Trichothecenes in Cereal Grains
Abstract
:1. Introduction
2. Trichothecenes
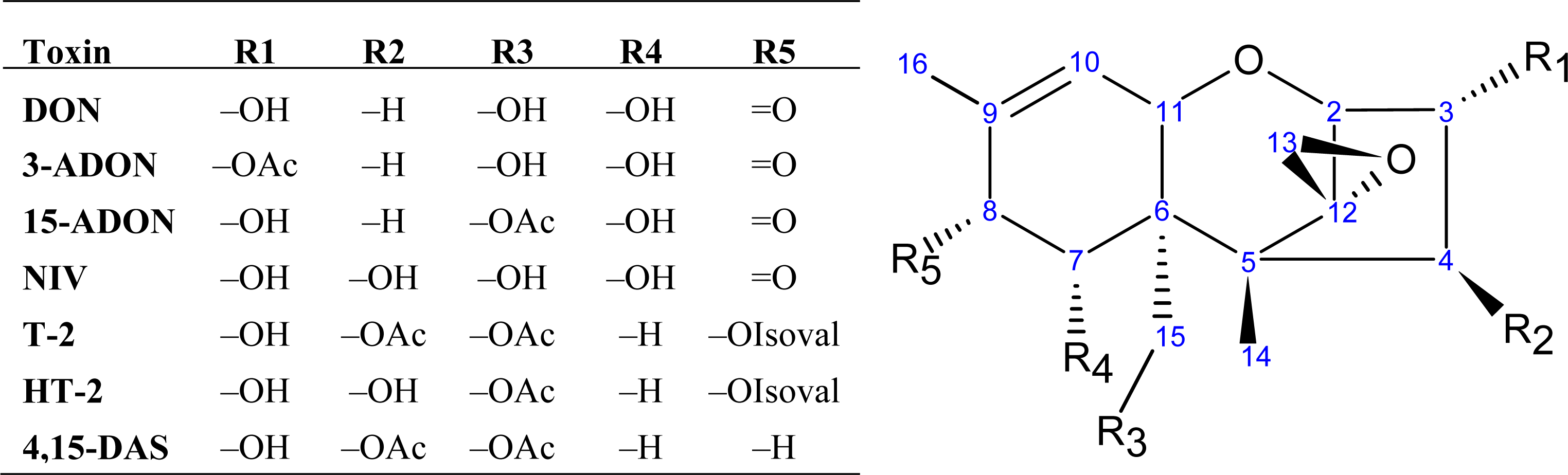
2.1. Trichothecene biosynthesis and structure
2.2. Trichothecene Toxicity: Food Safety and Quality
2.3. Trichothecenes as Aggressiveness Factors in Fusarium Head Blight
3. Fusarium Head Blight Resistance
3.1. Mechanisms of Resistance
3.2. Sources of Resistance
3.3. Breeding for Resistance
3.2. Engineering for Resistance
4. Conclusions
Acknowledgments
References and Notes
- Parry, DW; Jenkinson, P; McLeod, L. Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol 1995, 44, 207–238. [Google Scholar]
- Tóth, B; Mesterházy, Á; Horvath, Z; Bartók, T; Varga, M; Varga, J. Genetic Variability of Central European Isolates of the Fusarium graminearum Species Complex. Eur. J. Plant Pathol 2005, 113, 35–45. [Google Scholar]
- Eudes, F; Comeau, A; Rioux, S; Collin, J. Impact of trichothecenes on fusarium head blight (Fusarium graminearum) development in spring wheat (Triticum aestivum). Can. J. Plant Pathol 2001, 23, 318–322. [Google Scholar]
- Harris, LJ; Desjardins, AE; Plattner, RD; Nicholson, P; Butler, G; Young, YC; Weston, G; Proctor, RH; Hohn, TM. Possible role of trichothecene mycotoxins in virulence of Fusarium graminearum on maize. Plant Dis 1999, 83, 954–960. [Google Scholar]
- Langevin, F; Eudes, F; Comeau, A. Effect of trichothecenes produced by Fusarium graminearum during fusarium head blight development in six cereal species. Eur. J. Plant Pathol 2004, 110, 735–746. [Google Scholar]
- Proctor, RH; Hohn, TM; McCormick, SP. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact 1995, 8, 593–601. [Google Scholar]
- Eriksen, GS; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol 2004, 114, 205–239. [Google Scholar]
- Stack, RW. History of fusarium head blight with emphasis on North America. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 1; pp. 1–34. [Google Scholar]
- Gilbert, J; Tekauz, A. Review: Recent developments in research on fusarium head blight of wheat in Canada. Can. J. Plant Pathol 2000, 22, 1–8. [Google Scholar]
- Moschini, RC; Fortugno, C. Predicting wheat head blight incidence using models based on meteorological factors in Pergamino, Argentina. Eur. J. Plant Pathol 1996, 102, 211–218. [Google Scholar]
- McMullen, M. Impacts of fusarium head blight on North American agricultural community: The power of one disease to catapult change. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 18; pp. 484–503. [Google Scholar]
- Bai, G-H; Chen, L-F; Shaner, GE. Breeding for resistance to fusarium head blight of wheat in China. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 11; pp. 296–317. [Google Scholar]
- Bhat, RV; Beedu, SR; Ramakrishna, Y; Munshi, KL. Outbreak of Trichothecene mycotoxicosis associated with consumption of mold-damaged wheat products in Kashmir Valley India. Lancet 1989, 1, 35–37. [Google Scholar]
- Burgess, LW; Klein, TA; Bryden, WL; Tobin, NF. Head Blight of Wheat Caused by Fusarium graminearum Group 1 in New South Wales Australia in 1983. Australas. Plant Pathol 1987, 16, 72–78. [Google Scholar]
- Xu, XM; Nicholson, P; Thomsett, MA; Simpson, D; Cooke, BM; Doohan, FM; Brennan, J; Monaghan, S; Moretti, A; Mule, G; Homok, L; Beki, E; Tatnell, J; Ritieni, A; Edwards, SG. Relationship between the fungal complex causing fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar]
- Kriel, WM; Pretorius, ZA. The FHB challenge to irrigation wheat production in South Africa. Cereal Res. Commun 2008, 36, 569–571. [Google Scholar]
- Scott, DB; De Jager, EJH; Van Wyk, PS. Head blight of irrigated wheat in South Africa. Phytophylactica 1988, 20, 317–319. [Google Scholar]
- McMullen, M; Jones, R; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devasting impact. Plant Dis 1997, 81, 1340–1348. [Google Scholar]
- Xu, X. Effects of environmental conditions on the development of Fusarium ear blight. Eur. J. Plant Pathol 2003, 109, 683–689. [Google Scholar]
- Hongxiang, M; Yao, J; Zhou, M; Zhang, X; Ren, L; Yu, G; Lu, W. Molecular breeding for wheat fusarium head blight resistance in China. Cereal Res. Commun 2008, 36, 1–730. [Google Scholar]
- Dill-Macky, R; Jones, RK. The effect of previous crop residues and tillage on fusarium head blight of wheat. Plant Dis 2000, 84, 71–76. [Google Scholar]
- Gilbert, J; Fernando, WGD. Epidemiology and biological control of Gibberella zeae Fusarium graminearum. Can. J. Plant Pathol 2004, 26, 464–472. [Google Scholar]
- Horsley, RD; Pederson, JD; Schwarz, PB; McKay, K; Hochhalter, MR; McMullen, MP. Integrated use of tebuconazole and fusarium head blight–resistant barley genotypes. Agron. J 2006, 98, 194–197. [Google Scholar]
- Jones, RK. Assessments of fusarium head blight of wheat and barley in response to fungicide treatment. Plant Dis 2000, 84, 1021–1030. [Google Scholar]
- Khan, MR; Fischer, S; Egan, D; Doohan, FM. Biological control of Fusarium seedling blight disease of wheat and barley. Phytopathology 2006, 96, 386–394. [Google Scholar]
- Miller, JD; Culley, J; Fraser, K; Hubbard, S; Meloche, F; Ouellet, T; Seaman, WL; Seifert, KA; Turkington, K; Voldeng, H. Effect of tillage practice on fusarium head blight of wheat. Can. J. Plant Pathol 1998, 20, 95–103. [Google Scholar]
- Paul, PA; Lipps, PE; Hershman, DE; McMullen, MP; Draper, MA; Madden, LV. A Quantitative review of tebuconazole effect on fusarium head blight and deoxynivalenol content in wheat. Phytopathology 2007, 97, 211–220. [Google Scholar]
- De Wolf, ED; Madden, LV; Lipps, PE. Risk assessment models for wheat fusarium head blight epidemics based on within-season weather data. Phytopathology 2003, 93, 428–435. [Google Scholar]
- Prandini, A; Sigolo, S; Filippi, L; Battilani, P; Piva, G. Review of predictive models for fusarium head blight and related mycotoxin contamination in wheat. Food Chem. Toxicol. 2008. [epub]. [Google Scholar]
- McMullen, M; Halley, S; Schatz, B; Meyer, S; Jordahl, J; Ransom, J. Integrated strategies for fusarium head blight management in the United States. Cereal Res. Commun 2008, 36, 563–568. [Google Scholar]
- Miedaner, T; Reinbrecht, C; Lauber, U; Schollenberger, M; Geiger, HH. Effects of genotype and genotype-environment interaction on deoxynivalenol accumulation and resistance to fusarium head blight in rye, triticale, and wheat. Plant Breed 2001, 120, 97–105. [Google Scholar]
- Foroud, N; MacMillan, T; Ellis, B; Eudes, F. The role of trichothecene-chemotypes in fusarium head blight disease spread in wheat. Cereal Res. Commun 2008, 36, 489–490. [Google Scholar]
- Mesterházy, Á. Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to fusarium head blight. Eur. J. Plant Pathol 2002, 108, 675–684. [Google Scholar]
- Desjardins, AE; Hohn, TM; McCormick, SP. Trichothecene biosynthesis in Fusarium species: Chemistry, genetics, and significance. Microbiol. Rev 1993, 57, 595–604. [Google Scholar]
- Mirocha, CJ; Xie, W; Filho, ER. Chemistry and detection of Fusarium mycotoxins. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 6; pp. 144–164. [Google Scholar]
- Liddell, CM. Systematics of Fusarium species and allies associated with fusarium head blight. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 2; pp. 35–43. [Google Scholar]
- Ueno, Y. Trichothecenes: Chemical, Biological and Toxicological Aspects. Elsevier Scientific Publishers: Amsterdam, 1983. [Google Scholar]
- Sudakin, DL. Trichothecenes in the environment: Relevance to human health. Toxicol. Lett 2003, 143, 97–107. [Google Scholar]
- Desjardins, AE; Proctor, RH. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol 2007, 119, 47–50. [Google Scholar]
- Harris, LJ; Alexander, NJ; Saparno, A; Blackwell, B; McCormick, SP; Desjardins, AE; Robert, LS; Tinker, N; Hattori, J; Piche, C; Schernthaner, JP; Watson, R; Ouellet, T. A novel gene cluster in Fusarium graminearum contains a gene that contributes to butenolide synthesis. Fungal Genet. Biol 2007, 44, 293–306. [Google Scholar]
- Hohn, TM; Vanmiddlesworth, F. Purification and characterization of the sesquiterpene cyclase trichodiene synthetase from Fusarium sporotrichioides. Arch. Biochem. Biophys 1986, 251, 756–761. [Google Scholar]
- Hohn, TM; Desjardins, AE; McCormick, SP. The Tri4 gene of Fusarium sporotrichioides encodes a cytochrome P450 monooxygenase involved in trichothecene biosynthesis. Mol. Gen. Genet 1995, 248, 95–102. [Google Scholar]
- McCormick, SP; Alexander, NJ; Proctor, RH. Fusarium Tri4 encodes a multifunctional oxygenase required for trichothecene biosynthesis. Can. J. Microbiol 2006, 52, 636–642. [Google Scholar]
- McCormick, SP; Taylor, SL; Plattner, RD; Beremand, MN. Bioconversion of possible T-2 toxin precursors by a mutant strain of Fusarium sporotrichioides NRRL 3299. Appl. Environ. Microbiol 1990, 56, 702–6. [Google Scholar]
- McCormick, SP; Alexander, NJ; Trapp, SE; Hohn, TM. Disruption of TRI101, the gene encoding trichothecene 3-O-acetyltransferase, from Fusarium sporotrichioides. Appl. Environ. Microbiol 1999, 65, 5252–5256. [Google Scholar]
- Alexander, NJ; Hohn, TM; McCormick, SP. The TRI11 gene of Fusarium sporotrichioides encodes a cytochrome P-450 monooxygenase required for C-15 hydroxylation in trichothecene biosynthesis. Appl. Environ. Microbiol 1998, 64, 221–225. [Google Scholar]
- McCormick, SP; Hohn, TM; Desjardins, AE. Isolation and characterization of Tri3, a gene encoding 15-O-acetyltransferase from Fusarium sporotrichioides. Appl. Environ. Microbiol 1996, 62, 353–9. [Google Scholar]
- Desjardins, AE. Natural product chemistry meets genetics: when is a genotype a chemotype? J. Agric. Food Chem 2008, 56, 7587–7592. [Google Scholar]
- Lee, T; Han, YK; Kim, KH; Yun, SH; Lee, YW. Tri13 and Tri7 determine deoxynivalenol- and nivalenol-producing chemotypes of Gibberella zeae. Appl. Environ. Microbiol 2002, 68, 2148–2154. [Google Scholar]
- Brown, DW; McCormick, SP; Alexander, NJ; Proctor, RH; Desjardins, AE. A genetic and biochemical approach to study trichothecene diversity in Fusarium sporotrichioides and Fusarium graminearum. Fungal Genet. Biol 2001, 32, 121–133. [Google Scholar]
- Rocha, O; Ansari, K; Doohan, FM. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam 2005, 22, 369–378. [Google Scholar]
- McLaughlin, CS; Vaughn, MH; Campbell, JM; Wei, CM; Stafford, ME. Mycotoxins in Human and Health; Rodricks, JV, Hesseltine, CW, Mehlman, MA, Eds.; Pathotox Publishers: Park Forest South, Illinois, 1977; pp. 263–275. [Google Scholar]
- Ueno, Y. Mode of action of trichothecenes. Ann. Nutr. Aliment 1977, 31, 885–900. [Google Scholar]
- Ueno, Y. The toxicology of mycotoxins. Crit. Rev. Toxicol 1985, 14, 99–133. [Google Scholar]
- Minervini, F; Fornelli, F; Flynn, KM. Toxicity and apoptosis induced by the mycotoxins nivalenol, deoxynivalenol and fumonisin B1 in a human erythroleukemia cell line. Toxicol. In Vitro 2004, 18, 21–28. [Google Scholar]
- Thompson, WL; Wannemacher, RWJ. Structure-function relationships of 12,13–epoxytrichothecene mycotoxins in cell culture comparison to whole animal lethality. Toxicon 1986, 24, 985–994. [Google Scholar]
- Pace, JG; Watts, MR; Canterbury, WJ. T-2 Mycotoxin inhibits mitochondrial protein synthesis. Toxicon 1988, 26, 77–86. [Google Scholar]
- Bunner, DL; Morris, ER. Alteration of multiple cell Membrane functions in L-6 myoblasts by T-2 toxin an important mechanism of action. Toxicol. Appl. Pharmacol 1988, 92, 113–121. [Google Scholar]
- Kang, Z; Buchenauer, H. Immunocytochemical localization of Fusarium toxins in infected wheat spikes by Fusarium culmorum. Physiol. Mol. Plant Pathol 1999, 55, 275–288. [Google Scholar]
- Islam, Z; Pestka, JJ. Role of IL-1beta in endotoxin potentiation of deoxynivalenol-induced corticosterone response and leukocyte apoptosis in mice. Toxicol. Sci 2003, 74, 93–102. [Google Scholar]
- Shifrin, VI; Anderson, P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J. Biol. Chem 1999, 274, 13985–13992. [Google Scholar]
- Yoshino, N; Takizawa, M; Tashiro, F; Honda, M; Ueno, Y. T-2 toxin induced apoptosis in human peripheral blood lymphocytes in vitro. Res. Commun. Biochem. Cell Mol. Biol 1997, 1, 218–228. [Google Scholar]
- Mayer, CF. Endemic panmyelotoxicosis in the Russian grain belt. I. The clinical aspects of alimentary toxic aleukia (ATA); a comprehensive review. Mil. Surg 1953, 113, 173–189. [Google Scholar]
- Yagen, B; Joffe, AZ. Screening of toxic isolates of Fusarium poae and Fusarium sporotrichioides involved in causing alimentary toxic aleukia. Appl. Environ. Microbiol 1976, 32, 423–427. [Google Scholar]
- Del, PEM; Fernandes, JMC; Bergstrom, GC. Influence of growth stage on fusarium head blight and deoxynivalenol production in wheat. J. Phytopathol 2007, 155, 577–581. [Google Scholar]
- Bushnell, WR; Hazen, BE; Pritsch, C. Histology and physiology of fusarium head blight. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 3; pp. 44–83. [Google Scholar]
- Steffenson, BJ. Fusarium head blight of barley: Impact, epidemics, management, and strategies for identifying and utilizing genetic resistance. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 10; pp. 241–295. [Google Scholar]
- Hart, LP; Pestka, JJ; Liu, MT. Effect of kernel development and wet periods on production of deoxynivalenol in wheat infected with Gibberella zeae. Phytopathology 1984, 74, 1415–1418. [Google Scholar]
- Pestka, JJ; Smolinski, AT. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B Crit. Rev 2005, 8, 36–39. [Google Scholar]
- Rotter, BA; Prelusky, DB; Pestka, JJ. Toxicology of deoxynivalenol (Vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar]
- Wolpert, TJ; Dunkle, LD; Ciuffetti, LM. Host-selective toxins and avirulence determinants: What’s in a name? Annu. Rev. Phytopathol 2002, 40, 251–85. [Google Scholar]
- Tanaka, A; Shiotani, H; Yamamoto, M; Tsuge, T. Insertional mutagenesis and cloning of the genes required for biosynthesis of the host-specific AK-toxin in the Japanese pear pathotype of Alternaria alternata. Mol. Plant-Microbe Interact 1999, 12, 691–702. [Google Scholar]
- Navarre, DA; Wolpert, TJ. Victorin induction of an apoptotic/senescence-like response in oats. Plant Cell 1999, 11, 237–249. [Google Scholar]
- Baker, SE; Kroken, S; Inderbitzin, P; Asvarak, T; Li, BY; Shi, L; Yoder, OC; Turgeon, BG. Two polyketide synthase-encoding genes are required for biosynthesis of the polyketide virulence factor, T-toxin, by Cochliobolus heterostrophus. Mol. Plant-Microbe Interact 2006, 19, 139–149. [Google Scholar]
- Reino, JL; Hernandez, GR; Duran, PR; Collado, IG. Virulence-toxin production relationship in isolates of the plant pathogenic fungus Botrytis cinerea. J. Phytopathol 2004, 152, 563–566. [Google Scholar]
- Cotty, PJ; Misaghi, IJ. Zinniol Production by Alternaria Species. Phytopathology 1984, 74, 785–788. [Google Scholar]
- Beremand, MN; Desjardins, AE; Hohn, TM; Vanmiddlesworth, FL. Survey of Fusarium sambucinum Equals Gibberella pulicaris for Mating Type Trichothecene production and other selected tTraits. Phytopathology 1991, 81, 1452–1458. [Google Scholar]
- Miedaner, T; Reinbrecht, C; Schilling, AG. Association among aggressiveness, fungal colonization, and mycotoxin production of 26 isolates of Fusarium graminearum in winter rye head blight. Z. Pflanzenkr. Pflanzenschutz 2000, 107, 124–134. [Google Scholar]
- Perkowski, J; Kiecana, I; Schumacher, U; Mueller, HM; Chelkowski, J; Golinski, P. Head blight and biosynthesis of Fusarium toxins in barley kernels field inoculated with Fusarium culmorum. Eur. J. Plant Pathol 1996, 102, 491–496. [Google Scholar]
- Snijders, CHA; Perkowski, J. Effects of head blight caused by Fusarium culmorum on toxin content and weight of wheat kernels. Phytopathology 1990, 80, 570. [Google Scholar]
- Mesterházy, Á; Bartók, T; Mirocha, CG; Komoroczy, R. Nature of wheat resistance to fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed 1999, 118, 97–110. [Google Scholar]
- Arseniuk, E; Góral, T; Czembor, HJ. Reaction of triticale, wheat and rye accessions to graminaceous Fusarium spp. infection at the seedling and adult plant growth stages. Euphytica 1993, 70, 175–183. [Google Scholar]
- Boutigny, AL; Richard, FF; Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol 2008, 121, 411–423. [Google Scholar]
- Bai, G-H; Desjardins, AE; Plattner, RD. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–8. [Google Scholar]
- Maier, FJ; Miedaner, T; Hadeler, B; Felk, A; Salomon, S; Lemmens, M; Kassner, H; Schaefer, W. Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol. Plant Pathol 2006, 7, 449–461. [Google Scholar]
- Jansen, C; von, WD; Schaefer, W; Kogel, KH; Felk, A; Maier, FJ. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar]
- Mesterházy, Á. Types and components of resistance to fusarium head blight of wheat. Plant Breed 1995, 114, 377–386. [Google Scholar]
- Reid, LM; Mather, DE; Hamilton, RI; Bolton, AT. Genotypic differences in the resistance of maize silk to Fusarium graminearum. Can. J. Plant Pathol 1992, 14, 211–214. [Google Scholar]
- Chungu, C; Mather, DE; Reid, LM; Hamilton, RI. Inheritance of kernel resistance to Fusarium graminearum in maize. J. Hered 1996, 87, 382–385. [Google Scholar]
- Schroeder, HW; Christensen, JJ. Factors affecting resistance of wheat to scab by Gibberella zeae. Phytopathology 1963, 42, 720–727. [Google Scholar]
- Dill-Macky, R. Inoculation methods and evaluation of fusarium head blight resistance in wheat. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 8; pp. 184–210. [Google Scholar]
- Rudd, JC; Horsley, RD; McKendry, AL; Elias, EM. Host plant resistance genes for fusarium head blight: sources, mechanisms, and utility in conventional breeding systems. Crop Sci 2001, 41, 620–627. [Google Scholar]
- Eudes, F; Zhou, W; Badea, A; Laroche, A. Toward the development of fusarium head blight resistance and reduced levels of mycotoxin in wheat and barley. Recent Res. Devel. Plant Pathology 2004, 3, 1–33. [Google Scholar]
- Mesterházy, Á; Tóth, B; Bartók, T; Varga, M. Breeding strategies against FHB in winter wheat and their relation to type I resistance Cereal Res. Commun 2008, 36, 37–43. [Google Scholar]
- Kang, Z; Buchenauer, H. Ultrastructural immunocytochemical investigation of pathogen development and host responses in resistant and susceptible wheat spikes infected by Fusarium culmorum. Physiol. Mol. Plant Pathol 2000, 57, 255–268. [Google Scholar]
- Ilgen, P; Maier, FJ; Schäfer, W. Trichothecenes and lipases are host-induced and secreted virulence factors of Fusarium graminearum. Cereal Res. Commun 2008, 36, 421–428. [Google Scholar]
- Miller, JD; Young, JC; Sampson, DR. Deoxynivalenol and fusarium head blight resistance in spring cereals. J. Phytopathol 1985, 113, 359–367. [Google Scholar]
- Wang, YZ; Miller, JD. Effects of Fusarium graminearum metabolites on wheat tissue in relation to fusarium head blight resistance. J. Phytopathol 1988, 122, 118–125. [Google Scholar]
- Mesterházy, Á. Breeding wheat for fusarium head blight resistance in Europe. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 13; pp. 363–381. [Google Scholar]
- Miedaner, T. Breeding wheat and rye for resistance to Fusarium diseases. Plant Breed 1997, 116, 201–220. [Google Scholar]
- Tekauz, A; McCallum, B; Gilbert, J. Review: Fusarium head blight of barley in western Canada. Can. J. Plant Pathol 2000, 22, 9–16. [Google Scholar]
- Tekauz, A; McCallum, B; Ames, N; Fetch, JM. Fusarium head blight of oat—current status in western Canada. Can. J. Plant Pathol 2004, 26, 473–479. [Google Scholar]
- Tekauz, A; Mitchell Fetch, JW; Rossnagel, BG; Savard, ME. Progress in assessing the impact of fusarium head blight on oat in western Canada and screening of avena germplasm for resistance Cereal Res. Commun 2008, 36, 49–56. [Google Scholar]
- Bjørnstad, Å; Skinnes, H. Resistance to Fusrium infection in oats ( Avena sativa L.) Cereal Res. Commun 2008, 36, 57–62. [Google Scholar]
- Cooper, B; Skoglund, L; Askelson, S; Van Horn, J. In 2nd International Symposium on Fusarium. Head Blight: Orlando, FL, USA, 2004; pp. 44–48. [Google Scholar]
- de la Pena, RC; Smith, KP; Capettini, F; Muehlbauer, GJ; Gallo, MM; Dill, MR; Somers, DA; Rasmusson, DC. Quantitative trait loci associated with resistance to fusarium head blight and kernel discoloration in barley. Theor. Appl. Genet 1999, 99, 561–569. [Google Scholar]
- Ma, Z; Steffenson, BJ; Prom, LK; Lapitan, NL. Mapping of Quantitative Trait Loci for Fusarium Head Blight Resistance in Barley. Phytopathology 2000, 90, 1079–1088. [Google Scholar]
- Zhu, H; Gilchrist, L; Hayes, P; Kleinhofs, A; Kudrna, D; Liu, Z; Prom, L; Steffenson, B; Toojinda, T; Vivar, H. Does function follow form? Principal QTLs for fusarium head blight (FHB) resistance are coincident with QTLs for inflorescence traits and plant height in a doubled-haploid population of barley. Theor. Appl. Genet 1999, 99, 1221–1232. [Google Scholar]
- Oliver, RE; Cal, X; Friesen, TL; Halley, S; Stack, RW; Xu, SS. Evaluation of fusarium head blight resistance in tetraploid wheat (Triticum turgidum L.). Crop Sci 2008, 48, 213–222. [Google Scholar]
- Bürstmayr, H; Ban, T; Anderson, AJ. QTL mapping and marker-assisted selection for fusarium head blight resistance in wheat: A review. Plant Breed 2008. In press. [Google Scholar]
- Anderson, JA. Marker-assisted selection for fusarium head blight resistance in wheat. Int. J. Food Microbiol 2007, 119, 51–53. [Google Scholar]
- Liu, S; Pumphrey, MO; Gill, BS; Trick, HN; Zhang, JX; Dolezel, J; Chalhoub, B; Anderson, JA. Toward positional cloning of Fhb1, a major QTL for fusarium head blight resistance in wheat Cereal Res. Commun 2008, 36, 195–201. [Google Scholar]
- Han, FP; Fedak, G; Ouellet, T; Dan, H; Somers, DJ. Mapping of genes expressed in Fusarium graminearum-infected heads of wheat cultivar ‘Frontana’. Genome 2005, 48, 88–96. [Google Scholar]
- Mardi, M; Pazouki, L; Delavar, H; Kazemi, MB; Ghareyazie, B; Steiner, B; Nolz, R; Lemmens, M; Buerstmayr, H. QTL analysis of resistance to fusarium head blight in wheat using a ‘Frontana’-derived population. Plant Breed 2006, 125, 313–317. [Google Scholar]
- Steiner, B; Lemmens, M; Griesser, M; Scholz, U; Schondelmaier, J; Bürstmayr, H. Molecular mapping of resistance to fusarium head blight in the spring wheat cultivar Frontana. Theor. Appl. Genet 2004, 109, 215–24. [Google Scholar]
- Couture, L. Susceptibility of spring cereal cultivars to seed contamination by Fusarium-Spp during inflorescence. Can. J. Plant Sci 1982, 62, 29–34. [Google Scholar]
- Klahr, A; Zimmermann, G; Wenzel, G; Mohler, V. Effects of environment disease progress, plant height and heading date on the detection of QTLs or resistance to fusarium head blight in an European winter wheat cross. Euphytica 2007, 154, 17–28. [Google Scholar]
- Draeger, R; Gosman, N; Steed, A; Chandler, E; Thomsett, M; Srinivasachary; Schondelmaier, J; Bürstmayr, H; Lemmens, M; Schmolke, M; Mesterházy, Á; Nicholson, P. Identification of QTLs for resistance to fusarium head blight, DON accumulation and associated traits in the winter wheat variety Arina. Theor. Appl. Genet 2007, 115, 617–25. [Google Scholar]
- Zhang, X; Yang, S; Zhou, Y; He, Z; Xia, X. Distribution of the Rht-B1b, Rht-D1b and Rht8 reduced height genes in autumn-sown Chinese wheats detected by molecular markers. Euphytica 2006, 152, 109–116. [Google Scholar]
- Nicholson, P; Sriniv Asachary, N; Gosman, N; Steed, A; Chen, X. Role of phytohormone signalling in resistance of wheat to fusarium head blight Cereal Res. Commun 2008, 36, 213–216. [Google Scholar]
- Reid, LM; McDiarmid, G; Parker, AJ; Woldemariam, T. CO441 corn inbred line. Can. J. Plant Sci 2003, 83, 79–80. [Google Scholar]
- Ali, ML; Taylor, JH; Jie, L; Sun, G; William, M; Kasha, KJ; Reid, LM; Pauls, KP. Molecular mapping of QTLs for resistance to Gibberella ear rot, in corn, caused by Fusarium graminearum. Genome 2005, 48, 521–533. [Google Scholar]
- Pè, ME; Gianfranceschi, L; Taramino, G; Tarchini, R; Angelini, P; Dani, M; Binelli, G. Mapping quantitative trait loci (QTLs) for resistance to Gibberella zeae infection in maize. Mol. Gen. Genet 1993, 241, 11–16. [Google Scholar]
- Reinprecht, Y; Wu, X; Yan, S; Labey, L; Dasilva, E; Martin, JC; Pauls, KP. A microarray-based approach for identifying genes for resistance to Fusarium graminearum in maize (Zea Mays L.) Cereal Res. Commun 2008, 36, 253–259. [Google Scholar]
- Dodds, PN; Lawrence, GJ; Catanzariti, AM; Teh, T; Wang, CIA; Ayliffe, MA; Kobe, B; Ellis, JG. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. USA 2006, 103, 8888–8893. [Google Scholar]
- Zhang, G; Mergoum, M. Molecular mapping of kernel shattering and its association with fusarium head blight resistance in a Sumai3 derived population. Theor. Appl. Genet 2007, 115, 757–766. [Google Scholar]
- Bai, GH; Plattner, R; Desjardins, A; Kolb, F. Resistance to fusarium head blight and deoxynivalenol accumulation in wheat. Plant Breed 2001, 120, 1–6. [Google Scholar]
- Geddes, J; Eudes, F; Tucker, JR; Legge, WG; Selinger, LB. Evaluation of inoculation methods on infection and deoxynivalenol production by Fusarium graminearum on barley. Can. J. Plant Pathol 2008, 30, 66–73. [Google Scholar]
- Bruins, MBM; Karsai, I; Schepers, J; Snijders, CHA. Phytotoxicity of deoxynivalenol to wheat tissue with regard to in vitro selection for fusarium head blight resistance. Plant Sci 1993, 94, 195–206. [Google Scholar]
- Fadel, F; Wenzel, G. In vitro selection for tolerance to Fusarium in F-1 microspore populations of wheat. Plant Breed 1993, 110, 89–95. [Google Scholar]
- Eudes, F; Comeau, A; Rioux, S; Collin, J. Trichothecene-mediated in vitro selection in wheat for reduced mycotoxin accumulation caused by Fusarium graminearum. Trichothecene-mediated in vitro selection in wheat for reduced mycotoxin accumulation caused by Fusarium graminearum. 2008; In Press. [Google Scholar]
- Campbell, MA; Fitzgerald, HA; Ronald, PC. Engineering pathogen resistance in crop plants. Transgenic Res 2002, 11, 599–613. [Google Scholar]
- Dahleen, LS; Okubara, PA; Blechl, AE. Transgenic approaches to combat fusarium head blight in wheat and barley. Crop Sci 2001, 41, 628–637. [Google Scholar]
- Munkvold, GP. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Pathol 2003, 109, 705–713. [Google Scholar]
- Ehrlich, KC; Daigle, KW. Protein Synthesis Inhibition by 8 Oxo-12 13-Epoxytrichothecenes. Biochim. Biophys. Acta 1987, 923, 206–213. [Google Scholar]
- Kimura, M; Kaneko, I; Komiyama, M; Takatsuki, A; Koshino, H; Yoneyama, K; Yamaguchi, I. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Cloning and characterization of Tri101. J. Biol. Chem 1998, 273, 1654–1661. [Google Scholar]
- Eriksen, GS; Pettersson, H; Lundh, T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem. Toxicol 2004, 42, 619–624. [Google Scholar]
- Alexander, NJ. The TRI101 story: engineering wheat and barley to resist fusarium head blight. World Mycotoxin J 2008, 1, 31–37. [Google Scholar]
- Ohsato, S; Ochiai, FT; Nishiuchi, T; Takahashi, AN; Koizumi, S; Hamamoto, H; Kudo, T; Yamaguchi, I; Kimura, M. Transgenic rice plants expressing trichothecene 3-O-acetyltransferase show resistance to the Fusarium phytotoxin deoxynivalenol. Plant Cell Rep 2007, 26, 531–538. [Google Scholar]
- Okubara, PA; Blechl, AE; McCormick, SP; Alexander, NJ; Dill, MR; Hohn, TM. Engineering deoxynivalenol metabolism in wheat through the expression of a fungal trichothecene acetyltransferase gene. Theor. Appl. Genet 2002, 106, 74–83. [Google Scholar]
- Alexander, NJ; McCormick, SP; Hohn, TM. TRI12, a trichothecene efflux pump from Fusarium sporotrichioides: Gene isolation and expression in yeast. Mol. Gen. Genet 1999, 261, 977–984. [Google Scholar]
- Poppenberger, B; Berthiller, F; Lucyshyn, D; Sieberer, T; Schuhmacher, R; Krska, R; Kuchler, K; Glossl, J; Luschnig, C; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem 2003, 278, 47905–47914. [Google Scholar]
- Berthiller, F; Dall’Asta, C; Schuhmacher, R; Lemmens, M; Adam, G; Krska, R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem 2005, 53, 3421–3425. [Google Scholar]
- Dall’Asta, C; Berthiller, F; Schuhmacher, R; Adam, G; Lemmens, M; Krska, R. DON-glycosides: Characterisation of synthesis products and screening for their occurrence in DON-treated wheat samples. Mycotox. Res 2005, 21, 123–127. [Google Scholar]
- Lemmens, M; Scholz, U; Berthiller, F; Dall’Asta, C; Koutnik, A; Schuhmacher, R; Adam, G; Bürstmayr, H; Mesterházy, Á; Krska, R; Ruckenbauer, P. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for fusarium head blight resistance in wheat. Mol. Plant-Microbe Interact 2005, 18, 1318–24. [Google Scholar]
- Gareis, M; Bauer, J; Thiem, J; Plank, G; Grabley, S; Gedek, B. Cleavage of zearalenone-glycoside, a masked mycotoxin, during digestion in swine. Zentralbl. Veterinarmed. B 1990, 37, 236–240. [Google Scholar]
- Savard, ME. Deoxynivalenol fatty acid and glucoside conjugates. J. Agric. Food Chem 1991, 39, 570–574. [Google Scholar]
- Tanaka, T; Yoneda, A; Inoue, S; Sugiura, Y; Ueno, Y. Simultaneous determination of trichothecene mycotoxins and zearalenone in cereals by gas chromatography-mass spectrometry. J. Chromatogr. A 2000, 882, 23–28. [Google Scholar]
- Krska, R; Baumgartner, S; Josephs, R. The state-of-the-art in analysis of type-A and -B trichothecene mycotoxins in cereals. Fresenius J. Anal. Chem 2001, 371, 285–299. [Google Scholar]
- Berthiller, F; Schuhmacher, R; Buttinger, G; Krska, R. Rapid simultaneous determination of major type A- and B-trichothecenes as well as zearalenone in maize by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2005, 1062, 209–216. [Google Scholar]
- Krska, R; Welzig, E; Berthiller, F; Molinelli, A; Mizaikoff, B. Advances in the analysis of mycotoxins and its quality assurance. Food Addit. Contam 2005, 22, 345–353. [Google Scholar]
- Zöllner, P; Mayer-Helm, B. Trace mycotoxin analysis in complex biological and food matrices by liquid chromatography–atmospheric pressure ionisation mass spectrometry. J. Chromatogr. A 1136, 123–169.
- Krska, R; Welzig, E; Boudra, H. Analysis of Fusarium toxins in feed. Anim. Feed Sci. Technol 2007, 137, 241–264. [Google Scholar]
- Beremand, MN. Isolation and characterization of mutants blocked in T-2 toxin biosynthesis. Appl. Environ. Microbiol 1987, 53, 1855–1859. [Google Scholar]
- Beremand, MN; Desjardins, AE. Trichothecene biosynthesis in Gibberella pulicaris: Inheritance of C-8 hydroxylation. J. Indus. Microbiol 1989, 3, 167–174. [Google Scholar]
- Meek, IB; Peplow, AW; Ake, C, Jr; Phillips, TD; Beremand, MN. Tri1 encodes the cytochrome P450 monooxygenase for C-8 hydroxylation during trichothecene biosynthesis in Fusarium sporotrichioides and resides upstream of another new Tri gene. Appl. Environ. Microbiol 2003, 69, 1607–1613. [Google Scholar]
- Tokai, T; Koshino, H; Takahashi, AN; Sato, M; Fujimura, M; Kimura, M. Fusarium Tri4 encodes a key multifunctional cytochrome P450 monooxygenase for four consecutive oxygenation steps in trichothecene biosynthesis. Biochem. Biophys. Res. Commun 2007, 353, 412–417. [Google Scholar]
- Hohn, TM; Beremand, PD. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene 1989, 79, 131–138. [Google Scholar]
- McCormick, SP; Alexander, NJ. Fusarium Tri8 encodes a trichothecene C-3 esterase. Appl. Environ. Microbiol 2002, 68, 2959–2964. [Google Scholar]
- McCormick, SP; Hohn, TM. Accumulation of trichothecenes in liquid cultures of a Fusarium sporotrichioides mutant lacking a functional trichothecene C-15 hydroxylase. Appl. Environ. Microbiol 1997, 63, 1685–1688. [Google Scholar]
- Kim, HS; Lee, T; Dawlatana, M; Yun, SH; Lee, YW. Polymorphism of trichothecene biosynthesis genes in deoxynivalenol- and nivalenol-producing Fusarium graminearum isolates. Mycol. Res 2003, 107, 190–197. [Google Scholar]
- Peplow, AW; Meek, IB; Wiles, MC; Phillips, TD; Beremand, MN. Tri16 is required for esterification of position C-8 during trichothecene mycotoxin production by Fusarium sporotrichioides. Appl. Environ. Microbiol 2003, 69, 5935–5940. [Google Scholar]
- Kimura, M; Tokai, T; O’Donnell, K; Ward, TJ; Fujimura, M; Hamamoto, H; Shibata, T; Yamaguchi, I. The trichothecene biosynthesis gene cluster of Fusarium graminearum F15 contains a limited number of essential pathway genes and expressed non-essential genes. FEBS Lett 2003, 539, 105–110. [Google Scholar]
- Proctor, RH; Hohn, TM; McCormick, SP; Desjardins, AE. Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides. Appl. Environ. Microbiol 1995, 61, 1923–1930. [Google Scholar]
- Hohn, TM; Krishna, R; Proctor, RH. Characterization of a transcriptional activator controlling trichothecene toxin biosynthesis. Fungal Genet. Biol 1999, 26, 224–235. [Google Scholar]
- Tag, AG; Garifullina, GF; Peplow, AW; Ake, C, Jr; Phillips, TD; Hohn, TM; Beremand, MN. A novel regulatory gene, Tri10, controls trichothecene toxin production and gene expression. Appl. Environ. Microbiol 2001, 67, 5294–5302. [Google Scholar]
- Alexander, NJ; Hohn, TM; McCormick, SP. Molecular characterization of TRI12 which encodes an apparent transport protein involved in trichothecene production by Fusarium sporotrichioides. Cereal Res. Commun 1997, 25, 347–348. [Google Scholar]
- Brown, DW; Dyer, RB; McCormick, SP; Kendra, DF; Plattner, RD. Functional demarcation of the Fusarium core trichothecene gene cluster. Fungal Genet. Biol 2004, 41, 454–462. [Google Scholar]
- Hohn, TM; McCormick, SP; Desjardins, AE. Evidence for a gene cluster involving trichothecene-pathway biosynthetic genes in Fusarium sporotrichioides. Curr. Genet 1993, 24, 291–295. [Google Scholar]
- Kimura, M; Tokai, T; Takahashi, AN; Ohsato, S; Fujimura, M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem 2007, 71, 2105–2123. [Google Scholar]
- Ward, TJ; Bielawski, JP; Kistler, HC; Sullivan, E; O’Donnell, K. Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proc. Natl. Acad. Sci. USA 2002, 99, 9278–9283. [Google Scholar]
- McCormick, SP. The role of DON in pathogenicity. In Fusarium Head Blight of Wheat and Barley; Leonard, KJ, Bushnell, WR, Eds.; The American Phytopathological Society: St. Paul, Minnesota, USA, 2003; Chapter 7; pp. 165–183. [Google Scholar]
- Gottschalk, C; Barthel, J; Engelhardt, G; Bauer, J; Meyer, K. Occurrence of type A trichothecenes in conventionally and organically produced oats and oat products. Mol. Nutr. Food Res 2007, 51, 1547–1553. [Google Scholar]
- Brown, DW; Proctor, RH; Dyer, RB; Plattner, RD. Characterization of a Fusarium 2-gene cluster involved in trichothecene C-8 modification. J. Agric. Food Chem 2003, 51, 7936–7944. [Google Scholar]
- McCormick, SP; Alexander, NJ; Proctor, RH. Heterologous expression of two trichothecene P450 genes in Fusarium verticillioeds. Can. J. Microbiol 2006, 52, 220–226. [Google Scholar]


| Gene | Cluster | Description | References |
|---|---|---|---|
| Enzymes: for pathway reactions see also Scheme 1 | |||
| Tri1 | Tri1-Tri16 | C-7 monooxygenase (F. graminearum); C-8 monooxygenase (F. graminearum, F. sporotrichioides) | [154–156, 174–175] |
| Tri3 | Core Tri | 15-O-acetyltransferase | [47] |
| Tri4 | Core Tri | monooxygenase | [41–42, 157] |
| Tri5 | Core Tri | sesquiterpene cyclase, ‘trichodiene synthase’ 4-O-acetyltransferase; functional F. graminearum TRI7 required for | [6, 40, 158] |
| Tri7 | Core Tri | NIV-chemotype; functional F. sporotrichioides TRI7 required for T-2 toxin production | [49–50] |
| Tri8 | Core Tri | C-3 deacetylase; functional F. sporotrichioides TRI8 required for T-2 toxin production | [50, 159] |
| Tri9 | Core Tri | [50] | |
| Tri11 | Core Tri | C-15 monooxygenase | [45, 160] |
| Tri13 | Core Tri | monooxygenase; functional F. graminearum TRI13 required for NIV-chemotype | [49, 161] |
| Tri14 | Core Tri | ||
| Tri16 | Tri1-Tri16 | [162] | |
| Tri101 | None | 15-O-acetyltransferase | [44, 136, 162] |
| Transcription Factors | |||
| Tri6 | Core Tri | zinc-finger DNA binding protein; required for T-2 toxin production; binding motif (YNAGGCC) found in most promoter regions within Tri5 cluster | [164, 165, 50] |
| Tri10 | Core Tri | [166] | |
| Other | |||
| Tri12 | Core Tri | major facilitator superfamily (MFS) transporter involved in trichothecene efflux | [166, 141] |
| Resistance | Description | ||
|---|---|---|---|
| Resistance in Small Grain Cereals (as defined in reference 99) | |||
| Type I | Resistance to initial infection [90] | ||
| Type II | Resistance to disease spread [90] | ||
| Type III | Resistance to kernel infection [87] | ||
| Type IV | Tolerance against FHB and trichothecenes [87] | ||
| Type V | Resistance to trichothecene accumulation [97] | ||
| class 1 | by chemical modification of trichothecenes [83] | ||
| class 2 | by inhibition of trichothecene synthesis [83] | ||
| Resistance in Maize | |||
| Silk Resistance | Resistance to silk penetration [88] | ||
| Kernel Resistance | Resistance to kernel disease spread [89] | ||
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/). This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Foroud, N.A.; Eudes, F. Trichothecenes in Cereal Grains. Int. J. Mol. Sci. 2009, 10, 147-173. https://doi.org/10.3390/ijms10010147
Foroud NA, Eudes F. Trichothecenes in Cereal Grains. International Journal of Molecular Sciences. 2009; 10(1):147-173. https://doi.org/10.3390/ijms10010147
Chicago/Turabian StyleForoud, Nora A., and François Eudes. 2009. "Trichothecenes in Cereal Grains" International Journal of Molecular Sciences 10, no. 1: 147-173. https://doi.org/10.3390/ijms10010147




