Results and Discussion
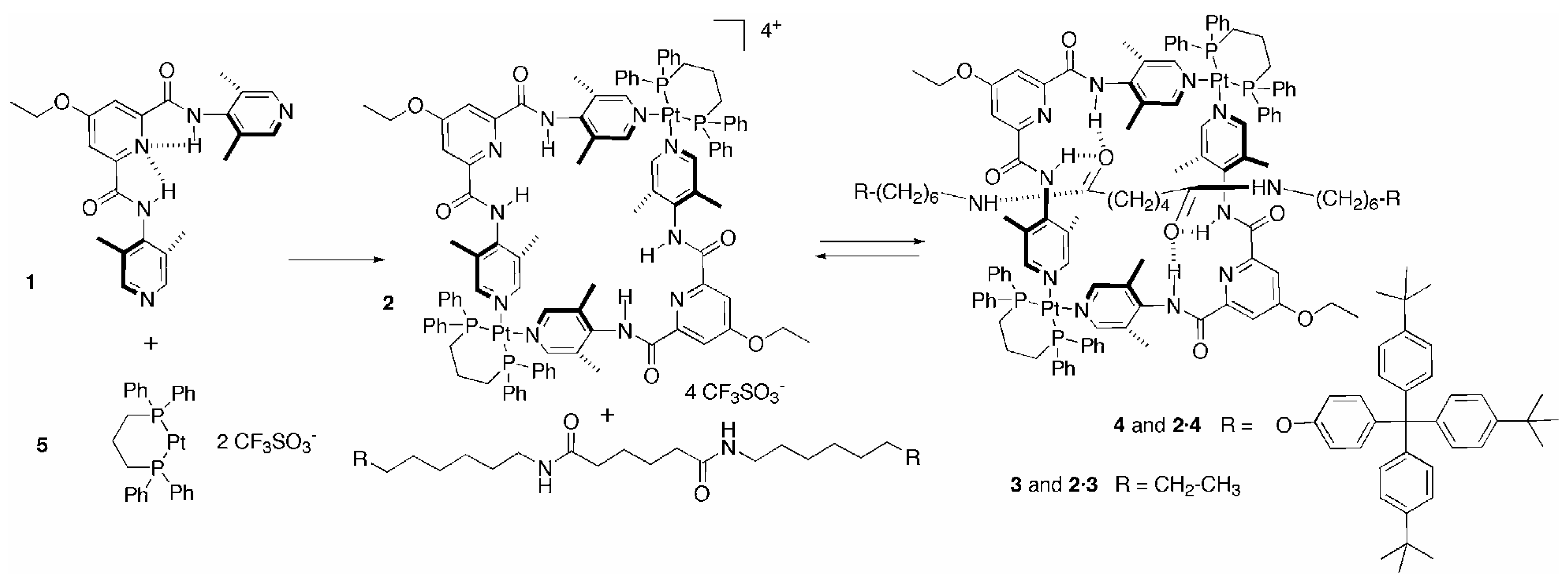
The dinuclear macrocycle
2, the macrocycle component of the rotaxane, was quantitatively self-assembled through Pt(II)-N coordination bonds [
8] from a mixture of a 1:1 of
cis-Pt-(dppp)(OTf)
2 and bispyridyl ligand
1. The ethoxy group of the bispyridyl ligand was introduced in order to increase the solubility of
1 in organic solvents. The macrocycle
2 contains a square cavity in which four hydrogen donors (NHs) converge by virtue of internal (pyridine N∙∙∙H-N amide) intramolecular hydrogen bonds [
9].
Scheme 1.
Self-assembly of macrocyle 2 and formation of [2]rotaxane 2·4 and [2]pseudo rotaxane 2·3.
Scheme 1.
Self-assembly of macrocyle 2 and formation of [2]rotaxane 2·4 and [2]pseudo rotaxane 2·3.
During the course of our studies a similar macrocycle containing Pd(II) metal centers has been described and its X-ray structure solved [
10]. Due to the similarity of our system with those reported [
6,
7] and since the efficient constructions of [2]rotaxanes require a high affinity between the wheel and the axle molecules, we decided to use axle molecules containing an adipamide unit. When macrocycle 2 (4mM) and four equivalents of the adipamide axle 3 were mixed in CDCl
3 at room temperature the
1H-NMR spectrum immediately showed a slight downfield shift of the NH resonances of the self-assembled macrocycle 2, indicating the formation of an hydrogen bonded-complex even in the presence of competing triflate anions. Synthetic receptors for anions are based on the amide H-bond donor properties. Particularly, a luminiscent rhenium (I) 2,6-pyridinecarboxamide, N, N´-di-4-pyridyl-based receptor has been recently described to bind anions with binding constants as high as 10
4-10
5 M
-1 in CH
2Cl
2 solution [
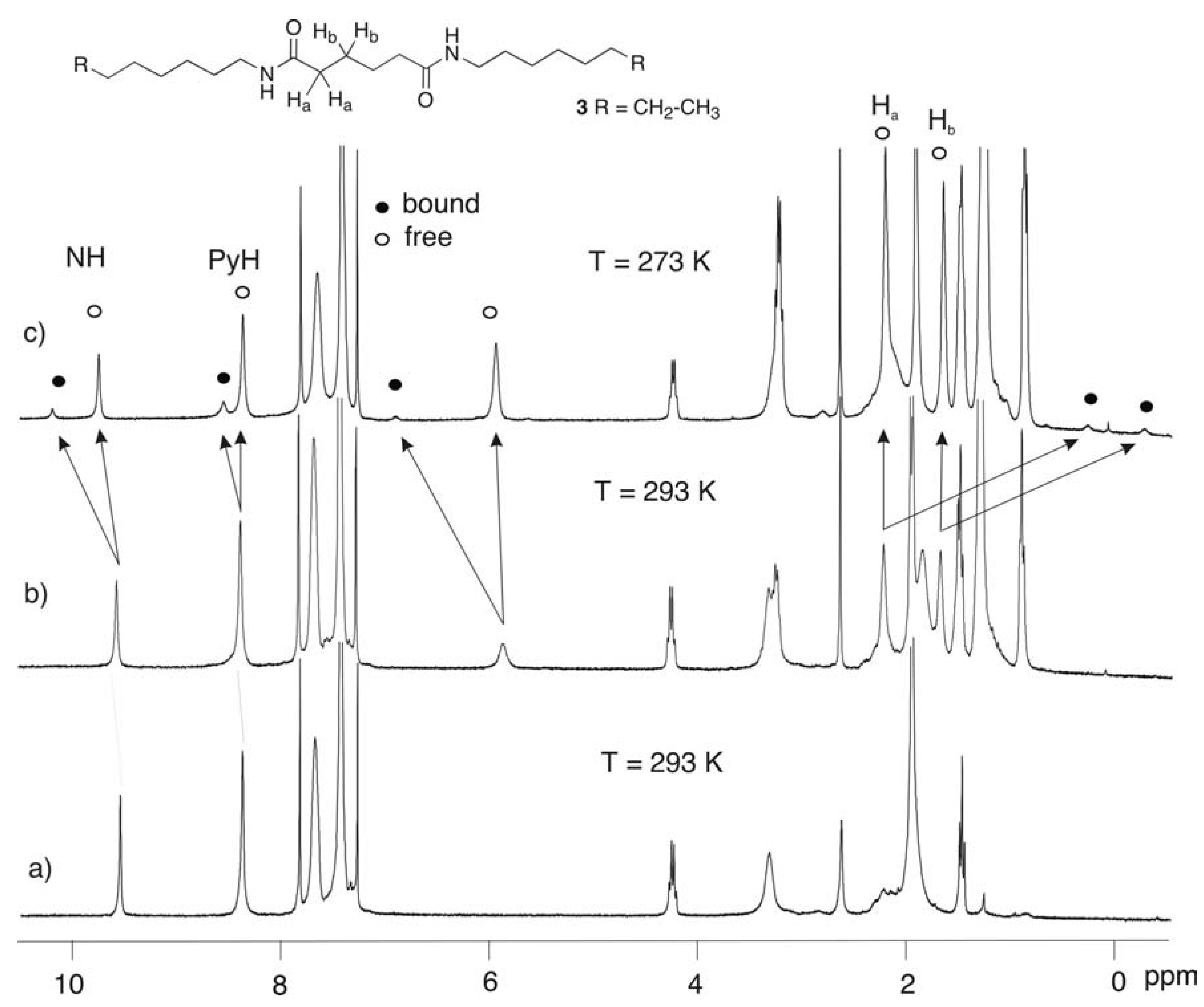
11]. We were worried that due to the structural resemblance to the binding sites of 2, the triflate anions acting as metal counter ions will inhibit the hydrogen-bonded complex formation between 2 and 3. Upon cooling the solution of 2 and 3 at 273 K a new set of signals assigned to the 2●3 complex could be seen (
Figure 1).
Figure 1.
1H-NMR spectra (300 MHz) in CDCl3 of a) 2 (4mM), b) 2 (4mM) + 3 (16 mM) at 293 K, c) 2 (4mM) + 3 (16 mM) at 273 K.
Figure 1.
1H-NMR spectra (300 MHz) in CDCl3 of a) 2 (4mM), b) 2 (4mM) + 3 (16 mM) at 293 K, c) 2 (4mM) + 3 (16 mM) at 273 K.
The
1H-NMR spectrum of the
2●
3 complex is clearly different from that of the free components. The amide NH and the proton of the dimethylpyridyl are shifted downfield, the first as a result of hydrogen bonding to the adipamide carbonyl and the second due to its diamagnetic anisotropy. In addition, the hydrogens α and β to the carbonyls in the adipamide
3 (H
a and H
b in
Figure 1) appear very far upfield at δ = 0.242 and δ = - 0.341 ppm, respectively, compared to δ = 2.195 and δ = 1.636 ppm for free
3.
The upfield shift of the H
a and H
b signals strongly suggests that the adipic moiety of
3 is inserted into the cavity of
2. The
31P-NMR spectrum of the mixture at 273 K also reveals the existence of two different phosphorus signals at δ = -15.16 ppm for the
2●
3 complex and δ = -13.98 ppm for the free macrocycle
2. On the basis of NMR integration (
1H,
31P) of several stock solutions an average association constant was calculated to be Ka
(2+3 ⇔ 2●3) = 20 ± 2M
-1. Jeong has recently reported that the association constants for tetralactam macrocycles, derived from pyridine 2,6-carboxamide, binding adipamides were highly sensitive to the substituents at the
para position of the pyridine ring [
12]. Electron donating groups (NMe
2, OMe) decrease the binding affinities. Even so, the calculated association constant Ka
(2+3 ⇔ 2●3) = 20 ± 2M
-1 is one order of magnitude lower than the reported values in the literature for similar systems. The energy barrier Δ
G# for the dissociation process of the
2●
3 complex is 14 kcal mol
-1, based on the coalescence temperature (290 K) [
13] of the NH signals of the wheel component
2, in complete agreement with the literature values for similar systems [
6]. Taken together, these results suggest the existence of a competitive binding of the wheel component
2 between the axle molecule
3 and the triflate anions.
The pseudorotaxane geometry for the
2●
3 complex was confirmed using an adipamide based axle molecule
4, which posses 4-[tris-(4-tert-butylphenyl)-phenoxyl] stopper end groups. The axle
4, with its bulkier stoppers, showed a different behaviour from
3 in the
1H-NMR and
31P-NMR spectra. First, after addition of axle
4 no immediate changes were observed in any of the resonances of the self-assembled macrocycle
2. It was necessary to heat the CDCl
3 solution of
2 (4 mM) and
4 (4 equivalents) during 48 h at 50º C in an oil bath to achieve equilibrium between the
2●
4 complex and its components. Second, with axle
4 and after the equilibration time it was possible to observe even at room temperature sharp and widely separated signals for the
2●
4 complex, very similar to those observed for the
2●
3 complex relative to free
3 at 273 K. This is indicative of slow exchange on the NMR time scale between complex
2●
3 and its components. The NHs are downfield shifted from δ = 9.50 ppm to δ = 10.10 ppm, and the H
a and H
b hydrogen atoms of the axle
4 shifted upfield to δ = 0.247 and δ = -0.236 ppm. The
31P-NMR spectrum showed a new signal at δ = -15.26 ppm corresponding to the [2]rotaxane complex
2●
4 and the signal for the free macrocycle
2 at δ = -14.07 ppm (
Figure 2). No coalescence could be observed up to the temperature limit of the solvent (320 K). Thus, although it was not possible to determine the corresponding Δ
G# for the exchange process here, clearly it must be significantly higher than 15.8 kcal mol
-1 consistent with a higher kinetic barrier to exchange introduced by the stoppering. The binding constant between wheel
2 and axle
4 measured at room temperature by
1H and
31P integration after the equilibration period was estimated to be Ka
(2+4 ⇔2●4) = 18 ± 2 M
-1. The magnitude of this value indicates that the apparent association constant is only slightly affected by the size of the end groups.
Figure 2.
1H-NMR (300 MHz) spectra in CDCl3 at 293 K of 2 (4mM) + 4 (16mM) showing free and bound species. Inset corresponding 31P-NMR (121 MHz).
Figure 2.
1H-NMR (300 MHz) spectra in CDCl3 at 293 K of 2 (4mM) + 4 (16mM) showing free and bound species. Inset corresponding 31P-NMR (121 MHz).
Figure 3.
ESI-MS in acetone of 2·4 [2]rotaxane showing the peak corresponding to the molecular ion [2·4 (CF3SO3)2-]2+. a) Observed isotopic distribution. b) Calculated isotopic distribution.
Figure 3.
ESI-MS in acetone of 2·4 [2]rotaxane showing the peak corresponding to the molecular ion [2·4 (CF3SO3)2-]2+. a) Observed isotopic distribution. b) Calculated isotopic distribution.
Further evidence for the existence of the [2]rotaxane
2●
4 in solution came from electrospray mass spectroscopy (ESI-MS). Ions corresponding to the di-cation of the
2●
4 complex, resulting from the loss of two triflate counter ions, were observed with a mass-to-charge ratio (m/z) of 1834.7319 (cald. 1834.7279) (
Figure 3).
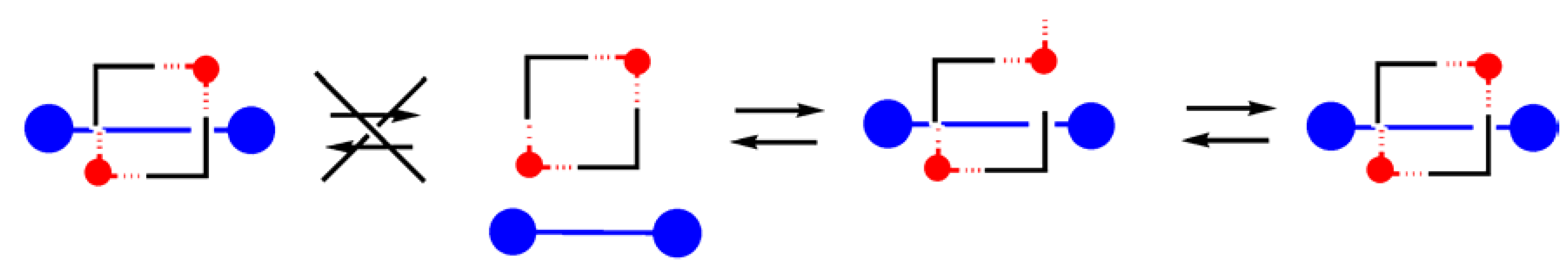
On the one hand, the observed changes in the kinetics of the formation and also in the
1H-NMR dynamic behavior of the
2●
3 and
2●
4 complexes can be interpreted as evidences for the existence of different exchange pathways leading to complex formation. On the other hand, the similarities observed for the chemical shift changes (proton and phosphorous) upon formation of both hydrogen bonded complexes and for its stability constants strongly suggest a very close geometry. Thus, the
2●
3 complex forms a [2]pseudorotaxane where the barrier to exit or entry the wheel component
2 by the axle
3 is only controlled by the formation or disruption of the hydrogen bonds which hold together the axle and the wheel, while the
2●
4 complex forms a [2]rotaxane stabilized by the same set of hydrogen bonds but where exit or entry of the axle
4 requires, due to the bulky stoppers attached at its ends, the opening of the wheel component
2 by breaking the coordinative bonds (
Scheme 2).
Scheme 2.
Schematic pathways involved in the exchange process between the free and the bound species for the [2]rotaxane. Due to the bulky stoppers of 4 the only operative pathway for the formation and dissociation of the 2·4 complex requires the disruption of a coordinative bond. The slipping process which is the main pathway for the exchange process in the 2·3 complex is inhibited due to the bulky stoppers.
Scheme 2.
Schematic pathways involved in the exchange process between the free and the bound species for the [2]rotaxane. Due to the bulky stoppers of 4 the only operative pathway for the formation and dissociation of the 2·4 complex requires the disruption of a coordinative bond. The slipping process which is the main pathway for the exchange process in the 2·3 complex is inhibited due to the bulky stoppers.
It is well known that Pt(II) complexes with nitrogen ligands have high affinities and consequently are kinetically stable on the NMR time scale. The fact that the dissociation of the highly stable self-assembled macrocycle
2, based on Pt(II)-pyridine coordinative bonds, controls the exchange dynamics for the
2●
4 complex explains its kinetic stability on the NMR time scale. For this reason, we can observe a different set of signals for free and bound
2 and
4 at room temperature on the
1H-NMR and
31P NMR spectrum (
Figure 2)