google Scholar]
- Wang, JK; Wang, M; Li, ZX. A lattice Boltzmann algorithm for fluid-solid conjugate heat transfer. Int. J. Therm. Sci 2007, 46, 228–234. [Google Scholar]
- Kuo, LS; Chen, PH. A unified approach for nonslip and slip boundary conditions in the lattice Boltzmann method. Comput. Fluids 2009, 38, 883–887. [Google Scholar]
- Yamamoto, K; Takeuchi, H; Hyakutake, T. Effect of wall characteristics on the behaviors of reflected gas molecules in a thermal problem. In Rarefied Gas Dynamics: 23rd International Symposium; Ketsdever, AD, Muntz, EP, Eds.; AIP Press: New York, NY, USA, 2003; pp. 1008–1015. [Google Scholar]
- Takeuchi, H; Yamamoto, K; Hyakutake, T. Behavior of the reflected molecules of a diatomic gas at a solid surface. In Rarefied Gas Dynamics: 24th International Symposium; Capitelli, M, Ed.; AIP Press: New York, NY, USA, 2005; pp. 987–992. [Google Scholar]
- Hyakutake, T; Yamamoto, K; Takeuchi, H. Flow of gas mixtures through micro channel. In Rarefied Gas Dynamics: 24th International Symposium; Capitelli, M, Ed.; AIP Press: New York, NY, USA, 2005; pp. 780–788. [Google Scholar]
- Yamamoto, K; Takeuchi, H; Hyakutake, T. Characteristics of reflected gas molecules at a solid surface. Phys. Fluids 2006, 18, 046103. [Google Scholar]
- Nedea, SV; Markvoort, AJ; van Steenhoven, AA; Hilbers, PAJ. Heat transfer predictions for micro/nano-channels at atomistic level using combined molecular dynamics and Monte Carlo techniques. ICNMM2007: Proceedings of the 5th International Conference on Nanochannels, Microchannels, and Minichannels, Puebla, Mexico; 2007; pp. 755–762. [Google Scholar]
- Nedea, SV; Markvoort, AJ; Spijker, P; van Steenhoven, AA. Heat transfer predictions using accommodation coefficients for a dense gas in a micro/nano-channel. 6th International Conference on Nanochannels, Microchannels, and Minichannels, Darmstadt, Germany; 2008; pp. 929–936. [Google Scholar]
- Nedea, SV; Markvoort, AJ; van Steenhoven, AA; Hilbers, PAJ. Heat transfer predictions for micro-/nanochannels at the atomistic level using combined molecular dynamics and Monte Carlo techniques. J. Heat Tran 2009, 131, 033104–033108. [Google Scholar]
- Chirita, V; Pailthorpe, BA; Collins, RE. Molecular-dynamics study of low-energy Ar scattering by the ni(001) surface. J. Phys. D-Appl. Phys 1993, 26, 133–142. [Google Scholar]
- Chirita, V; Pailthorpe, BA; Collins, RE. Non-equilibrium energy and momentum accommodation coefficients of ar atoms scattered from Ni(001) in the thermal regime: A molecular dynamics study. Nucl. Instrum. Methods Phys. Res. B - Beam Interact. Mater. Atoms 1997, 129, 465–473. [Google Scholar]
- Finger, GW; Kapat, JS; Bhattacharya, A. Molecular dynamics simulation of adsorbent layer effect on tangential momentum accommodation coefficient. J. Fluids Eng.-T. ASME 2007, 129, 31–39. [Google Scholar]
- Arya, G; Chang, HC; Maginn, EJ. Molecular simulations of Knudsen wall-slip: Effect of wall morphology. Mol. Simulat 2003, 29, 697–709. [Google Scholar]
- Celestini, F; Mortessagne, F. Cosine law at the atomic scale: Toward realistic simulations of Knudsen diffusion. Phys. Rev. E 2008, 77, 021202. [Google Scholar]
- Cao, BY; Chen, M; Guo, ZY. Temperature dependence of the tangential momentum accommodation coefficient for gases. Appl. Phys. Lett 2005, 86, 091905. [Google Scholar]
- Spijker, P; Markvoort, AJ; Nedea, SV; Hilbers, PAJ. Velocity correlations between impinging and reflecting particles using md simulations and different wall models. 6th International Conference on Nanochannels, Microchannels, and Minichannels, Darmstadt, Germany; 2008; pp. 959–968. [Google Scholar]
- Sun, J; Li, ZX. Three-dimensional molecular dynamic study on accommodation coefficients in rough nanochannels. Proceedings of the 7th International Conference on Nanochannels, Microchannels, and Minichannels, Pohang, Korea; 2009. [Google Scholar]
- Eckert, ER; Drake, RM. Analysis of Heat and Mass Transfer; Hemisphere Publishing Co: New York, NY, USA, 1987. [Google Scholar]
- Cao, BY; Chen, M; Guo, ZY. Rarefied gas flow in rough microchannels by molecular dynamics simulation. Chin. Phys. Lett 2004, 21, 1777–1779. [Google Scholar]
- Cao, BY; Chen, M; Guo, ZY. Effect of surface roughness on gas flow in microchannels by molecular dynamics simulation. Int. J. Eng. Sci 2006, 44, 927–937. [Google Scholar]
- Chai, ZH; Guo, ZL; Zheng, L; Shi, BC. Lattice Boltzmann simulation of surface roughness effect on gaseous flow in a microchannel. J. Appl. Phys 2008, 104, 014902. [Google Scholar]
- Ziarani, AS; Mohamad, AA. Effect of wall roughness on the slip of fluid in a microchannel. Nanoscale Microscale Thermophys. Eng 2008, 12, 154–169. [Google Scholar]
- Liu, CF; Ni, YS. The fractal roughness effect of micro Poiseuille flows using the lattice Boltzmann method. Int. J. Eng. Sci 2009, 47, 660–668. [Google Scholar]
- Liu, CF; Ni, YS; Rao, Y. Roughness effect of different geometries on micro gas flows by lattice Boltzmann simulation. Int. J. Mod. Phys. C 2009, 20, 953–956. [Google Scholar]
- Sofos, FD; Karakasidis, TE; Liakopoulos, A. Effects of wall roughness on flow in nanochannels. Phys. Rev. E 2009, 79, 026305. [Google Scholar]
- Mo, G; Rosenberger, F. Molecular-dynamics simulation of flow in a two-dimensional channel with atomically rough walls. Phys. Rev. A 1990, 42, 4688–4692. [Google Scholar]
- Cao, BY. Non-Maxwell slippage induced by surface roughness for microscale gas flow: A molecular dynamics simulation. Mol. Phys 2007, 105, 1403–1410. [Google Scholar]
- Sokhan, VP; Quirke, N. Slip coefficient in nanoscale pore flow. Phys. Rev. E 2008, 78, 015301. [Google Scholar]
- Cook, SR; Hoffbauer, MA. Absolute momentum transfer in gas-surface scattering. Phys. Rev. E 1997, 55, R3828–R3831. [Google Scholar]
- Notter, RH; Sather, NF. Normal momentum transfer on ideal crystalline surfaces. AIAA J 1971, 9, 965–966. [Google Scholar]
- Knuth, EL. Free-molecule normal-momentum transfer at satellite surfaces. AIAA J 1979, 18, 602–605. [Google Scholar]
- Collins, FG; Knox, EC. Method for determining wall boundary conditions for DSMC calculations at high speed ratios. . AIAA-94-0036, 1994. [Google Scholar]
- Cook, SR; Cross, JB; Hoffbauer, M. Hypersonic gas-surface energy accommodation test facility. . AIAA-94-2637, 1994. [Google Scholar]
- Polikarpov, PJ; Borisov, SF; Kleyn, A; Taran, JP. Normal momentum transfer study by a dynamic technique. J. Appl. Mech. Tech. Phys 2003, 44, 298–303. [Google Scholar]
- Ambaye, H; Manson, JR. Calculations of accommodation coefficients for diatomic molecular gases. Phys. Rev. E 2006, 73, 031202. [Google Scholar]
- Cook, SR; Hoffbauer, MA. Analyzing gas-surface interactions using the reduced force coefficients. Phys. Rev. E 1998, 58, 504–511. [Google Scholar]
- Gad-el-Hak, M. Liquid: The holy grail of microfluidic modeling. Phys. Fluids 2005, 17, 100612. [Google Scholar]
- Liang, XG. Some effects of interface on fluid flow and heat transfer on micro- and nanoscale. Chin. Sci. Bull 2007, 52, 2457–2472. [Google Scholar]
- Lauga, E; Stone, HA. Effective slip in pressure-driven Stokes flow. J. Fluid Mech 2003, 489, 55–77. [Google Scholar]
- Schnell, E. Slippage of water over nonwettable surfaces. J. Appl. Phys 1956, 27, 1149–1152. [Google Scholar]
- Churaev, NV; Sobolev, VD; Somov, AN. Slippage of liquids over lyophobic solid surfaces. J. Colloid Interf. Sci 1984, 97, 574–581. [Google Scholar]
- Watanabe, K; Udagawa, Y; Udagawa, H. Drag reduction of Newtonian fluid in a circular pipe with a highly water-repellant wall. J. Fluid Mech 1999, 381, 225–238. [Google Scholar]
- Kiseleva, OA; Sobolev, VD; Churaev, NV. Slippage of the aqueous solutions of cetyltrimethylammonium bromide during flow in thin quartz capillaries. Colloid J 1999, 61, 263–264. [Google Scholar]
- Cheng, JT; Giordano, N. Fluid flow through nanometer-scale channels. Phys. Rev. E 2002, 65, 031206. [Google Scholar]
- Choi, CH; Westin, KJA; Breuer, KS. Apparent slip flows in hydrophilic and hydrophobic microchannels. Phys. Fluids 2003, 15, 2897–2902. [Google Scholar]
- Cheikh, C; Koper, G. Stick-slip transition at the nanometer scale. Phys. Rev. Lett 2003, 91, 156102. [Google Scholar]
- Qu, J; Perot, B; Rothstein, JP. Laminar drag reduction in microchannels using ultrahydrophobic surfaces. Phys. Fluids 2004, 16, 4635–4643. [Google Scholar]
- Choi, CH; Ulmanella, U; Kim, J; Ho, CM; Kim, CJ. Effective slip and friction reduction in nanograted superhydrophobic microchannels. Phys. Fluids 2006, 18, 087105. [Google Scholar]
- Ulmanella, U; Ho, CM. Molecular effects on boundary condition in micro/nanoliquid flows. Phys. Fluids 2008, 20, 101512. [Google Scholar]
- Pfahler, J; Harley, J; Bau, H; Zemel, J. Liquid transport in micron and submicron channels. Sensor. Actuat. A 1990, 23, 431–434. [Google Scholar]
- Hasegawa, T; Suganuma, M; Watanabe, H. Anomaly of excess pressure drops of the flow through very small orifices. Phys. Fluids 1997, 9, 1–3. [Google Scholar]
- Tabor, D; Winterton, RHS. The direct measurement of normal and retarded van der Waals forces. P. Roy. Soc. Lond. A 1969, 312, 435–450. [Google Scholar]
- Israelachvili, JN; Tabor, D. The measurement of van der Waals dispersion forces in the range 1.5 to 130 nm. P. Roy. Soc. Lond. A 1972, 331, 19–38. [Google Scholar]
- Chan, DYC; Horn, RG. The drainage of thin liquid films between solid surfaces. J. Chem. Phys 1985, 83, 5311–5324. [Google Scholar]
- Israelachvili, JN. Measurement of the viscosity of liquids in very thin films. J. Colloid Interf. Sci 1986, 110, 263–271. [Google Scholar]
- Horn, RG; Smith, DT; Haller, W. Surface forces and viscosity of water measured between silica sheets. Chem. Phys. Lett 1989, 162, 404–408. [Google Scholar]
- Georges, JM; Millot, S; Loubet, JL; Tonck, A. Drainage of thin liquid films between relatively smooth surfaces. J. Chem. Phys 1993, 98, 7345–7360. [Google Scholar]
- Zhu, YX; Granick, S. Rate-dependent slip of Newtonian liquid at smooth surfaces. Phys. Rev. Lett 2001, 87, 096105. [Google Scholar]
- Baudry, J; Charlaix, E; Tonck, A; Mazuyer, D. Experimental evidence for a large slip effect at a nonwetting fluid-solid interface. Langmuir 2001, 17, 5232–5236. [Google Scholar]
- Zhu, YX; Granick, S. Limits of the hydrodynamics no-slip boundary condition. Phys. Rev. Lett 2002, 88, 106102. [Google Scholar]
- Zhu, YX; Granick, S. Apparent slip of Newtonian fluids past adsorbed polymer layers. Macromolecules 2002, 35, 4658–4663. [Google Scholar]
- Zhu, YX; Granick, S. No-slip boundary condition switches to partial slip when fluid contains surfactant. Langmuir 2002, 18, 10058–10063. [Google Scholar]
- Cottin-Bizonne, C; Jurine, S; Baudry, J; Crassous, J; Restagno, F; Chariaix, E. Nanotechnology: An investigation of the boundary condition at hydrophobic and hydrophilic interfaces. Europ. Phys. J. E 2002, 9, 47–53. [Google Scholar]
- Cottin-Bizonne, C; Cross, B; Steinberger, A; Charlaix, E. Boundary slip on smooth hydrophobic surfaces: Intrinsic effects and possible artifacts. Phys. Rev. Lett 2005, 94, 056102. [Google Scholar]
- Craig, VSJ; Neto, C; Wiiliams, RM. Shear-dependent boundary slip in an aqueous Newtonian liquid. Phys. Rev. Lett 2001, 87, 054504. [Google Scholar]
- Bonaccurso, E; Kappl, M; Butt, HS. Hydrodynamic force measurements: Boundary slip of water on hydrophilic surfaces and electrokinetics effects. Phys. Rev. Lett 2002, 88, 076103. [Google Scholar]
- Sun, GX; Bonaccurso, E; Franz, V; Butt, HJ. Confined liquid: Simultaneous observation of a molecularly layered structure and hydrodynamics slip. J. Chem. Phys 2002, 17, 10311–10314. [Google Scholar]
- Bonaccurso, E; Butt, HJ; Craig, VSJ. Surface roughness and hydrodynamics boundary slip of a Newtonian fluid in a completely wetting system. Phys. Rev. Lett 2003, 90, 144501. [Google Scholar]
- Neto, C; Craig, VSJ; Williams, DRM. Evidence of shear-dependent boundary slip in Newtonian liquids. Eur. Phys. J. E 2003, 12, S71–S74. [Google Scholar]
- Vinogradova, OI; Yakubov, GE. Dynamic effects on force measurements, 2. Lubrication and the atomic force microscope. Langmuir 2003, 19, 1227–1234. [Google Scholar]
- Cho, JH; Law, BM; Rieutord, F. Dipole-dependent slip on Newtonian liquids at smooth solid hydrophobic surfaces. Phys. Rev. Lett 2004, 92, 166102. [Google Scholar]
- Henry, CL; Neto, C; Evans, DR; Biggs, S; Craig, VSJ. The effect of surfactant adsorption on liquid boundary slippage. Physica A 2004, 339, 60–65. [Google Scholar]
- Vinogradova, OI; Yakubov, GE. Surface roughness and hydrodynamics boundary conditions. Phys Rev E 2006, 73, 045302(R). [Google Scholar]
- Boehnke, UC; Remmler, T; Motschmann, H; Wurlitzer, S; Hauwede, J; Fischer, MT. Partial air wetting on solvophobic surfaces in polar liquids. J. Colloid Interf. Sci 1999, 211, 243–251. [Google Scholar]
- Churaev, NV; Ralston, J; Sergeeva, IP; Sobolev, VD. Electrokinetic properties of methylated quartz capillaries. Adv. Colloid Interf. Sci 2002, 96, 265–278. [Google Scholar]
- Gogte, S; Vorobieff, P; Truesdell, R; Mammoli, A; van Swol, F; Shah, P; Brinker, CJ. Effective slip on textured superhydrophobic surfaces. Phys. Fluids 2005, 17, 051701. [Google Scholar]
- Choi, CH; Kim, CJ. Large slip of aqueous liquid flow over a nanoengineered superhydrophobic surface. Phys. Rev. Lett 2006, 96, 066001. [Google Scholar]
- Joly, L; Ybert, C; Bocquet, L. Probing the nanohydrodynamics at liquid-solid interfaces using thermal motion. Phys. Rev. Lett 2006, 96, 046101. [Google Scholar]
- Stanislas, M; Kompenhans, J; Westerwell, J. Particle Image Velocimetry: Progress Towards Industrial Application; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Tretheway, DC; Meinhart, CD. Apparent fluid slip at hydrophobic microhannel walls. Phys. Fluids 2002, 14, L9–L12. [Google Scholar]
- Tretheway, DC; Meinhart, CD. A generating mechanism for apparent fluid slip in hydrophobic microchannels. Phys. Fluids 2004, 16, 1509–1515. [Google Scholar]
- Joseph, P; Tabeling, P. Direct measurement of the apparent slip length. Phys Rev E 2005, 71, 035303(R). [Google Scholar]
- Truesdell, R; Mannoli, A; Vorobieff, P; van Swol, F; Brinker, CJ. Drag reduction on a patterned superhydrophobic surface. Phys. Rev. Lett 2006, 97, 044504. [Google Scholar]
- Joseph, P; Cottin-Bizonne, C; Benoit, JM; Ybert, C; Journet, C; Tabeling, P; Bocquet, L. Slippage of water past superhydrophobic carbon nanotube forests in microchannels. Phys. Rev. Lett 2006, 97, 156104. [Google Scholar]
- Byun, D; Kim, J; Ko, HS; Park, HC. Direct measurement of slip flows in superhydrophobic microchannels with transverse grooves. Phys. Fluids 2008, 20, 113601. [Google Scholar]
- Pit, R; Hervet, H; Leger, L. Friction and slip of a simple liquid at a solid surface. Trib. Lett 1999, 7, 147–152. [Google Scholar]
- Pit, R; Hervet, H; Leger, L. Direct experimental evidence of slip in hexadecane: Solid interfaces. Phys. Rev. Lett 2000, 85, 980–983. [Google Scholar]
- Schmatko, T; Hervet, H; Leger, L. Friction and slip at simple fluid-solid interfaces: The roles of the molecular shape and the solid-liquid interaction. Phys. Rev. Lett 2005, 94, 244501. [Google Scholar]
- Lumma, D; Best, A; Gansen, A; Feuillebois, F; Rädler, JO; Vinogradoval, OI. Flow profile near a wall measured by double- focus fluorescence cross-correlation. Phys. Rev. E 2003, 67, 056313. [Google Scholar]
- Jin, S; Huang, P; Park, J; Yoo, JY; Breuer, KS. Near-surface velocimetry using evanescent wave illumination. Exp. Fluids 2004, 37, 825–833. [Google Scholar]
- Huang, P; Guasto, JS; Breuer, KS. Direct measurement of slip velocities using three-dimensional total internal reflection velocimetry. J. Fluid Mech 2006, 556, 447–464. [Google Scholar]
- Huang, P; Breuer, KS. Direct measurement of slip length in electrolyte solutions. Phys. Fluids 2007, 19, 028104. [Google Scholar]
- Bouzigues, CI; Tabeling, P; Bocuet, L. Nanofluidics in the Debye layer at hydrophilic and hydrophobic surfaces. Phys. Rev. Lett 2008, 101, 114503. [Google Scholar]
- Lasne, D; Maali, A; Amarouchene, Y; Cognet, L; Lounis, B; Kellay, H. Velocity profiles of water flowing past solid glass surfaces using fluorescent nanoparticles and molecules as velocity probes. Phys. Rev. Lett 2008, 100, 214502. [Google Scholar]
- Zhu, LD; Tretheway, D; Petzold, L; Meinhart, C. Simulation of fluid slip at 3D hydrophobic microchannel walls by the lattice Boltzmann method. J. Comput. Phys 2005, 202, 181–195. [Google Scholar]
- Sbragaglia, M; Succi, S. Analytical calculation of slip flow in lattice Boltzmann models with kinetic boundary conditions. Phys. Fluids 2005, 17, 093602. [Google Scholar]
- Zhang, J; Kwok, DY. Study of contact angles, contact line dynamics and interfacial liquid slip by a mean-field free-energy lattice Boltzmann model. Contact Angle, Wettability and Adhesion 2006, 4, 3–28. [Google Scholar]
- Wolf, FG; dos Santos, LOE; Philippi, PC. Modeling and simulation of the fluid-solid interaction in wetting. J Stat Mech-Theor Exp 2009, P06008. [Google Scholar]
- Hyvaluoma, J; Harting, J. Slip flow over structured surfaces with entrapped microbubbles. Phys. Rev. Lett 2008, 100, 246001. [Google Scholar]
- O’Connell, ST; Thompson, PA. Molecular dynamics-continuum hybrid computations: A tool for studying complex fluid flows. Phys. Rev. E 1995, 52, R5792–R5795. [Google Scholar]
- Flekkey, EG; Wagner, G; Feder, J. Hybrid model for combined particle and continuum dynamics. Europhys. Lett 2000, 52, 271–276. [Google Scholar]
- Nie, XB; Chen, SY; E, WN; Robbins, MO. A continuum and molecular dynamics hybrid method for micro- and nano-fluid flow. J. Fluid Mech 2004, 500, 55–64. [Google Scholar]
- Liu, J; Chen, SY; Nie, XB; Robbins, MO. A continuum-atomistic simulation of heat transfer in micro- and nano-flows. J. Comput. Phys 2007, 227, 279–291. [Google Scholar]
- Wang, YC; He, GW. A dynamic coupling model for hybrid atomistic-continuum computations. Chem. Eng. Sci 2007, 62, 3574–3579. [Google Scholar]
- Li, Q; He, GW. An atomistic-continuum hybrid simulation of fluid flows over superhydrophobic surfaces. Biomicrofluidics 2009, 3, 022409. [Google Scholar]
- Sun, J; He, YL; Tao, WQ. Molecular dynamics-continuum hybrid simulation for condensation of gas flow in a microchannel. Microfluid. Nanofluid 2009, 7, 407–422. [Google Scholar]
- Tehver, R; Toigo, F; Koplik, J; Banavar, JR. Thermal walls in computer simulations. Phys. Rev. E 1998, 57, R17–R20. [Google Scholar]
- Koplik, J; Banavar, JR; Willemsen, JF. Molecular dynamics of fluid flow at solid surface. Phys. Fluids A 1989, 1, 781–794. [Google Scholar]
- Maruyama, S. Molecular dynamics method for microscale heat transfer. In Advances in Numerical Heat Transfer; Minkowycz, WJ, Sparrow, EM, Eds.; Taylor and Francis: New York, NY, USA, 2000; pp. 189–226. [Google Scholar]
- Hook, JR; Hall, HE. Solid State Physics, 2nd ed; Wiley: Chichester, UK, 1991. [Google Scholar]
- Barrat, JL; Bocquet, L. Large slip effect at a nonwetting fluid-solid interface. Phys. Rev. Lett 1999, 82, 4671–4674. [Google Scholar]
- Koplik, J; Banavar, JR; Willemsen, JF. Molecular dynamics of Poiseuille flow and moving contact lines. Phys. Rev. Lett 1988, 60, 1282–1285. [Google Scholar]
- Heinbuch, U; Fischer, J. Liquid flow in pores-slip, no-slip, or multilayer sticking. Phys. Rev. A 1989, 40, 1144–1146. [Google Scholar]
- Thompson, PA; Robbins, MO. Simulations of contact-line motion: Slip and the dynamics contact angle. Phys. Rev. Lett 1989, 63, 766–769. [Google Scholar]
- Thompson, PA; Robbins, MO. Shear flow near solids: Epitaxial order and flow boundary conditions. Phys. Rev. A 1990, 41, 6830–6837. [Google Scholar]
- Sun, M; Ebner, C. Molecular dynamics study of flow at a fluid-wall interface. Phys. Rev. Lett 1992, 69, 3491–3494. [Google Scholar]
- Thompson, PA; Troian, SM. A general boundary condition for liquid flow at solid surface. Nature 1997, 389, 360–362. [Google Scholar]
- Jabbarzadeh, A; Atkinson, JD; Tanner, RL. Effects of the wall roughness on slip and rheological properties of hexadecane in molecular dynamics simulation of Couette shear flow between two sinusoidal walls. Phys. Rev. E 2000, 61, 690–699. [Google Scholar]
- Cieplak, M; Koplik, J; Banavar, JR. Boundary conditions at a fluid-solid interface. Phys. Rev. Lett 2001, 86, 803–806. [Google Scholar]
- Sokhan, VP; Nicholson, D; Quirke, N. Fluid flow in nanopores: An examination of hydrodynamic boundary conditions. J. Chem. Phys 2001, 115, 3878–3887. [Google Scholar]
- Fan, XJ; Phan-Thien, N; Yong, NT; Diao, X. Molecular dynamics simulation of a liquid in a complex nano channel flow. Phys. Fluids 2002, 14, 1146–1153. [Google Scholar]
- Sokhan, VP; Nicholson, D; Quike, N. Fluid flow in nanopores: Accurate boundary conditions for carbon nanotubes. J. Chem. Phys 2002, 117, 8531–8539. [Google Scholar]
- Cottin-Bizonne, C; Barrat, JL; Bocquet, L; Charlaix, E. Low-friction flows of liquid at nanopatterned interfaces. Nat. Mater 2003, 2, 237–242. [Google Scholar]
- Nagayama, G; Cheng, P. Effects of interface wettability on microscale flow by molecular dynamics simulation. Int. J. Heat Mass Tran 2004, 47, 501–513. [Google Scholar]
- Galea, TM; Attard, P. Molecular dynamics study of the effect of atomic roughness on the slip length at the fluid-solid boundary during shear flow. Langmuir 2004, 20, 3477–3482. [Google Scholar]
- Cottin-Bizonne, C; Barentin, C; Charlaix, E; Bocquet, L; Barrat, JL. Dynamics of simple liquids at heterogeneous surfaces: Molecular-dynamics simulations and hydrodynamic description. Eur. Phys. J. E 2004, 15, 427–438. [Google Scholar]
- Priezjev, NV; Troian, SM. Molecular origin and dynamic behavior of slip in sheared polymer films. Phys. Rev. Lett 2004, 92, 018302. [Google Scholar]
- Walther, JH; Werder, T; Jaffe, RL; Koumoutsakos, P. Hydrodynamic properties of carbon nanotubes. Phys. Rev. E 2004, 69, 062201. [Google Scholar]
- Soong, CY; Wang, SH; Tzeng, PY. Molecular dynamics simulation of rotating fluids in a cylindrical container. Phys. Fluids 2004, 16, 2814–2827. [Google Scholar]
- Yang, SC; Fang, LB. Effect of surface roughness on slip flows in hydrophobic and hydrophilic microchannels by molecular dynamics simulation. Mol. Simul 2005, 31, 971–977. [Google Scholar]
- Yang, SC. Effects of surface roughness and interface wettability on nanoscale flow in a nanochannel. Microfluid. Nanofluid 2006, 2, 501–511. [Google Scholar]
- Guo, ZL; Zhao, TS; Shi, Y. Temperature dependence of the velocity boundary condition for nanoscale fluid flows. Phys. Rev. E 2005, 72, 036301. [Google Scholar]
- Cao, BY; Chen, M; Guo, ZY. Velocity slip of liquid flow in nanochannels. Acata Phys. Sinica 2006, 55, 5305–5310. [Google Scholar]
- Cao, BY; Chen, M; Guo, ZY. Liquid flow in surface-nanostructured channels studied by molecular dynamics simulation. Phys. Rev. E 2006, 74, 066311. [Google Scholar]
- Voronov, RS; Papavassiliou, DV; Lee, LL. Boundary slip and wetting properties of interfaces: Correlation of the contact angle with the slip length. J. Chem. Phys 2006, 124, 204701. [Google Scholar]
- Cieplak, M; Koplik, J; Banavar, JR. Nanoscale fluid flows in the vicinity of patterned surfaces. Phys. Rev. Lett 2006, 96, 114502. [Google Scholar]
- Li, YX; Xu, JL. A new criterion number for the boundary conditions at the solid/liquid interface in nanoscale. Nanosc. Microsc. Thermophys. Eng 2006, 10, 109–141. [Google Scholar]
- Lichter, S; Martini, A; Snurr, RQ; Wang, Q. Liquid slip in nanoscale channels as a rate process. Phys. Rev. Lett 2007, 98, 226001. [Google Scholar]
- Soong, CY; Yen, TH; Tzeng, PY. Molecular dynamics simulation of nanochannel flows with effects of wall lattice-fluid interactions. Phys. Rev. E 2007, 76, 036303. [Google Scholar]
- Yen, TH; Soong, CY; Tzeng, PY. Hybrid molecular dynamics-continuum simulation for nano/mesoscale channel flows. Microfluid. Nanofluid 2007, 3, 665–675. [Google Scholar]
- Martini, A; Hsu, HY; Patankar, NA; Lichter, S. Slip at high shear rates. Phys. Rev. Lett 2008, 100, 206001. [Google Scholar]
- Huang, DM; Sendner, C; Horinek, D; Netz, RR; Bocquet, L. Water slippage versus contact angle: A quasiuniversal relationship. Phys. Rev. Lett 2008, 101, 226101. [Google Scholar]
- Martini, A; Roxin, A; Snurr, RQ; Wang, Q; Lichter, S. Molecular mechanisms of liquid slip. J. Fluid Mech 2008, 600, 257–269. [Google Scholar]
- Sofos, FD; Karakasidis, TE; Liakopoulos, A. Effects of wall roughness on flow in nanochannels. Phys. Rev. E 2009, 79, 026305. [Google Scholar]
- Priezjev, N. Shear rate threshold for the boundary slip in dense polymer films. Phys. Rev. E 2009, 80, 031608. [Google Scholar]
- de Gennes, PG. Wetting-statics and dynamics. Rev. Mod. Phys 1985, 57, 827–863. [Google Scholar]
- Good, RJ. Contact angle, wetting, and adhesion – A critical review. J. Adhes. Sci. Technol 1992, 6, 1269–1302. [Google Scholar]
- Young, T. An essay on the cohesion of fluids. Philos. T. Roy. Soc. Lond 1805, 95, 65–87. [Google Scholar]
- Tolstoi, DM. Molecular theory for slippage of liquids over solid surfaces. DAN SSSR 1952, 85, 1089–1092. (in Russian).. [Google Scholar]
- Frenkel, J. Kinetic Theory of Liquids; Dover: New York, NY, USA, 1955. [Google Scholar]
- Blake, TD. Slip between a liquid and a solid-D.M. Tolstoi (1952) theory reconsidered. Colloids Surf 1990, 47, 135–145. [Google Scholar]
- Ellis, JS; McHale, G; Hayward, G; Thompson, M. Contact angle-based predictive model for slip at the solid-liquid interface of a transverse-shear mode acoustic wave device. J. Appl. Phys 2003, 94, 6201–6207. [Google Scholar]
- Bocquet, L; Barrat, JL. Hydrodynamic boundary conditions and correlation functions of confined fluids. Phys. Rev. Lett 1993, 70, 2726–2729. [Google Scholar]
- Bocquet, L; Barrat, JL. Hydrodynamic boundary conditions, correlation functions, and Kubo relations for confined fluids. Phys. Rev. E 1996, 49, 3079–3092. [Google Scholar]
- Bocquet, L; Barrat, JL. Influence of wetting properties on hydrodynamic boundary conditions at a fluid/solid interface. Faraday Discuss 1999, 112, 119–127. [Google Scholar]
- Rowlinson, J; Widom, B. Molecular Theory of Capillarity; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- Richardson, S. On the no-slip boundary condition. J. Fluid Mech 1973, 59, 707–719. [Google Scholar]
- Nye, JF. A calculation on sliding of ice over a wavy surface using a Newtonian viscous approximation. P. Roy. Soc. Lond. A 1969, 311, 445–467. [Google Scholar]
- Nye, JF. Glacier sliding without cavitation in a linear viscous approximation. P. Roy. Soc.Lond. A 1970, 315, 381–403. [Google Scholar]
- Jansons, KM. Determination of the macroscopic (partial) slip boundary condition for a viscous flow over a randomly rough surface with a perfect slip microscopic boundary condition. Phys. Fluids 1988, 31, 15–17. [Google Scholar]
- Sarkar, K; Prosperetti, A. Effective boundary conditions for Stokes flow over a rough surface. J. Fluid Mech 1996, 316, 223–240. [Google Scholar]
- Ponomarev, IV; Meyerovich, AE. Surface roughness and effective stick-slip motion. Phys. Rev. E 2003, 67, 026302. [Google Scholar]
- Hu, YD; Werner, C; Li, DQ. Influence of three-dimensional roughness on pressure-driven flow through microchannels. ASME J. Fluids Eng 2003, 125, 871–879. [Google Scholar]
- Rawool, AS; Mitra, SK; Kandlikar, SG. Numerical simulation of flow through microchannels with designed roughness. Microfluid. Nanofluid 2006, 2, 215–221. [Google Scholar]
- Wang, HL; Wang, Y. Flow in microchannels with rough walls: Flow pattern and pressure drop. J. Micromech. Microeng 2007, 17, 586–596. [Google Scholar]
- Barthlott, W; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surface. Planta 1997, 202, 1–8. [Google Scholar]
- Gao, XF; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar]
- von Baeyer, HC. The lotus effect-the secret of the self-cleaning leaves of the lotus plant, like the subtlest applications of high technology, is simplicity itself. Science World 2000, 40, 12–15. [Google Scholar]
- Nun, E; Oles, M; Schleich, B. Lotus-effect surfaces. Macromol. Symp 2002, 187, 677–682. [Google Scholar]
- Barthlott, W; Neinhuis, C. The lotus effect: A self-cleaning surface based on a model taken from nature. Tekstil 2001, 50, 461–465. [Google Scholar]
- Marmur, A. The lotus effect: Superhydrophobicity and metastability. Langmuir 2004, 20, 3517–3519. [Google Scholar]
- Patankar, NA. Mimicking the lotus effect: Influence of double roughness structures and slender pillars. Langmuir 2004, 20, 8209–8213. [Google Scholar]
- Gao, LC; McCarthy, TJ. The “lotus effect” explained: Two reasons why two length scales of topography are important. Langmuir 2006, 22, 2966–2967. [Google Scholar]
- Lee, HJ; Micheilsen, S. Lotus effect: Superhydrophobicity. J. Textile Inst 2006, 97, 455–462. [Google Scholar]
- Namavar, F; Cheung, CL; Sabirianov, RF; Mei, WN; Zeng, XC; Wang, GH; Haider, H; Garvin, KL. Lotus effect in engineered zirconis. Nano Lett 2008, 8, 988–996. [Google Scholar]
- Su, CH; Chen, QM. Fabrication of lotus effect coatings. Chin. J. Inorg. Chem 2008, 24, 298–302. [Google Scholar]
- Spori, DM; Drobek, T; Zurcher, S; Ochsner, M; Sprecher, C; Muehlebach, A; Spencer, ND. Beyond the lotus effect: Roughness, influences on wetting over a wide surface-energy range. Langmuir 2009, 24, 5411–5417. [Google Scholar]
- Cassie, ABD; Baxter, S. Wettability of porous surfaces. T. Faraday Soc 1944, 40, 546–551. [Google Scholar]
- Dammer, SM; Lohse, D. Gas enrichment at liquid-wall interfaces. Phys. Rev. Lett 2006, 96, 206101. [Google Scholar]
- Biben, T; Joly, L. Wetting on nanorough surfaces. Phys. Rev. Lett 2008, 100, 186103. [Google Scholar]
- Watanabe, K; Mizunuma, YH. Slip of Newtonian fluids at solid boundary. JSME Int. J. B 1998, 44, 525–529. [Google Scholar]
- Watanabe, K; Ogata, S. Drag reduction for a rotating disk with highly water-repellent wall. JSME Int. J. B 1998, 44, 556–560. [Google Scholar]
- Yu, YS; Wer, QD. Experimental study on physical mechanism of drag reduction of hydrophobic materials in laminar flow. Chin. Phys. Lett 2006, 23, 1634–1637. [Google Scholar]
- Wang, CY. Flow over a surface with parallel grooves. Phys. Fluids 2003, 15, 1114–1121. [Google Scholar]
- Andrienko, D; Dunweg, B; Vinogradova, OI. Boundary slip as a result of a prewetting transition. J. Chem. Phys 2003, 119, 13106–13112. [Google Scholar]
- Lauga, E; Brenner, MP. Dynamic mechanisms for apparent slip on hydrophobic surfaces. Phys. Rev. E 2004, 70, 026311. [Google Scholar]
- Sbragalia, M; Prosperetti, A. A note on the effective slip properties for microchannels flows with ultrahydrophobic surfaces. Phys. Fluids 2007, 19, 043603. [Google Scholar]
- Ybert, C; Barentin, C; Cottin-Bizonne, C; Joseph, P; Bocquet, L. Achieving large slip with superhydrophobic surfaces: Scaling laws for generic geometries. Phys. Fluids 2007, 19, 123601. [Google Scholar]
- Feuillebois, F; Bazant, MZ; Vinogradova, OI. Effective slip over superhydrophobic surfaces in thin channels. Phys. Rev. Lett 2009, 102, 026001. [Google Scholar]
- Steinberger, A; Cottin-Bizonne, C; Kleimann, P; Charlaix, E. High friction on a bubble mattress. Nat. Mater 2007, 6, 665–668. [Google Scholar]
- Govardhan, RN; Srinivas, GS; Asthana, A; Bobji, MS. Time dependence of effective slip on textured hydrophobic surfaces. Phys. Fluids 2009, 21, 052001. [Google Scholar]
- Buschnell, DM. Drag reduction in nature. Annu. Rev. Fluid Mech 1991, 23, 65–79. [Google Scholar]
- Atwood, BT; Schowalter, WR. Measurements of slip at the wall during flow of high-density polyethylene through a rectangular conduit. Rheol. Acta 1989, 28, 134–146. [Google Scholar]
- Spikes, HA. The half-wetted bearing. Part 1: Extended Reynolds equation. Proc. IME J: J. Eng. Tribol 2003, 217, 1–14. [Google Scholar]
- Spikes, HA; Granick, S. Equation for slip of simple liquids at smooth solid surfaces. Langmuir 2003, 19, 5065–5071. [Google Scholar]
- Ishida, N; Inoue, T; Miyahara, M; Higashitani, K. Nano bubbles on a hydrophobic surface in water observed by tapping-mode atomic force microscopy. Langmuir 2000, 16, 6377–6380. [Google Scholar]
- Tyrrell, JWG; Attard, P. Images of nanobubbles on hydrophobic surfaces and their interactions. Phys. Rev. Lett 2001, 87, 176104. [Google Scholar]
- Tyrrell, JWG; Attard, P. Atomic force microscope images of nanobubbles on a hydrophobic surface and corresponding force separation data. Langmuir 2002, 18, 160–167. [Google Scholar]
- Steitz, R; Gutberlet, T; Hauss, T; Klösgen, B; Krastev, R; Schemmel, S; Simonsen, AC; Findenegg, GH. Nanobubbles and their precursor layer at the interface of water against a hydrophobic substrate. Langmuir 2003, 19, 2409–2418. [Google Scholar]
- Ruckenstein, E; Rajora, P. On the no-slip boundary condition of hydrodynamics. J. Colloid Interf. Sci 1983, 96, 488–491. [Google Scholar]
- Ruckenstein, E; Churaev, N. A possible hydrodynamic origin of the forces of hydrophobic attraction. J. Colloid Interf. Sci 1991, 147, 535–538. [Google Scholar]
- Lum, K; Chandler, D; Weeks, JD. Hydrophobicity at small and large length scales. J. Phys. Chem 1999, 103, 4570–4577. [Google Scholar]
- Epstein, PS; Plesset, MS. On the stability of gas bubbles in liquid-gas solutions. J. Chem. Phys 1950, 18, 1505–1513. [Google Scholar]
- Ljunggren, S; Eriksson, JC. The lifetime of a colloid-sized bubble in water and the cause of the hydrophobic attraction. Colloids Surf A 1997, 129–130, 151–155. [Google Scholar]
- Christenson, HK; Claesson, PM. Direct measurements of the force between hydrophobic surfaces in water. Adv. Colloid Interf. Sci 2001, 91, 391–436. [Google Scholar]
- Agrawal, A; Park, J; Ryu, DY; Hammond, PT; Russell, TP; McKinley, GH. Controlling the location and spatial extent of nanobubbles using hydrophobically nanopatterned surfaces. Nano Lett 2005, 5, 1751–1756. [Google Scholar]
- Koishi, T; Yasuoka, K; Ebisuzaki, T; Yoo, S; Zeng, XC. Large-scale molecular-dynamics simulation of nanoscale hydrophobic interaction and nanobubble formation. J. Chem. Phys 2005, 123, 204707. [Google Scholar]
- Zhang, XH; Maeda, N; Craig, VSJ. Physical properties of nanobubbles on hydrophobic surfaces in water and aqueous solutions. Langmuir 2006, 22, 5025–5035. [Google Scholar]
- Borkent, BM; Dammer, SM; Schonherr, H; Vancso, GJ; Lohse, D. Superstability of surface nanobubbles. Phys. Rev. Lett 2007, 98, 204502. [Google Scholar]
- Yang, SJ; Dammer, SM; Bremond, N; Zandvliet, HJW; Kooij, ES; Lohse, D. Characterization of nanobubbles on hydrophobic surfaces in water. Langmuir 2007, 23, 7072–7077. [Google Scholar]
- Zhang, XH; Quinn, A; Ducker, WA. Nanobubbles at the interface between water and a hydrophobic solid. Langmuir 2008, 24, 4756–4764. [Google Scholar]
- Vinogradova, OI. Drainage of a thin liquid-film confined between hydrophobic surfaces. Langmuir 1995, 11, 2213–2220. [Google Scholar]
- de Gennes, PG. On fluid/wall slippage. Langmuir 2002, 18, 3413–3414. [Google Scholar]
- Hampton, MA; Donose, BC; Taran, E; Nguyen, AV. Effect of nanobubbles on friction forces between hydrophobic surfaces in water. J. Colloid Interf. Sci 2009, 329, 202–207. [Google Scholar]
- Hendy, SC; Lund, NJ. Effective slip lengths for flows over surfaces with nanobubbles: The effects of finite slip. J. Phys.-Condens. Mat 2009, 21, 144202. [Google Scholar]
- Israelachvili, JN. Measurement of viscosity of liquids in very thin films. J. Colloid Interf. Sci 1986, 110, 263–271. [Google Scholar]
- Israelachvili, JN; McGuiggan, PM; Honola, AM. Dynamic properties of molecularly thin liquid films. Science 1988, 240, 189–191. [Google Scholar]
- Klein, J; Kumacheva, E. Simple liquids confined to molecularly thin layers. I. confinement-induced liquid to solid transitions. J. Chem. Phys 1998, 108, 6996–7009. [Google Scholar]
- Ravivi, U; Giasson, S; Frey, J; Klein, J. Viscosity of ultr-thin water films confined between hydrophobic and hydrophilic surfaces. J. Phys.-Condens. Mat 2002, 14, 9275–9283. [Google Scholar]
- Perkin, S; Goldberg, R; Chai, L; Kampf, N; Klein, J. Dynamic properties of confined hydration layers. Faraday Discuss 2009, 141, 399–413. [Google Scholar]
- Becher, T; Mugele, F. Nanofluidics: Molecularly thin lubricant layers under confinement. Mol. Simul 2005, 31, 489–494. [Google Scholar]
- Tretheway, D; Stone, S; Meinhart, C. Effects of absolute pressure and dissolved gases on apparent fluid slip in hydrophobic microchannels. Bull. Am. Phys. Soc 2004, 49, 215–217. [Google Scholar]
- Ruckenstein, E; Rajora, P. On the no-slip boundary condition of hydrodynamics. J. Colloid Interf. Sci 1983, 96, 488–491. [Google Scholar]
- Majumder, M; Chopra, N; Andrews, R; Hinds, BJ. Enhanced flow in carbon nanotubes. Nature 2005, 438, 44. [Google Scholar]
- Holt, JK; Park, HG; Wang, YM; Stadermann, M; Artyukhin, AB; Grigoropoulos, CP; Noy, A; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar]
- Verweij, H; Schillo, MC; Li, J. Fast mass transport through carbon nanotube membranes. Small 2007, 3, 1996–2004. [Google Scholar]
- Whitby, M; Cagnon, L; Thanou, M; Quirke, N. Enhanced fluid flow through nanoscale carbon pipes. Nano Lett 2008, 8, 2632–2637. [Google Scholar]
- Supple, S; Quirke, N. Rapid imbibition of fluids in carbon nanotubes. Phys. Rev. Lett 2003, 90, 214501. [Google Scholar]
- Chen, X; Cao, GX; Han, AJ; Punyamuitula, VK; Liu, L; Culligan, PJ; Kim, T; Qiao, Y. Nanoscale fluid transport: Size and rate effects. Nano Lett 2008, 8, 2988–2992. [Google Scholar]
- Thomas, JA; McGaughey, AJH. Reassessing fast water transport through carbon nanotubes. Nano Lett 2008, 8, 2788–2793. [Google Scholar]
- Joseph, S; Aluru, NR. Why are carbon nanotubes fast transporters of water? Nano Lett 2008, 8, 452–458. [Google Scholar]
- Whitby, M; Quirke, N. Fluid flow in carbon nanotubes and nanopipes. Nat. Nanotechnol 2007, 2, 87–94. [Google Scholar]
- Noy, A; Park, HG; Fornasiero, F; Holt, JK; Grigoropoulos, CP; Bakajin, O. Nanofluidics in carbon nanotubes. Phys. Today 2007, 2, 22–29. [Google Scholar]
- Mattia, D; Gogotsi, Y. Review: Static and dynamic behavior of liquids inside carbon nanotubes. Microfluid. Nanofluid 2008, 5, 289–305. [Google Scholar]
- Moraney, RM; White, RM; Howe, RT. Microtransport induced by ultrasonic Lamb waves. Appl Phys Lett 1991, 59, 774–776. [Google Scholar]
- Insepov, Z; Wolf, D; Hassanein, A. Nanopumping using carbon nanotubes. Nano Lett 2006, 6, 1893–1895. [Google Scholar]
- Natsuki, T; Ni, QQ; Hayashi, T; Endo, M. Wave propagation in double-walled carbon nanotubes conveying fluid. J. Appl. Phys 2008, 103, 094312. [Google Scholar]
- Kong, J; Xu, Y; Yung, KL; Xie, YC; He, L. Enhanced polymer melts flow through nanoscale channels under vibration. J. Phys. Chem. C 2009, 113, 624–629. [Google Scholar]
- Longhurst, MJ; Quirke, N. Temperature-driven pumping of fluid through single-walled carbon nanotubes. Nano Lett 2007, 7, 3324–3328. [Google Scholar]
- Shiomi, J; Maruyama, S. Water transport inside a sing-walled carbon nanotube driven by a temperature gradient. Nanotechnology 2009, 20, 055708. [Google Scholar]
- Zambrano, HA; Walther, JH; Koumoutsakos, P; Sbalzarini, IF. Thermophoretic motion of water nanodroplets confined inside carbon nanotubes. Nano Lett 2009, 9, 66–71. [Google Scholar]
- Dresselhaus, MS; Dresselhaus, G; Avouris, P. Carbon Nanotubes: Synthesis, Structure, Properties, and Applications; Springer: New York, NY, USA, 2000. [Google Scholar]
- O’Connell, MJ. Carbon Nanotubes: Properties and Applications; CRC Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Baughman, RH; Zakhidov, AA; de Heer, WA. Carbon nanotubes-the route toward applications. Science 2002, 297, 787–792. [Google Scholar]
- Livi, R; Lepri, S. Heat in one dimension. Nature 2003, 421, 327–327. [Google Scholar]
- Popov, VN. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R 2004, 43, 61–102. [Google Scholar]
- Hu, YH; Shenderova, OA; Hu, ZS; Padgett, CW; Brenner, DW. Carbon nanostructures for advanced composites. Rep. Prog. Phys 2006, 69, 1847–1895. [Google Scholar]

| C1 | C2 | References |
|---|---|---|
| 1 | 0 | Maxwell [52] |
| 1 | 5π/12 | Schamberg (from [66]) |
| 1 | −0.5 | Karniadakis and Beskok [66] |
| 1.1466 | 0.9756 or 0.647 | Cercignani [68] |
| 1.1466 | 0 | Albertoni et al. [89] |
| ≈1 | ≈0.5 | Chapman and Cowling [90] |
| 0.7252 | 0 | Loyalka [91] |
| 1.0299 | 0 | Loyalka et al. [92] |
| 1.11 | 0.61 | Hadjiconstantinou [93] |
| 1.1466 | 0.14 | Sreekanth (from [95,96]) |
| 1 | 9/8 | Deissler (from [66,97]) |
| 1 | 0.5 | Hsia and Domoto (from [66,97]) |
| 1 | 2/9 | Mitsuya [98] |
| 1.125 | 0 | Pan et al. [99] |
| 1 | 0.145–0.19 | Lockerby [100] |
| 4/3 | 0.25 | Wu (Kn < 1) [101] |
| C1 | C2 | References |
|---|---|---|
| 0 | Maxwell [52] | |
| Karniadakis and Beskok [66] | ||
| Cercignani [68] | ||
| 0 | Loyalka [91] | |
| 0 | Loyalka et al. [92] | |
| Lockerby [100] | ||
| Wu [101] (f = min[1/Kn,1]) |
| Authors | Gases | Walls | Temperature (K) | TMAC |
|---|---|---|---|---|
| 1969 [118] Knechtel and Pitts | Ar+ | Au | — | 0.5–0.95 |
| Al | 0.42–0.95 | |||
| 1969 [125] Doughty and Schaetzle | Ar | Al with varnish | — | 0.7–1.4 |
| N2 | 0.4–1.3 | |||
| 1974 [126] Seidl and Steinheil | He | Polished Cu | 300 | 0.67–0.96 |
| Cu with 5μm grooves | 0.96–1.16 | |||
| Cu with adsorbents | 0.49–1.2 | |||
| W (100) | 0.77–0.93 | |||
| Au (111) | 0.68–0.87 | |||
| Glass | 0.71–0.79 | |||
| 1979 [127] Liu et al. | He | Al | Room temperature | ~1 |
| Al2O3 | ||||
| 1998 [128] Rettner | N2 | C | 273 | > 1 |
| Pt | 273 | 0.82–0.96 | ||
| Glass | 293 | 0.80–0.98 | ||
| Disk | 293 | 0.84–0.96 | ||
| Authors | Gases | Walls | Temperature(K) | Kn | TMAC |
|---|---|---|---|---|---|
| 1974 [160] Thomas and Lord | He Ne Ar Xe | polished steel rough steel | 298 | — | 0.824, 1.040 0.918, 1.035 0.931, 1.049 0.943, 1.075 |
| 1977 [88,127] Lord | He Ne Ar Kr Xe | Mo W Ta Pt Ti | — | — | 0.2 (He, Mo) 0.46 (He, Ta) 0.67 (Ar, Mo) 0.9 (contaminated) |
| 1980 [132] Comsa et al. | He, Ne, Ar, Kr, Xe, CH4, N2, H2, O2, CO, CO2 | steel | — | > 1 | 0.994–1.027 |
| 1996 [133] Gabis et al. | Ne, Ar, Kr, CH4, N2, C2H6 | steel | 293 | 0.01-1 | 0.83–1.01 |
| 1996 [88,134] Tekasakul et al. | He Ar Kr | steel | 297 | 0.00464–0.583 0.00167–0.210 0.0013–0.163 | 0.8836–0.9714 0.8470–0.9381 0.8044–0.9563 |
| 1997 [88,135] Bentz et al. | N2 CH4 | steel | 294 | 0.00163–0.0258 0.0013–0.0215 | 0.83–0.89 0.98–1.11 |
| 2001 [138] Bentz et al. | He Ar | steel | 293 | Slip regime | 0.8134–0.8412 0.7826–0.8005 |
| 2003 [139] Jousten | N2 | stainless steel etched or with H2O covered | 290–313 | — | 1.158–1.166 |
| Authors | Gases | Walls(Roughness) | Temperature(K) | Kn | TMAC |
|---|---|---|---|---|---|
| 1969 [88] Sreekanth | N2 | brass | — | 0.007–0.237 | 0.9317 |
| 1998 [161] Veijola et al. | air | Si(1 nm) | — | — | 0.621–0.661 |
| Si(30 nm) | 0.749–0.803 | ||||
| 2001 [140] Arkilic et al. | Ar | Si(0.8 nm) | 293 | 0.1–0.41 | 0.8 ± 0.1 |
| N2 | 0.1–0.34 | 0.83 ± 0.05 | |||
| CO2 | 0.1–0.44 | 0.88 ± 0.06 | |||
| 2001 [162,163] Sazhin et al. | He, Ne, Ar, Kr | Ag | — | > 100 | 0.71–0.92 |
| Ti | 0.71–0.92 | ||||
| Ti with O2 adsorbed | 0.96–1.00 | ||||
| 2003 [94] Maurer et al. | He | glass, Si | 297-301 | 0.06–0.8 | 0.91 ± 0.03 |
| N2 | 0.002–0.59 | 0.87 ± 0.03 | |||
| 2003 [164] Jang et al. | air | glass, Si(35 nm) | 298 | 0.00115(outlet) | 0.204 |
| 2004 [141] Colin et al. | He, N2 | glass, Si | 294.2 | 0.029–0.22 | 0.93 |
| 0.002–0.008 | 1 | ||||
| 0.005–0.03 | 0.93 | ||||
| 0.027–0.09 | 0.93 | ||||
| 2004 [142] Hsieh et al. | N2 | glass, Si(1.47 μm) | ≈300 | 0.001–0.024 (outlet) | 0.3–0.7 |
| 2004 [146] Copper et al. | Ar | carbon nanotubes | — | — | 0.52±0.01 |
| N2 | |||||
| O2 | |||||
| 2006 [143] Jang and Wereley | air | glass (2.0 nm) | 297 | 0.0017(outlet) | 0.85 |
| Si(6.43 nm) | |||||
| 2007 [124] Ewart et al. | He | Si(25.2 nm) | — | 0.009–0.309 | 0.914 ± 0.009 |
| Ar | 0.003–0.302 | 0.871 ± 0.017 | |||
| N2 | 0.003–0.291 | 0.908 ± 0.041 | |||
| 2007 [144] Jang and Wereley | N2 | glass(2.0 nm) | 295.5 | 0.0137 (outlet) | 0.96 |
| SiO2(6.8 nm) | |||||
| 2007 [145] Huang et al. | air | glass(0.07 μm) | — | 0.018 | 0.90 |
| 2007 [147] Blanchard and Ligrani | He, air | Disk(10 nm) | 301 | 0.0025–0.031 | 0.915, 0.885 |
| Disk(404 nm) | 0.357, 0.346 | ||||
| Disk(770 nm) | 0.253, 0.145 | ||||
| 2007 [165] Ewart et al. | He | Si(20 nm) | 293.45–297.46 | 0.03–0.7 | 0.910 ± 0.004 |
| 2008 [166] Ewart et al. | He | Si(20 nm) | — | 0.003–30 | 1.001 ± 0.019 |
| Ar | 0.947 ± 0.010 | ||||
| Xe | 0.947 ± 0.015 | ||||
| N2 | 0.954 ± 0.005 | ||||
| Authors | Gases | Walls | Temperature (K) | Kn | TMAC |
|---|---|---|---|---|---|
| 1949 [61] Kuhlthau | air | Forged | alloy299 | 0.04–0.1 | 0.72–1.07 |
| Duralumin | |||||
| 0.1–8.3 | 0.71–0.77 [92] | ||||
| ST-14 | |||||
| 1973 [88,152] Suetin et al. | He | glass | room temperature | slip flow regime | 0.895 ± 0.004 |
| free molecular regime | 0.935 ± 0.004 | ||||
| Ne | slip flow regime | 0.865 ± 0.004 | |||
| free molecular regime | 0.929 ± 0.003 | ||||
| Ar | |||||
| slip flow regime | 0.927 ± 0.028 | ||||
| free molecular regime | 0.975 ± 0.006 | ||||
| 1974 [88,153] Porodnov et al. | Kr | 0.00049–0.0096 | 0.995 ± 0.026 | ||
| Xe | 0.00036–0.007 | 1.010 ± 0.040 | |||
| H2 | glass | 77.2 | 0.0011–0.022 | 0.957 ± 0.015 | |
| D2 | (0.05–1.5 μm) | 293 | 0.0011–0.022 | 0.934 ± 0.006 | |
| N2 | 0.0006–0.012 | 0.925 ± 0.014 | |||
| CO2 | 0.0004–0.0078 | 0.993 ± 0.009 | |||
| 1975 [154] 1980 [155] 1983 [156] Shields | He | 298 | — | ||
| Ne | Pt, Ag, W | 0.375–0.96 | |||
| O2 | rough(254 nm) | 0.06–0.84 | |||
| CO2 | adsorbents | ||||
| N2 | |||||
| 2004 [157] Gronych et al. | Xe | Bronze | 300.3 | free molecular regime | 0.90 |
| Ar | |||||
| 0.95 | |||||
| H2 | 0.94 | ||||
| He | 1.0 | ||||
| 2008 [151] Maali and Bhushan | Air | Glass | Room temperature | 0.01–10 | 0.72 |
| Authors | Gases | Walls | Temperature (K) | Kn | TMAC |
|---|---|---|---|---|---|
| 1997 [182] Chirita et al. | Ar | Ni(001) | 150 | — | −0.3~0.5 |
| 300 | −0.6~0.15 | ||||
| 2001 [119] Yamamoto | Ar | Pt(111) | 300–450 | 0.2 | 0.19 |
| Xe | 0.81 | ||||
| 2003 [184] Arya et al. | LJ potential | FCC(110) | 200–400 | — | 0–1 |
| 2005 [186] Cao et al. | Ar | Pt(111) | 100–300 | 0.02-0.16 | 0.2–0.4 |
| 2005 [175] Takeuchi et al. | N2 | Pt(111) | 300 | 0.2 | |
| smooth | 0.29–0.33 | ||||
| Xe adsorbed | |||||
| 0.84–0.88 | |||||
| 2005 [176] Hyakutake et al. | Ar | Pt(111) | 300, 600 | 0.2 | 0.89, 0.41 |
| Xe | 0.95, 0.80 | ||||
| 2007 [183] Finger et al. | He | Cu with adsorbent layer | ––– | — | 0.25–1.2 |
| 2008 [117] Sun and Li | Ar | Pt | 100–500 | 0.031–0.061 | 0.04–0.8 |
| 2008 [185] Celestini et al. | LJ potential | LJ potential | — | — | ~inversed collision number |
| 2008 [187] Spijker et al. | LJ potential | LJ potential | — | 0.028 | 0.51–0.83 |
| 2009 [188] Sun and Li | Ar | Pt | 100–300 | 0.12 | 0.348–0.87 |
| Authors | Surfaces | Liquids | Wettability | Roughness | Slip length | Parameter dependence |
|---|---|---|---|---|---|---|
| 1956 [211] Schnell | Glass + DDS | Water | -- | -- | 1–10 μm | SRI |
| 1984 [212] Churaev et al. | Quartz + TMS | Water | 70–90° | -- | 30 nm | SRD/TD |
| Mercury | 115–130° | -- | 70 nm | SRD/TD | ||
| CCL4 | 0° | -- | No-slip | -- | ||
| Benzene | 0° | -- | No-slip | -- | ||
| 1999 [213] Watanabe et al. | Acrylic Resin + FAMAR | Tap water | 150° | -- | ~240 nm | -- |
| 1999 [214] Kiseleva et al. | Quartz + CTA(+) | CTAB solutions | 70° | -- | 10 nm | SRI |
| 2002 [215] Cheng et al. | Glass + photoresist | Water | -- | 0.5 nm (pp) | No-slip | -- |
| Hexane | -- | 10 nm | SRI | |||
| Hexadecane | -- | 25 nm | SRI | |||
| Decane | -- | 15 nm | SRI | |||
| Silicon oil | -- | 20 nm | SRI | |||
| 2003 [216] Choi et al. | Silicon | Water | ≈0° | 1.1 nm (rms) | 0–10 nm | SRD |
| Silicon + OTS | Water | ≧90° | 0.3 nm (rms) | 5–35 nm | SRD | |
| 2003 [217] Cheikh et al. | Poly(carbonate) + PVP | SDS solutions | <90° | -- | 20 nm | SRI |
| 2004 [218] Qu et al. | Silicon (SM) | Water | >90° | -- | No-slip | SRI |
| Silicon (SP) | Water | 130–174° | -- | >20 μm | SRI/PD | |
| 2006 [219] Choi et al. | Silicon + SiO2 (SP) | Water | <90° | -- | 30 nm (t) | SRI/PD |
| 0 (t) | SRI/PD | |||||
| Silicon + SiO2 + Teflon (SP) | ~130° | -- | 143 nm (p) | SRI/PD | ||
| 61 nm (p) | SRI/PD | |||||
| 2008 [220] Ulmanella et al. | Silicon | Isopropanol | -- | 8.5 nm | <5nm | -- |
| n-hexadecane | -- | <5 nm | -- | |||
| Isopropanol | -- | 0.5 nm | 5–30 nm | SRD | ||
| n-hexadecane | -- | 40–120 nm | SRD | |||
| Authors | Surfaces | Liquids | Wettability | Roughness | Slip length | Parameter dependence |
|---|---|---|---|---|---|---|
| Group A: using SFA technique | ||||||
| 1985 [225] Chan et al. | Mica | OMCTS | -- | -- | No-slip | -- |
| Tetradecane | -- | -- | No-slip | -- | ||
| Hexadecane | -- | -- | No-slip | -- | ||
| 1986 [226] Israelachvili | Mica | Water | -- | -- | No-slip | -- |
| Tetradecane | -- | -- | No-slip | -- | ||
| 1989 [227] Horn et al. | Silica | NaCl solutions | 45° | 0.5 nm (av) | No-slip | -- |
| 1993 [228] Georges et al. | 6 surfaces | 9 liquids | -- | 0.2-50 nm (pp) | No-slip | -- |
| 2001 [229] Zhu et al. | Mica + HDA | Tetradecane | 12° | ≈0.1 nm | 0–1 μm | SRD |
| Mica + OTE | Tetradecane | 44° | 0–1.5 μm | SRD | ||
| Water | 110° | 0–2.5 μm | SRD | |||
| 2001 [230] Baudry et al. | Cobalt | Glycerol | 20–60° | 1 nm (pp) | No-slip | -- |
| Gold + thiol | 90° | 40 nm | SRI | |||
| 2002 [231] Zhu et al. | Mica + OTS | Water | 75–105° | 6 nm (rms) | No-slip | -- |
| Tetradecane | 12–35° | 6 nm (rms) | No-slip | -- | ||
| Mica + .8 PPO | Water | 85–110° | 3.5 nm (rms) | 0–5 nm | SRD | |
| Tetradecane | 21–38° | 3.5 nm (rms) | 0–5 nm | SRD | ||
| Mica + .2 PPO | Water | 90–110° | 2 nm (rms) | 0–20 nm | SRD | |
| Tetradecane | -- | 2 nm (rms) | 0–20 nm | SRD | ||
| Mica + OTE | Water | 110° | 0.2 nm (rms) | 0–40 nm | SRD | |
| Tetradecane | 38° | 0.2 nm (rms) | 0–40 nm | SRD | ||
| 2002 [232] Zhu et al. | Mica + PVP/PB | Tetradecane | -- | ≈0.1 nm (th) | No-slip | -- |
| Mica + PVA | Water | -- | 0–80 nm | SRD | ||
| 2002 [233] Zhu et al. | Mica | n-Alkanes | Complete | -- | No-slip | -- |
| Mica + HDA | Octane | -- | -- | 0–2 nm | SRD | |
| Dodadecane | -- | -- | 0–10 nm | SRD | ||
| Tetradecane | 12° | -- | 0–15 nm | SRD | ||
| 2002 [234] Cottin-Bizonne et al. | Glass | Glycerol | <5° | 1 nm (pp) | No-slip | -- |
| Glass + OTS | Glycerol | 95° | 50–200 nm | SRI | ||
| Water | 100° | 50–200 nm | SRI | |||
| 2005 [235] Cottin-Bizonne et al. | Pyrex | Water | Hydrophilic | 1 nm (pp) | No-slip | -- |
| Dodecane | Hydrophilic | No-slip | -- | |||
| Pyrex + OTS | Water | 105° | -- | 19 nm | SRI | |
| Group B: using AFM technique | ||||||
| 2001 [236] Craig et al. | Silica + gold + thiols | Sucrose solutions | 40–70° | 0.6 nm (rms) | 0–15 nm | SRD |
| 2002 [237] Bonaccurso et al. | Mica/glass | NaCl solutions | Complete | 1 nm (rms) | 8–9 nm | SRI |
| 2002 [238] Sun et al. | Mica/glass | 1-propanol | <90° | 1 nm (rms) | 10–14 nm | -- |
| 2003 [239] Bonaccurso et al. | Silicon/glass | Sucrose solutions | Complete | 0.7 nm (rms) | 0–40 nm | SRD/RD |
| Silicon/glass + KOH | 4 nm (rms) | 80 nm | SRD/RD | |||
| 12.1 nm (rms) | 100–175 nm | SRD/RD | ||||
| 2003 [240] Neto et al. | Silica + gold + thiols | Sucrose solutions | 40–70° | 0.6 nm (rms) | 0–18 nm | SRD |
| 2003 [241] Vinogradova et al. | Silica/glass | NaCl solutions | Complete | 0.3 nm (rms) | No-slip | SRD |
| Polystyrene | 90° | 2.5 nm (rms) | 4–10 nm | SRD | ||
| 2004 [242] Cho et al. | Borosilicate + HTS | Octane | 13° | 0.3 nm (rms) | No-slip | -- |
| Dodecane | 32° | No-slip | -- | |||
| Tridecane | 35° | 10 nm | -- | |||
| Tetradecane | 37° | 15 nm | -- | |||
| Pentadecane | 39° | 10 nm | -- | |||
| Hexadecane | 39° | 20 nm | -- | |||
| Cyclohexane | 25° | 10 nm | -- | |||
| Benzene | 32° | 50 nm | -- | |||
| Aniline | 64° | 50 nm | -- | |||
| Water | 97° | 30 nm | -- | |||
| Benzaldehyde | 62° | 20 nm | -- | |||
| Nitrobenzene | 63° | 10 nm | -- | |||
| 2-Nitroanisole | 70° | No-slip | -- | |||
| 2004 [243] Henry et al. | Silica/mica | Water | Complete | -- | 80–140 nm | SRD |
| Silica/mica + CTAB | CTAB solutions | >90° | -- | 50–80 nm | SRD | |
| 2006 [244] Vinogradova et al. | Glass + Gold | NaCl solutions | 90° (a) | 0.5–11 nm (rms) | No-slip | SRI |
| 63° (r) | ||||||
| Authors | Surfaces | Liquids | Wettability | Roughness | Slip length | Parameter dependence |
|---|---|---|---|---|---|---|
| ST: 1999 [245] Boehnke et al. | Silica | Proanediol | ≈0° | -- | No-slip | -- |
| Proanediol + Va | -- | 1 μm | -- | |||
| PDMS | -- | -- | No-slip | -- | ||
| Silica + DETMDS | Proanediol | 70–80° | -- | No-slip | -- | |
| Proanediol + Va | -- | 1 μm | -- | |||
| PDMS | -- | -- | No-slip | -- | ||
| SPT: 2002 [246] Churaev et al. | Quartz | KCl solutions | -- | 2 nm (pp) | No-slip | -- |
| Quartz + TMS | KCl solutions | 80–90° | 25 nm (pp) | 5–8 nm | -- | |
| DRS: 2005 [247] Gogte et al. | Acrylic polymer+TT Sandpaper+TT | water | 156° | Smooth | -- | -- |
| >90° | 8,15 μm | Slip | -- | |||
| CPT: 2006 [248] Choi et al. | Silicon (TOP) | Water | ~10° | 0.3 nm | Slip | VD |
| Silicon (Teflon) | ~120° | 0.6 nm | Slip | |||
| Silicon (SP + TOP) | ~0° | -- | Slip | |||
| Silicon (SP+ Teflon) | 175° | -- | ~20 μm | |||
| Silicon (TOP) | ||||||
| Silicon (Teflon) | 30wt% glycerin | -- | 0.3 nm | Slip | VD | |
| Silicon (SP + TOP) | -- | 0.6 nm | Slip | |||
| Silicon (SP + Teflon) | -- | -- | Slip | |||
| --° | -- | ~50 μm | ||||
| TM: | Silica | Aqueous | Hydrophilic | <1 nm (pp) | No-slip | -- |
| 2006 [249] | Silica + OTS | solutions | Hydrophobic | <1 nm (pp) | ~18 nm | -- |
| Joly et al. | Silica + OTS | Hydrophobic | 3 nm (rms) | No-slip | RD | |
| Authors | Surfaces | Liquids | Wettability | Roughness | Slip length | Parameter dependence |
|---|---|---|---|---|---|---|
| 2002 [251] 2004 [252] Tretheway et al. | Glass | Water | ≈0° | -- | No-slip | -- |
| Glass + OTS | 120° | 0.2 nm | 0.9 μm | -- | ||
| 2005 [253] Joseph et al. | Glass | Water | ≈0° | 0.5 nm(rms) | 50 nm | -- |
| Glass + OTS | 95° | No-slip | -- | |||
| Glass + CDOS | 95° | 50 nm | -- | |||
| 2006 [254] Truesdell et al. | PDMS | -- | 100° | -- | ~100 μm | SRI/PD |
| PDMS (SP) | 156° | -- | ~250 μm | SRI/PD | ||
| PDMS (SP+ ASC) | >150° | -- | ~1.25 mm | SRI/PD | ||
| 2006 [255] Joseph et al. | CNT forests | DI-water | >165° | 1.7 μm | ~0.47 μm | RD |
| 3.5 μm | ~0.98 μm | |||||
| 6 μm | ~1.68 μm | |||||
| 2008 [256] Byun et al. | Glass | Water | Hydrophilic | -- | No-slip | WD/PD |
| PDMS | 105° | -- | 2 μm | |||
| PDMS (SP) | 136–145° | -- | 0.4–5.4 μm | |||
| Authors | Surfaces | Liquids | Wettability | Roughness | Slip length | Parameter dependence |
|---|---|---|---|---|---|---|
| 1999 [257] 2000 [258] Pit et al. | Sapphire | Hexadecane | Complete | 0.4 nm (rms) | 175 nm | SRI |
| Sapphire + FDS | 65° | No-slip | -- | |||
| Sapphire + OTS | 40° | 400 nm | SRI | |||
| Sapphire + STA | 25° | 350 nm | SRI | |||
| 2005 [259] Schmatko et al. | Sapphire + Al2O3 | Squalane | 0° | 0.4 nm (rms) | 30 nm | WD/MSD |
| Sapphire + SiH | 20° | 0.4 nm (rms) | -- | |||
| Sapphire + OTS | 40° | 0.3 nm (rms) | 110 nm | |||
| Sapphire + Al2O3 | Hexadecane | 0° | 0.4 nm (rms) | 120 nm | WD/MSD | |
| Sapphire + SiH | 20° | 0.4 nm (rms) | 240 nm | |||
| Sapphire + OTS | 40° | 0.3 nm (rms) | 350 nm | |||
| Authors | Surfaces | Liquids | Wettability | Roughness | Slip length | Parameter dependence |
|---|---|---|---|---|---|---|
| 2003 [260] Lumma et al. | Mica | Water | -- | 15 nm (pp) | 0.5–0.86 μm | -- |
| Glass | Water | 5–10° | 0.6–1 μm | -- | ||
| NaCl solutions | 0.2–0.6 μm | -- |
| Authors | Surfaces | Liquids | Wettability | Roughness | Slip length | Parameter dependence |
|---|---|---|---|---|---|---|
| 2006 [262] Huang et al. | PDMS | DI-Water | Hydrophilic | 0.47 nm (rms) | 26–57 nm | SRD |
| PDMS + OTS | 120° | 0.35 nm (rms) | 37–96 nm | SRD | ||
| 2007 [263] Huang et al. | PDMS + OTS | DI-Water | 120° | 0.35 nm (rms) | 50–110 nm | SRD |
| 0.1mM NaCl | 30–100 nm | SRD | ||||
| 1mM NaCl | 50–110 nm | SRD | ||||
| 2008 [264] Bouzigues et al. | PDMS | Water | <20° | 0.33 nm (rms) | −3–3 nm | -- |
| PDMS + OTS | 95° | 0.44 nm (rms) | 21–29 nm | -- | ||
| 2008 [265] Lasne et al. | Glass | Water | Hydrophilic | -- | No-slip | -- |
| Glass + OTS | 90° | -- | 45 nm | -- | ||
| Authors | Solid/Liquid | Flow | Wetta-bility | Roughness | Slip length | Parameter dependence |
|---|---|---|---|---|---|---|
| 1988 [283] Koplik et al. | RL/LJ | PF+CL | 0–79° | No | Slip near CL | -- |
| 1989 [284] Heinbuch et al. | RL/LJ | PF | Complete | No | −2σ–0 | -- |
| 1989 [285] Thompson et al. | RL/LJ | CF+CL | 0–90° | No | Slip near CL | -- |
| 1989 [279] Koplik et al. | RL/LJ | PF | 0–80° | No | 0–10 σ | -- |
| CF | ||||||
| 1990 [286] Thompson et al. | RL/LJ | CF | <90° | No | 0–2σ | WD |
| 1992 [287] Sun et al. | RL/LJ | PF | -- | No | Slip for frozen walls | -- |
| 1997 [288] Thompson et al. | RL/LJ | CF | 0–140° | No | 0–60σ | SRD |
| 1999 [282] Barrat et al. | RL/LJ | PF+CL | 90–140° | No | 0–50σ | WD |
| CF+CL | ||||||
| 2000 [289] Jabbarzadeh et al. | RL/hexadecane | CF | Complete | 0.4-0.8 (SIN) | nm 0–10 nm | RD |
| 2001 [290] Cieplak et al. | RL/LJ | PF | -- | No | 0–15σ | WD |
| CF | ||||||
| 2001 [291] Sokhan et al. | CS/LJ | PF | -- | No | 1.8–10.4 nm | |
| 2002 [292] Fan et al. | RL/LJ | PF | Complete | No | 0–5σ | -- |
| 2002 [293] Sokhan et al. | CNT/LJ | PF | -- | No | 0–5 nm | -- |
| 2003 [294] Cottin-Bizonne et al. | RL/LJ | CF | 110–137° | ~10σ (GR) | 2–57 nm | RD |
| 2004 [295] Nagayama et al. | Platinum/LJ | PF | 0–180° | No | 0–100 nm | SRD/WD |
| 2004 [296] Galea et al. | RL/LJ | CF | Complete | Atomic | −4–4σ | RD |
| 2004 [297] Cottin-Bizonne et al. | RL/LJ | CF | 110-137° | ~10σ (GR) | 0–150σ | RD |
| 2004 [298] Priezjev et al. | RL/Polymer | CF | -- | No | 0–70σ | SRD/CLD |
| 2004 [299] Walther et al. | CNT/Water | PF | ~86° | No | −0.11–88 nm | CP |
| 2004 [300] Soong et al. | RL/LJ or WCA | RF | -- | No | 0–6σ | NLS |
| 2005 [301] 2006 [302] Yang et al. | TW/LJ | PF | 90-140° | 1.7-3.3σ (GR) | −3–8σ | RD |
| 2005 [303] Guo et al. | RL/LJ | CF | -- | No | −3–3σ | TD |
| 2006 [304] Cao et al. | Platinum/LJ | CF | 0-130° | No | −1–15σ | WD |
| 2006 [305] Cao et al. | Platinum/LJ | PF | 30-175° | 0-2.0 nm | −5–25σ | RD/WD |
| 2006 [306] Voronov et al. | CS/LJ | CF | 25-147° | No | 0–3.5 μm | WD |
| 2006 [307] Cieplak et al. | TW/Chains | PF | 0-130° | No | −4–12σ | PD |
| 2006 [308] Li et al. | RL/LJ | CF | -- | No | −2–8σ | WD |
| 2007 [309] Lichter et al. | TW/LJ | CF | -- | No | 0–2.5σ | VD |
| 2007 [310] Soong et al. | TW/LJ | PF | -- | No | −5–30σ | LPD |
| CF | ||||||
| 2007 [311] (HMDCS) Yen et al. | RL/LJ | PF | -- | No | 2–18σ | CSD |
| CF | ||||||
| 2008 [312] Martini et al. | TW/n-decane | CF | -- | No | 0–25 nm | SRD |
| 2008 [313] Huang et al. | Alkylsilane/Water | CF | 40-150° | No | 0–20 nm | WD |
| Diamon/Water | 40-150° | -- | ||||
| 2008 [314] Martini et al. | RL/n-decane | CF | -- | No | 0–2 nm | WSD |
| 2009 [315] Sofos et al. | Kr/Ar | PF | -- | Atomic | 0–0.8σ | RD |
| 2009 [316] Priezjev | RL/Polymer | CF | -- | No | −6–24σ | SRD |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cao, B.-Y.; Sun, J.; Chen, M.; Guo, Z.-Y. Molecular Momentum Transport at Fluid-Solid Interfaces in MEMS/NEMS: A Review. Int. J. Mol. Sci. 2009, 10, 4638-4706. https://doi.org/10.3390/ijms10114638
Cao B-Y, Sun J, Chen M, Guo Z-Y. Molecular Momentum Transport at Fluid-Solid Interfaces in MEMS/NEMS: A Review. International Journal of Molecular Sciences. 2009; 10(11):4638-4706. https://doi.org/10.3390/ijms10114638
Chicago/Turabian StyleCao, Bing-Yang, Jun Sun, Min Chen, and Zeng-Yuan Guo. 2009. "Molecular Momentum Transport at Fluid-Solid Interfaces in MEMS/NEMS: A Review" International Journal of Molecular Sciences 10, no. 11: 4638-4706. https://doi.org/10.3390/ijms10114638





