Experimental Section
General
Melting points were measured on an Electrothermal 88629 apparatus and are uncorrected. Infrared (IR) spectra were recorded on a Perkin Elmer FT-IR 1600 spectrometer. 1H- and 13C-NMR spectra were recorded in CDCl3 at 200 MHz and 50.289 MHz, respectively, on a Varian Mercury 200 Spectrometer with TMS as internal standard. Mass spectra were obtained on a Agilent 1100 series LC/MSD Trap, SL Spectrometer by electrospray insertion.
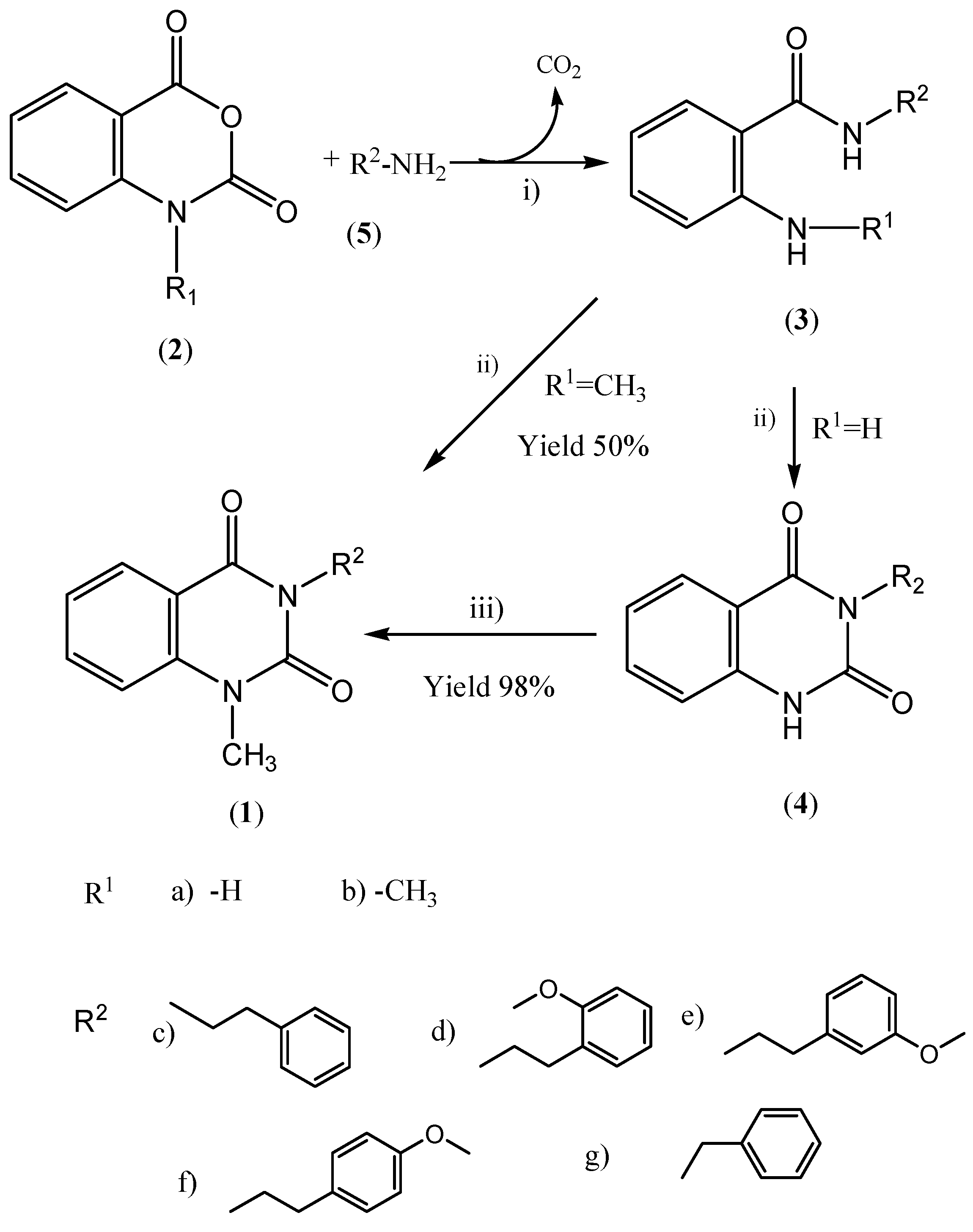
General Method for Preparing o-Aminobenzamides 3: 2-Amino-N-(2-phenylethyl)-benzamide (3ac).
A solution of phenylethylamine (5c, 1mmol) in DMF (30 mL) was stirred while isatoic anhydride (2a, 1.3 mmol) was added portionwise over a period of 30 minutes and then the temperature was maintained at ~50°C for an additional 30 minutes as CO2 was evolved. The mixture was stirred at room temperature for 2 hours more, hot water (100 mL) was added and then it was kept at room temperature without stirring for 6 hours. The solids were collected by filtration, dried under high vacuum and purified by silica gel chromatography column using dichloromethane as eluant. Finally, the excess of solvent was removed under reduced pressure to give compound 3ac as a pale brown solid. Yield 85%; mp 83-85°C; IR (KBr): 3291, 1631, 1596, 1303, 1155, 1030 cm-1; 1H-NMR: δ 7.40-7.13 (m, 7H, Ar-H), 6.67 (br, 1H, NH-CO), 6.07 (brs, 2H, NH-Ar), 5.50 (brs, 1H, NH-CO), 3.66 (q, 2H, J=6.8 Hz, CH2-NH), 2.91 (t, 2H, J=6.8 Hz, CH2-Ar) ppm; 13C NMR: δ 164.5, 148.6, 138.9, 132.2, 128.9, 128.8, 127.0, 126.6, 117.3, 116.6, 40.8, 35.8 ppm; ESI-MS (m/e): 263[M+Na]+.
The following compounds were similarly prepared:
2-Amino-N-[2-(2-methoxyphenyl)ethyl]benzamide (3ad). Yield 92%; mp 80-82°C; IR (KBr): 3468, 3032, 2937, 1664, 1596, 1255, 1093 cm-1; 1H-NMR: δ 7.21-7.16 (m, 4H, Ar-H), 6.92-6.85 (m, 2H, Ar-H), 6.65-6.59 (m, 2H, Ar-H), 6.42 (brs, 2H, NH-Ar), 5.20 (brs, 1H, NH-CO), 3.81 (s, 3H, CH3O), 3.60 (q, 2H, J=6.8Hz, CH2N), 2.93 (t, 2H, J=6.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 169.4, 157.4, 148.6, 132.1, 130.0, 127.9, 127.0, 120.94, 117.2, 116.4, 110.4, 55.3, 40.5, 29.9 ppm; ESI-MS (m/e) 263[M+ Na]+.
2-Amino-N-[2-(3-methoxyphenyl)ethyl]benzamide (3ae). Yield 84%; mp 69-71°C; (KBr): 3453, 3350, 1635,1587, 1255, 1041 cm-1; 1H-NMR: δ 7.27-7.14 (m, 3H, Ar-H), 6.84-6.71 (m, 3H, Ar-H), 6.67-6.55 (m, 3H, Ar-H), 6.11 (brs, 1H, NH-Ar), 4.5 (brs, 2H, NH-CO), 3.77 (s, 3H, CH3-O), 3.66 (q, 2H, J1=J2=6.8 Hz, NH-CH2), 2.88 (t, 2H, J1= 6.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 169.4, 159.9, 148.6, 140.6, 132.3, 129.7, 127.1, 121.2, 117.3, 116.6, 116.2, 114.4, 112.1, 55.2, 40.7, 35.7 ppm; ESI-MS (m/e): 293[M+Na]+, 271[M+H]+.
2-Amino-N-[2-(4-methoxyphenyl)ethyl]benzamide (3af) Yield 88%; mp 98-100°C; IR (KBr): 3483, 3230, 2922, 1671, 1244, 1030 cm-1; 1H-NMR: δ 7.25-7.11 (m, 4H, Ar-H), 6.89-6.55 (m, 2H, Ar-H), 6.68-6.55 (m, 2H, Ar-H), 6.07 (brs, 2H, NH-Ar), 5.0 (brs, 1H, NH-CO) 3.79(s, 3H, CH3-O), 3.62 (q, 2H, J1=J2=6.8 Hz, CH2-N), 2.85 (t, 2H, J=6.8 Hz, CH2-Ar) ppm; 13C-NMR: δ 169.3, 158.3, 148.6, 132.2, 129.7, 129.0, 117.2, 116.6, 114.13, 55.3, 40.9, 34.9 ppm; ESI-MS (m/e): 293[M+Na]+.
o-Amino-N-benzylbenzamide (3ag) Yield 93%; mp 104-106°C; IR (KBr): 3468, 3030, 2937, 1664, 1251, 1063 cm-1; 1H-NMR: δ 7.31-7.14 (m, 7H, Ar-H), 6.67-6.60 (m, 2H, Ar-H), 6.45 (brs, 2H, NH-Ar), 5.45 (brs, 2H, NH-CO) 4.57 (d, 2H, J=5.4 Hz, CH2-Ar) ppm; 13C-NMR: δ 169.1, 148.7, 138.2, 132.4, 128.7, 127.8, 127.5, 127.1 117.3, 116.7, 43.7 ppm; ESI-MS (m/e): 263[M+Na]+..
General Method for Preparing Quinazolinediones 4: 3-Phenylethyl-1H-quinazoline-2,4-dione (4c).
Triphosgene (10 mmol) in dichloromethane (10 mL) was added with stirring at room temperature to a solution of 2-amino-N-phenyl-ethylbenzamide (3ac, 5mmol) in dichloromethane (50 mL) and the resulting mixture was stirred for an additional 30 minutes. This was followed by the addition of triethylamine (30 mmol) and the mixture was refluxed for 2 hours. The reaction was quenched by addition of aqueous acid (5% HCl, 50 mL), and the mixture was extracted into dichloromethane. The organic phase was washed successively with saturated Na2CO3 solution (2 x 40 mL) and water (2 x 40 mL) and then dried over anhydrous Na2SO4 to give, after removal of excess solvent under reduced pressure, compound 4c as a yellow solid. Yield 75%; mp 173-175°C; IR (KBr): 2937, 1719, 1653, 1590, 1266 cm-1; 1H-NMR: δ 10.40 (s, 1H, NH-CO), 8.15 (dd, 1H, J1=1.6, J2=8.2 Hz, H-Ar), 7.67-7.59 (ddd, J1=1.6, J2=J3= 7.4 Hz, 1H, H-Ar), 7.34-7.15 (m, 7H, H-Ar) 4.33 (ddd, J1=5.6, J2=J3=8.0 Hz, 2H, CH2-NH), 3.05 (ddd, J1=5.6, J2=J3=8.0 Hz, 2H, CH2-Ar) ppm; 13C-NMR: δ 162.26, 151.9, 139.3, 138.6, 134.9, 128.95, 128.7, 128.6, 126.6, 115.3, 114.6, 42.3, 34.1 ppm; ESI-MS (m/e): 264.9[M- H]-.
The following compounds were similarly prepared:
3-[2-(2-Methoxyphenyl)ethyl]-lH-quinazoline-2,4-dione (4d). Yield 73%; mp 174-176°C; IR (KBr): 2937, 2800, 1719, 1654, 1477, 1243, 1028 cm-1; 1H-NMR: δ 10.65 (s, 1H, NH-Ar), 8.18 (dd, 1H, J1=1.6, J2=8.2 Hz, H-Ar), 7.53 (ddd, J1=1.6, J2=J3=7.4 Hz, 1H, H-Ar), 7.21-6.69 (m, 6H, H-Ar), 4.34 (ddd, J1=5.6, J2=J3=8.0 Hz, 2H, NH-CH2), 3.77 (s, 3H, CH3), 3.02 (ddd, J1=5.6, J2=J3=8.0 Hz, 2H, CH2-Ar) ppm; 13C-NMR: δ 162.4, 157.4, 151.0, 138.8, 134.3, 130.3, 127.7, 127.4, 126.9, 122.4, 120.1, 114.8, 114.2, 109.9, 55.1, 40.6, 28.5 ppm; ESI-MS (m/e): 295 [M –H]-.
3-[2-(3-Methoxyphenyl)ethyl]-lH-quinazoline-2,4-dione (4e). Yield 73%; mp 165-167°C; IR (KBr): 2928, 2593, 1728, 1671, 1604,1152 cm-1; 1H-NMR: δ 10.65 (s, 1H, NH-Ar), 7.93 (dd, J1=1.6 Hz, J2=8.2 Hz, 1H, H-Ar), 7.42 (ddd, J1=1.6, J2=J3= 7.4 Hz, 1H, H-Ar), 7.01-6.58 (m, 6H, H-Ar), 4.12 (ddd, 2H, J1=5.6, J2=J3=7.8 Hz, CH2-NH), 3.80 (s, 3H, CH3-O) 2.82 (ddd, J1=5.6 Hz, J2=J3=7.8 Hz, 2H, CH2-Ar) ppm; 13C-NMR: δ 159.4, 157.8, 135.2, 133.9, 130.6, 128.3, 127.7, 127.1, 126 0, 125.3, 120.9, 120.5, 118.8, 110.2, 55.5, 43.3, 28.5 ppm; ESI-MS (m/e): 295 [M –H]-.
3-[2'-(4'-Methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione (4f). Yield 78%; mp 76-78°C; IR (KBr): 1741, 1675, cm-1; 1H-NMR: δ 9.80 (s, 1H, NH-Ar) 8.13 (dd, J1=1.6 Hz, J2=8.2 Hz, 1H, H-Ar) 7.61 (ddd, J1=1.6, J2=J3=7.4 Hz, 1H, H-Ar) 7.26-6.81 (m, 6H, H-Ar), 4.25 (ddd, J1=5.6, J2=J3=7.4 Hz, 2H, CH2-NH), 3.76 (s, 3H, CH3-O), 2.95 (ddd, J1=5.6, J2=J3=7.4 Hz, 2H, CH2-Ar) ppm; 13C-NMR: δ 161.3, 159.1, 151.2, 138.1, 134.6, 130.2, 129.6, 128.1, 123.1, 114.6, 113.6, 55.1, 42.1, 32.8 ppm; ESI-MS (m/e): 295 [M- H]-.
3-Benzyl-1H,3H-quinazoline-2,4-dione (4g). Yield 89%; mp 163-165°C; IR (KBr): 2956, 1709, 1657, 1266, cm-1; 1H-NMR: δ 11.15 (s, 1H, NH), 7.92(d, 1H, J=8.2 Hz, H-Ar), 7.46-7.03 (m, 8H, H-Ar), 5.12(s, 2H, CH2-Ar) ppm; 13C-NMR: δ 169.9, 155.3, 144.1, 141.7, 139.2, 132.7, 132.3, 131.7.8, 127.1, 119.8, 118.6, 48.3 ppm; ESI-MS (m/e) 251 [M- H]-.
General Method for Preparing N-Methylquinazolinediones 1: 1-Methyl-3-(2'-phenylethyl)-1H,3H-quinazoline-2,4-dione (1c).
Tetramethylguanidine (0.064 g, 0.56 mmol) was added to a solution of 3-phenylethyl-1-H-quinazoline-2,4-dione (
4c, 0.015g, 0.56 mmol) in CHCl
3 (1 mL) and the mixture was stirred at room temperature for 15 min., then methyl iodide (0.15 g, 12 mmol) was added and the reaction was heated to 55°C for 45 minutes. Activated carbon was added and the mixture was stirred for 10 min. and then filtered. Removal of the solvent under reduced pressure gave the title compound
1c as a yellow solid. Yield >96%; mp 99-101°C (Lit. [
25], mp. 100-102°C); IR (KBr): 3042, 2929, 1701, 1654, 1610, 1481 cm
-1;
1H-NMR: δ 8.29 (dd, 1H, J
1=2.6, J
2=8.4 Hz, Ar-H), 7.69 (ddd, 1H, J
1= 5.4, J
2= J
3=7.4 Hz, Ar-H), 7.34-7.23 (m, 7H, Ar-H), 4.31 (ddd, J
1= 5.0, J
2= J
3=7.6 Hz, 2H, N-CH
2), 3.62 (s, 3H, N-CH
3), 2.98 (ddd, J
1= 5.0, J
2= J
3=7.6 Hz, Ar-CH
2) ppm;
13C-NMR: δ 161.3, 150.5, 140.2, 138.3, 134.8, 129.9, 128.7, 128.6, 128.2, 126.2, 122.7,120.9, 113.25, 43.3, 33.9, 30.6 ppm; ESI-MS (m/e): 289.1[M+ H]
+.
The following compounds were prepared in similar fashion:
1-Methyl-3-[2'-(2'-methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione (1d). Yield >98%; mp 155-163°C; IR (KBr): 2943, 1703, 1651, 1608, 1484, 1243, 1028 cm-1; 1H-NMR: δ 8.20 (dd, 1H, J1=2.2, J2=8.4 Hz, Ar-H), 7.63 (ddd, 1H, J1= 5.4, J3= J2=7.4 Hz, 1H, Ar-H), 7.25-6.75 (m, 6H, Ar-H), 4.33 (ddd, J1= 4, J2=J3=5.8 Hz, 2H, N-CH2), 3.62 (s, 3H, N-CH3), 2.98 (ddd, J1= 4, J2=J3=5.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 161.3, 157.5, 140.2, 134.6, 130.3, 128.6, 127.48, 126.87, 122.56, 120.17, 113.16, 110.0, 55.2, 41.8, 30.6, 28.7 ppm; ESI-MS (m/e): 310.9[M+ H]+; 332 [M +Na]+.
1-Methyl-3-[2'-(3'-methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione (1e). Yield 89%; mp 131-133°C; IR (KBr): 2945, 2833, 1699, 1656,1604 cm-1; 1H-NMR: δ 8.21 (dd, 1H, J1=2.1, J2=8.3 Hz, Ar-H), 7.63 (ddd, 1H, J1= 5.4, J3= J2=7.5 Hz, 1H, Ar-H), 7.25-6.62 (m, 6H, Ar-H), 4.29 (ddd, J1= 5.2, J2=J3=8.2 Hz, 2H, N-CH2), 3.79 (s, 3H, O-CH3), 3.61 (s, 3H, N-CH3), 2.98 (ddd, J1=4, J2=J3=5.8 Hz, Ar-CH2) ppm; 13C-NMR: δ 169.5, 159.9, 148.5, 140.5, 132.8, 132.4, 129.7, 127.0, 126.4, 122.7, 117.4, 116.8, 116.3, 112.1, 55.1, 40.7, 35.7 ppm; ESI-MS (m/e): 332.9 [M +Na]+.
1-Methyl-3-[2'-(4'-methoxyphenyl)ethyl]-lH,3H-quinazoline-2,4-dione (
1f). Yield >83%; mp. 134-136°C. (Lit. [
25] mp. 133-134°C); IR (KBr): 3301, 2928, 1700, 1647, 1600, 1400, 1261. cm
-1;
1H-NMR : δ 8.210 (dd, 1H, J
1=1.4, J
2=7.8 Hz, Ar-H), 7.69 (ddd, 1H, J
1= 1.4, J
3= J
2=7.4 Hz, 1H, Ar-H), 7.30-6.85 (m, 6H, Ar-H), 4.26 (ddd, J
1= 5.2, J
2=J
3=7.8 Hz, 2H, N-CH
2), 3.79 (s, 3H, O-CH
3), 3.61 (s, 3H, N-CH
3), 2.91 (ddd, J
1= 5.2, J
2=J
3=7.8 Hz, Ar-CH
2) ppm;
13C-NMR: δ 161.8, 158. 2, 140.5, 130.1, 129.2, 124.2, 114.0, 113.6, 55.5, 43.7, 33.4, 31.0 ppm; ESI-MS (m/e): 332.9 [M +Na]
+.
1-Methyl-3-(benzyl)-1H,3H-quinazoline-2,4-dione (1g). Yield 93%; mp.103-106°C; IR (KBr): 3416, 2918, 1700, 1652, 1604, 1480, 1266, cm-1; 1H-NMR: δ 7.95 (d, 1H, J=8.0 Hz, H-Ar), 7.51 (t, 1H, J=7.9 Hz, H-Ar), 7.25-6.90 (m, 7H, H-Ar) 5.04 (s, 2H, CH2-Ar), 3.05 (m, 3H, CH2-NH) ppm; 13C-NMR: δ 161.2, 150.2, 139.8, 136.5, 134.9, 128.1, 127.9, 127.8, 126.9, 122.5, 114.7, 44.5 ppm; ESI-MS (m/e) 288.9 [M+Na]+.