A Novel Approach to the Synthesis of 6-Amino-7-hydroxy-flavone
Abstract
:Introduction
Results and Discussion
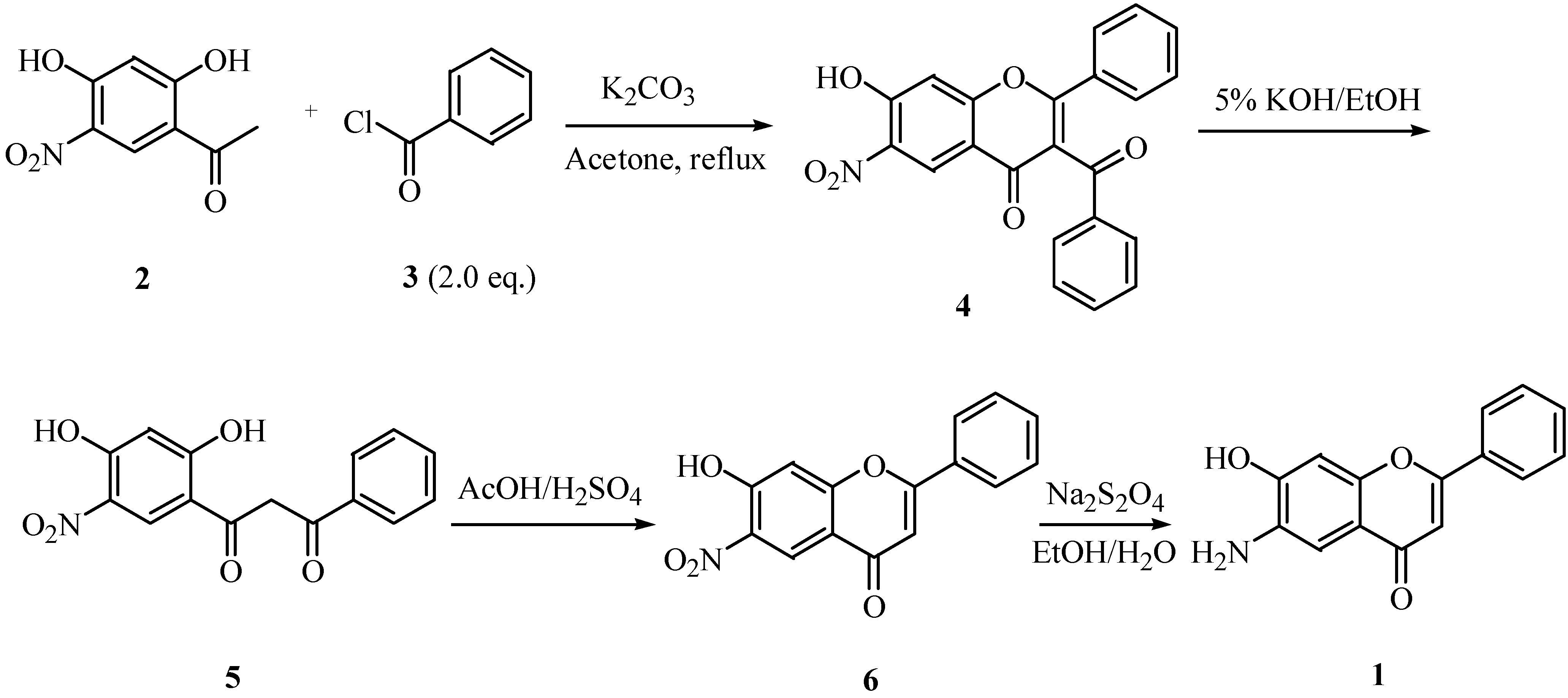
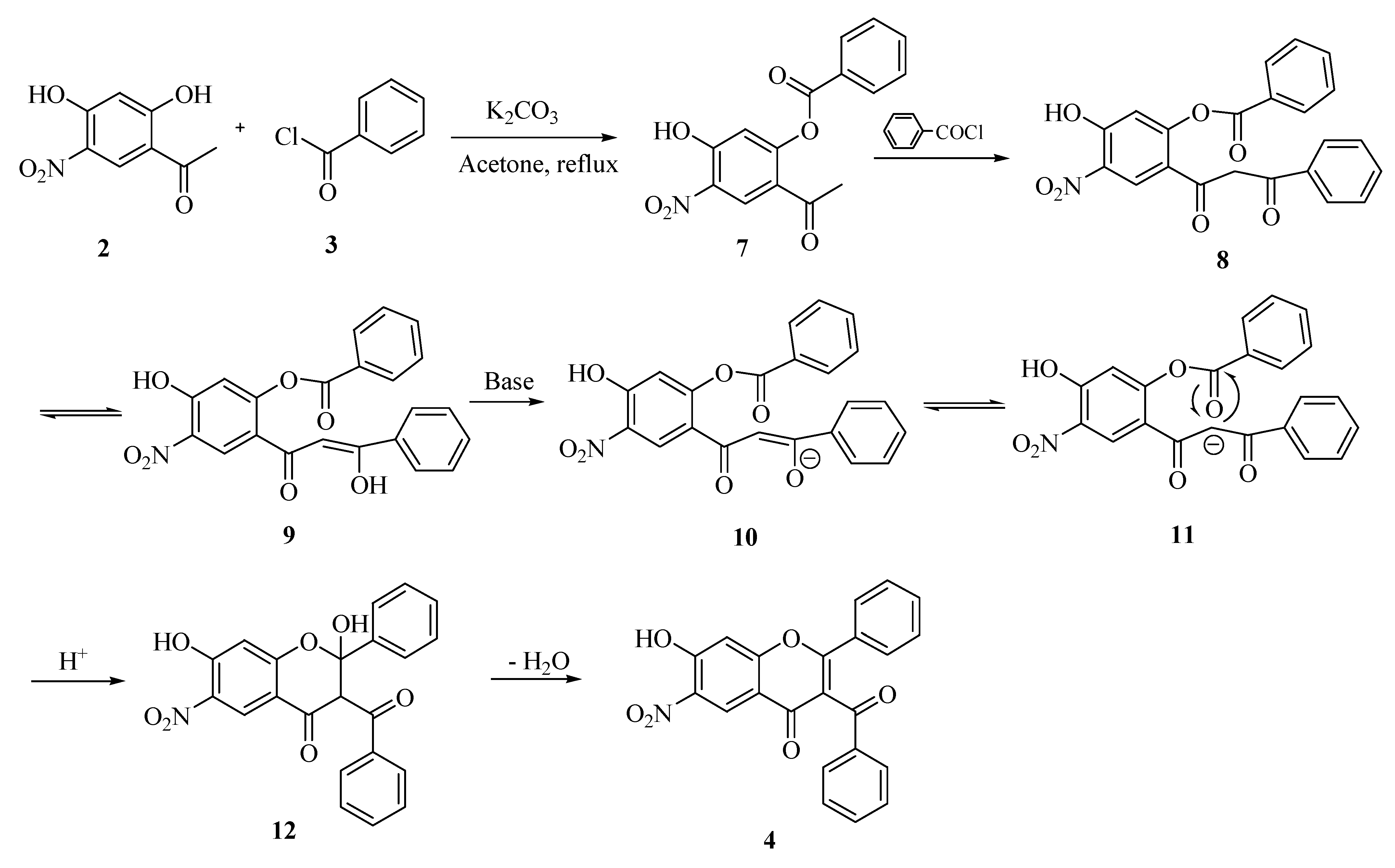
Conclusions
Experimental
General
Synthesis of 3-benzoyl-7-hydroxy-6-nitroflavone (4).
Synthesis of Compounds 5 and 9.
Preparation of 7-hydroxy-6-nitroflavone (6)
Synthesis of 6-amino-7-hydroxyflavone (1).
Acknowledgements
References
- Rao, K. V.; Chattopadhyay, S. K.; Reddy, G. C. Flavonoids with mosquito larval toxicity. J. Agric. Food. Chem. 1990, 38, 1427–1430. [Google Scholar]
- Weidenborner, M.; Jha, H. C. Antifungal activity of flavonoids and their mixtures against different fungi occurring on grain. Pestic. Sci. 1993, 38, 347–351. [Google Scholar]
- Silva, A. M.; Weidenbörner, M.; Cavaleiro, J. A. S. Growth control of different fusarium species by selected flavones and flavonoid mixtures. Mycol. Res. 1998, 102, 638–640. [Google Scholar]
- Wu, E. S. C.; Loch, J. T.; Toder, B. H.; Borrelli, A. R.; Gawlak, D.; Radov, L. A.; Gensmantel, N. P. Flavones. 3. Synthesis, biological activities, and conformational analysis of isoflavone derivatives and related compounds. J. Med. Chem. 1992, 35, 3519–3525. [Google Scholar]
- Wolfman, C.; Viola, H.; Marder, M.; Wasowski, C.; Ardenghi, P.; Izquierdo, I.; Paladini, A. C.; Medina, J. H. Anxioselective properties of 6,3'-dinitroflavone, a high-affinity benzodiazepine receptor ligand. Eur. J. Pharmacol. 1996, 318, 23–30. [Google Scholar]
- Akama, T.; Ishida, H.; Kimura, U.; Gomi, K.; Saito, H.; Fuse, E.; Kobayashi, S.; Yoda, N.; Kasai, M. Design and synthesis of potent antitumor 5,4'-diaminoflavone derivatives based on metabolic considerations. J. Med. Chem. 1997, 40, 1894–1900. [Google Scholar]
- Gee, J. M.; Johnson, I. T. Polyphenolic compounds: interactions with the gut and implications for human health. Curr. Med. Chem. 2001, 8, 1245–1255. [Google Scholar]
- Rice-Evans, C. A.; Miller, N. J.; Paganga, G. Structure antioxidant activity relationshipsof flavonoids and phenolic acids. Free. Rad. Biol. Med. 1996, 20, 933–956. [Google Scholar]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar]
- Pietta, P. G. Flavonoids as antioxidant. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar]
- Bois, F.; Beney, C.; Boumendjel, A.; Mariotte, A. M.; Conseil, G.; Di Pietro, A. Halogenated chalcones with high-affinity binding to P-glycoprotein: potential modulators of multidrug resistance. J. Med. Chem. 1998, 41, 4161–4164. [Google Scholar]
- Boumendjel, A.; Bois, F.; Beney, C.; Mariotte, A. M.; Conseil, G.; Di Pietro, A. B-ring substituted 5,7-dihydroxyflavonols with high-affinity binding to P-glycoprotein responsible for cell multidrug resistance. Bioorg. Med. Chem. Lett. 2001, 11, 75–77. [Google Scholar]
- Cushman, M.; Zhu, H.; Geahlen, R. L.; Kraker, A. J. Synthesis and biochemical evaluation of a series of aminoflavones as potential inhibitors of protein-tyrosine kinases p56lck, EGFr, and p60v-src. J. Med. Chem. 1994, 37, 3353–3362. [Google Scholar]
- Akama, T.; Ishida, H.; Kimura, U.; Gomi, K.; Saito, H. Structure-activity relationships of the 7-substituents of 5,4'-diamino-6,8,3'-trifluoroflavone, a potent antitumor agent. J. Med. Chem. 1998, 41, 2056–2067. [Google Scholar]
- Kulkarni, V. G.; Jadhav, G. V. Chalcones. Preparation of chalcones from 5-nitroresacetophenone and 3-bromo-5-nitroresacetophenones; their bromination and study of the reactivity of the chalcone dibromides. J. Indian. Chem. Soc. 1954, 31, 746–754. [Google Scholar]
- Costantino, L.; Rastelli, G.; Gamberini, M. C.; Vinson, J. A.; Bose, P.; Iannone, A.; Staffieri, M.; Antolini, L.; Corso, A. D.; Mura, U.; Albasini, A. 1-Benzopyran-4-one antioxidants as aldose reductase inhibitors. J. Med. Chem. 1999, 42, 1881–1893. [Google Scholar]
- Naik, R. M.; Thakor, V. M. Chromones. III. Kostanecki-Robinson acylation of some o-hydroxy-acetophenones. Proc. Indian Acad. Sci. 1953, 37A, 774–783. [Google Scholar]
- Sample Availability: Samples of compounds 1, 4, 5, 6 and 9 are available from the authors.
© 2004 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Tang, L.; Zhang, S.; Yang, J.; Gao, W.; Cui, J.; Zhuang, T. A Novel Approach to the Synthesis of 6-Amino-7-hydroxy-flavone. Molecules 2004, 9, 842-848. https://doi.org/10.3390/91000842
Tang L, Zhang S, Yang J, Gao W, Cui J, Zhuang T. A Novel Approach to the Synthesis of 6-Amino-7-hydroxy-flavone. Molecules. 2004; 9(10):842-848. https://doi.org/10.3390/91000842
Chicago/Turabian StyleTang, Lijun, Shufen Zhang, Jinzong Yang, Wentao Gao, Jian Cui, and Tianyu Zhuang. 2004. "A Novel Approach to the Synthesis of 6-Amino-7-hydroxy-flavone" Molecules 9, no. 10: 842-848. https://doi.org/10.3390/91000842



