Introduction
Calcium channel antagonists (calcium entry blockers) are known to be clinically useful agents in treating hypertension, angina pectoris, and certain cardiac arrythmias. Structurally they are classified into three groups: 1,4-dihydropyridines (nifedipine, nitrendipine), phenylalkylamines (verapamil) and benzothiazepines (diltiazem) (
Figure 1) [
1]. Their major pharmacological effects appear to be exerted by selectively inhibiting the influx of extracellular Ca
2+ through L-type voltage-dependent calcium channels and subsequently inhibiting the myocardial contraction and having the vasodilator effects as a result [
2,
3]. A considerable number of structure-activity relationship studies of 1,4-dihydropyridine series have been delineated [
4,
5], however, compared to the 1,4-dihydropyridine series, there has been limited information available on structure-activity relationships in the diltiazem series [
6]. Although diltiazem is widely used in therapy, it has a relatively short duration of action and is known to cause significant prolongation of cardiac P-R intervals [
7].
The 8-chloro derivative (benzothiazepinone numbering) TA 3090 (
Figure 1), a second generation diltiazem analogue, has recently been introduced into clinical use [
8,
9]. This compound is a more potent antihypertensive than diltiazem in animals. The combination of proven clinical utility, the need for improvements in potency and duration of action, and limited structure-activity data made the study of diltiazem and related compounds an attractive area for further research [
10,
11].
Figure 1.
Representative Ca2+ channel antagonists and compound 1.
Figure 1.
Representative Ca2+ channel antagonists and compound 1.
In the present paper an approach for designing a pyrrolopyridothiazepine derivative as a novel calcium channel antagonist is described. The plan was to use 4-aminopyridine as the starting material instead of the 4-chloroaniline part of TA 3080. Also, the replacement of the amide linkage of diltiazem with a bioisosteric pyrrole ring was attempted to improve the short duration of action, and the sulfur group was maintained for the activity. 2-(4-Methoxyphenyl)pyrrolo[2,1-d]pyrido[2,3-c][1,5]thiazepine-3(2H)-one (1) was prepared as a key intermediate of this pyrrolopyridothiazepine series.
Results and Discussion
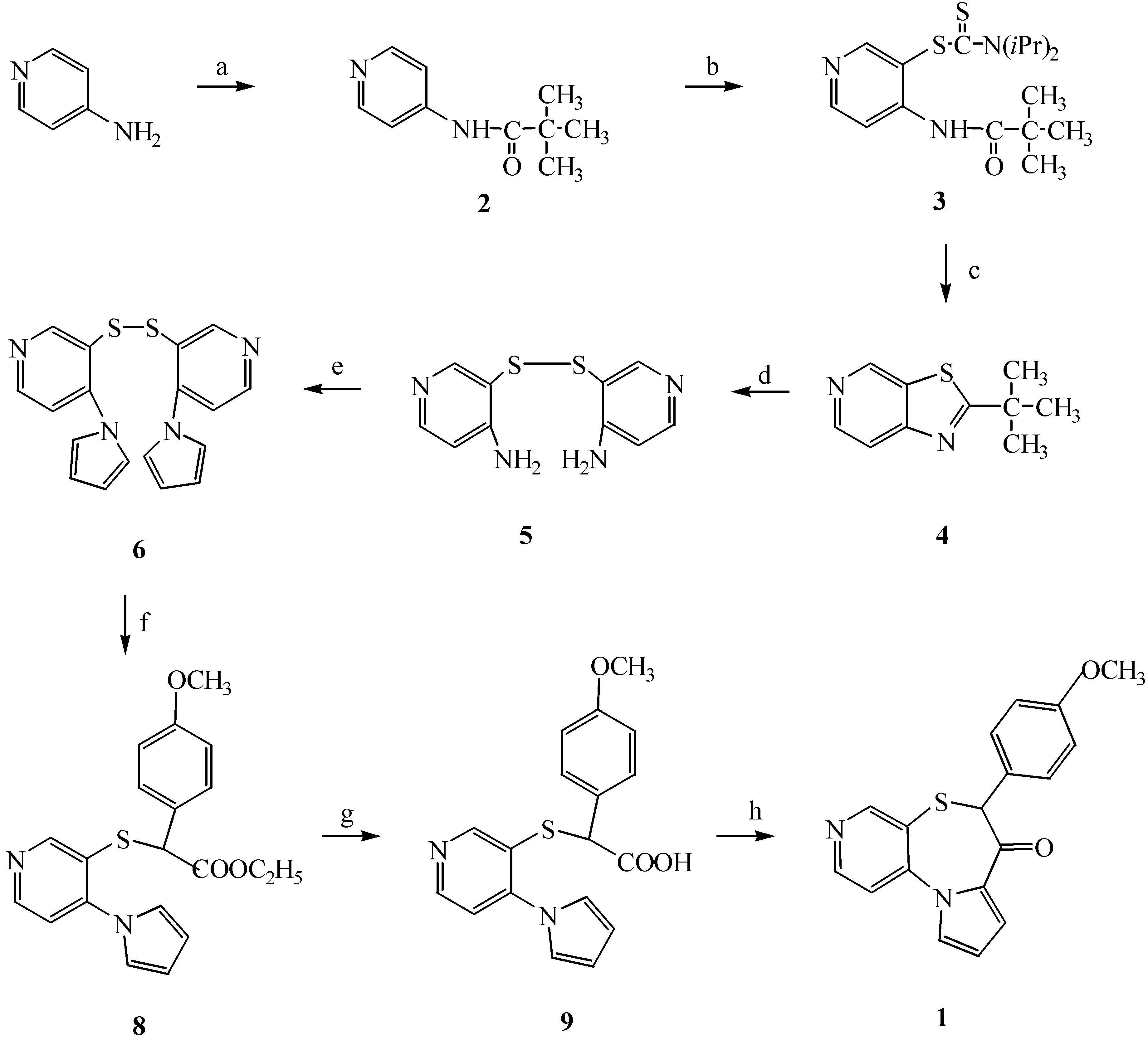
The preparation of compound
1 was accomplished by the reaction sequence shown in
Scheme 1. Because the sulfur group is known to act as an important pharmacophore exhibiting antihypertensive activity, we tried to replace the 3-hydrogen of 4-aminopyridine with a SH group. The literature contains various examples of the synthesis of thiols by the direct thiation of organomagnesium or organolithium reagents with elemental sulfur [
12,
13], but this type of reaction has been of limited utility, since clean products are usually not obtained, and sulfides are formed as significant by-products. 4-Aminopyridine was reacted with pivaloyl chloride at 0˚C in the presence of triethylamine to form 4-pivaloylaminopyridine (
2) in high yield. The pivaloyl group is a powerful auxiliary for direct lithiation [
14]. Double lithiation of 4-pivaloylaminopyridine using 2.5 M
n-butyllithium produced a white precipitate of the dilithio derivative. Reaction of this species with the electrophile tetraisopropylthiuram disulfide (TITD) produced 3-(
N,N-diisopropyldithiocarbamato)pyridine (
3) in good yield (87 %). TITD was directly synthesized from isopropylamine, carbon disulfide, and potassium ferricyanide at 0˚C [
15]. Attempted removal of the pivaloyl group using 5 M-HCl solution produced a 2-
tert-butylthiazolo[5,4-c]pyridine (
4) in 99 % yield as shown in
Scheme 1 [
16]. The subsequent alkaline hydrolysis of compound
4 produced the disulfide
5. Compound
6 was prepared by reaction of 2,5-dimethoxytetrahydrofuran with bis(2-amino-4-pyridyl)disulfide (
5). Bis(4
H-pyrrolo-3-pyridyl)disulfide(
6) was reduced with NaBH
4 in refluxing ethanol to give 3-mercapto-4
H-pyrrolopyridine and the latter subsequently reacted with α-bromo-4-methoxyphenylacetic acid ethyl ester (
7) to give compound
8. Compound
7 was obtained from 4-methoxyphenylacetic acid by an esterification reaction with absolute ethanol containing a catalytic amount of concentrated H
2SO
4, and then bromination with
N-bromosuccinimide. Hydrolysis of ester
8 with 5 % NaOH solution afforded 4-methoxy-α-[(4
H-pyrrolo-3-pyridyl)thio]phenyl acetic acid (
9). Intramolecular cyclization of the acid
9 using phosphorus pentachloride gave the target compound
1. All synthesized compounds were identified with IR, NMR, and mass spectra, respectively.
Scheme 1.
Reagents and conditions : (a) Pivaloyl chloride, triethylamine, anhydrous CH2Cl2, 0˚C; (b) TITD, 2.5 M n-butyllithium, -78˚C, anhydrous THF; (c) 5 M-HCl, reflux; (d) 5 M NaOH, solid NaOH, reflux; (e) 2,5-Dimethoxytetrahydrofuran, glacial acetic acid, 110˚C; (f) i) NaBH4, absolute ethanol, reflux, ii) α-bromo-4-methoxyphenyl acetic acid ethyl ester (7), ethanol, rt; (g) 5 % NaOH, methanol/THF (1:1), rt; (h) PCl5, anhydrous CH2Cl2, rt , 60˚C.
Experimental
General
Reactions were performed under an atmosphere of nitrogen. Solvents were purified and dried prior to use. Anhydrous MgSO4 was used for drying reaction mixtures. Melting points were measured on a Thomas-Hoover melting point apparatus in open capillary tubes and are not corrected. 1H-NMR spectra were obtained on a Gemini 200 MHz spectrometer in CDCl3 or D2O. Chemical shifts (δ) are reported in ppm relative to tetramethylsilane and coupling constants (J) in Hz. IR spectra were determined on a Jasco FT-IR 300E spectrometer as KBr pellet. GC-mass spectra were recorded on a Shimadzu GC-QP 5000 spectrometer. Analytical TLC was performed on Merck precoated silica gel 60 F254 plates. Column chromatography was carried out on Merck silica gel (9385, 230-400 mesh).
4-(Pivaloylamino)pyridine (2).
A solution of pivaloyl chloride (24.63 mL, 0.2 mol) in anhydrous CH2Cl2 (36 mL) was cautiously added at 0˚C to a solution of 4-aminopyridine (18.82 g, 0.2 mol) and triethylamine (27 mL) in anhydrous CH2Cl2 (90 mL). The reaction mixture was stirred in an ice bath for 1 hour and then allowed to warm to room temperature and stirred overnight. Cold water (100 mL) was added and the CH2Cl2 layer was separated from the aqueous one. The organic phase was washed with saturated NaHCO3 solution (2×40 mL) and water, dried over anhydrous MgSO4, filtered and concentrated to yield a crude brown product that was recrystallized from ethyl acetate/n-hexane (1:5) to yield the pure product as white needles, yield: 30.41 g (85 %); mp: 135-137 ˚C; IR (KBr) cm-1: 3507, 2967, 1701, 1591; 1H-NMR (CDCl3) δ: 1.33 (9H, s, -C(CH3)3), 7.51 (2H, d, J=6.1Hz, ArH), 7.61 (1H, br s, NH), 8.48 (2H, d, J=5.8Hz, ArH).
3-(N,N-Diisopropyldithiocarbamato)-4-(pivaloylamino)pyridine (3).
A solution of 4-(pivaloylamino)pyridine (2, 17.82 g, 0.1 mol) in dry tetrahydrofuran (160 mL) was cooled to -78℃ under N2 gas and 2.5 M n-butyllithium (84 mL, 0.21 mol) was slowly added. The reaction mixture was stirred for 4 hours at 0˚C and recooled to -78˚C. A solution of tetraisopropylthiuram disulfide (TITD, 35.26 g, 0.1 mol) in dry tetrahydrofuran (140 mL) was slowly added. After completion of the addition, the cooling bath was removed. After stirring for 2 hours, water (180 mL) was carefully added, followed by diethyl ether (3×180 mL). The combined organic solutions were washed with brine and dried over anhydrous MgSO4. Rotary evaporation of the solvent gave the crude product, which was recrystallized from ethyl acetate to provide pure white product (25.22 g, 71 %). Evaporation of the mother liquors gave a brown oil, which when purified by column chromatography (ethyl acetate/n-hexane=1:5) gave an additional 5.4 g (15 %) of product. Total yield: 30.62 g (87 %); mp: 117-119℃; IR (KBr) cm-1: 3450, 2965, 1686, 1591; 1H-NMR (CDCl3) δ: 1.25 (9H, s, -C(CH3) 3), 1.53 (12H, br s, -CH(CH3) 2×2), 4.80 (2H, very br s, -CH×2), 8.37 (1H, d, J=5.5Hz, ArH), 8.50 (1H, s, ArH), 8.60 (1H, d, J=5.5Hz, ArH).
2-t-Butylthiazolo[5,4-c]pyridine (4).
A mixture of 2-(N,N-diisopropyldithiocarbamato)-4-(pivaloylamino)pyridine (3, 16 g, 45.25 mmol) and 5 M HCl solution (155 mL) was refluxed for 5 hours. The mixture was allowed to reach room temperature, and the acidic solution was then washed with ethyl ether (70 mL) and then neutralized with 20 % NaOH solution in an ice bath. The aqueous layer was extracted with CH2Cl2 (3×100 mL), followed by drying over anhydrous MgSO4. The organic solvent was evaporated leaving a solid that was purified by recrystallization from diethyl ether/n-hexane (1:3) to yield a white amorphous solid; yield: 8.63 g (99 %); mp: 52-54˚C; IR (KBr) cm-1: 2960, 1580, 1510, 1440; 1H-NMR (CDCl3) δ: 1.60 (9H, s, -C(CH3) 3), 8.29 (1H, d, J=6.7Hz, ArH), 8.73 (1H, d, J=6.4Hz, ArH), 10.04 (1H, s, ArH).
Bis(4-amino-3-pyridyl)disulfide (5)
A mixture of 2-tert-butylthiazolo[5,4-c]pyridine (4, 5 g, 26 mmol) and 10 % aqueous NaOH (80 mL) was heated under reflux for 6 hours. After this time, if the starting material was still visible as an oil on the surface, the mixture was allowed to cool down and some NaOH pellets (8 g) were added and the mixture refluxed for another 6 hours. The reaction mixture was cooled down, washed with dichloromethane and the aqueous layer was neutralized with concentrated HCl. It was then continuously extracted with ethyl acetate for 48 hours and evaporated to yield a yellow solid (2.53 g). Evaporation of the solvent after several extractions yielded further disulfide (1.05 g). Yield: 3.58 g (55 %); mp: 136-138℃; IR (KBr) cm-1: 3389, 1634, 1536; 1H-NMR (CDCl3) δ: 6.55(2H, brs, NH2), 6.71 (2H, d, J=5.8Hz, ArH), 7.70 (1H, s, ArH); MS (m/z): 250 (M+).
Bis(4H-pyrrolo-3-pyridyl)disufide (6)
To a solution of bis(4-amino-3-pyridyl) disulfide (5, 1 g, 4 mmol) in glacial acetic acid (15 mL), 2,5-dimethoxytetrahydrofuran (1.04 g, 7.83 mol) in glacial acetic acid (5 mL) was slowly added and the mixture heated at 110˚C for 1 hour. Evaporation of the acetic acid provided a dark brown oily product which was extracted with diethyl ether (3×30 mL) and washed successively with saturated NaHCO3 and water and then dried over anhydrous MgSO4. After filtering the filtrate was concentrated to give the crude product, which was purified by column chromatography (ethyl acetate/n-hexane=1:1) to provide a pale yellow solid, yield: 630 mg (31 %); mp: 115.3-116.8˚C; IR (KBr) cm-1: 1648, 1568; 1H-NMR (CDCl3) δ: 6.35 (2H, t, pyrrole-H), 6.89 (2H, t, pyrrole-H), 7.16(1H, d, J=5.3Hz, ArH), 8.53 (1H, d, J=5.2Hz, ArH), 8.67 (1H, s, ArH); MS(m/z): 175 (M+/2).
4-Methoxy-α-[(4H-pyrrolo-3-pyridyl)thio]phenyl acetic acid ethyl ester (8)
A solution of bis(4H-pyrrolo-3-pyridyl)disulfide (6, 600 mg, 1.7 mmol) in 60 mL of absolute ethanol was heated to reflux. Sodium borohydride (132 mg, 3.4 mmol) was slowly added over 10 minutes. After completion of the addition the mixture was allowed to cool down, α-bromo-4-methoxyphenyl acetic acid ethyl ester 7 (936 mg, 3.4 mmol) was added and the resulting mixture was stirred for 5 hours at room temperature. The reaction mixture was concentrated to half its volume, water was added, and the aqueous layer was extracted with ethyl acetate (3×50 mL). The combined organic layers were dried and evaporated to give the crude product, which was purified by column chromatography (ethyl acetate/n-hexane=1:3) to afford the title compound, Yield: 507 mg (81 %); mp: 165.6-166.8˚C; IR (KBr) cm-1: 1725; 1H-NMR (CDCl3) δ: 1.09 (3H, t, CH3), 3.77 (3H, s, OCH3), 3.97-4.28 (2H, m, CH2), 4.28 (1H, s, CH) 6.41 (2H, t, pyrrole-H), 6.78 (2H, t, pyrrole-H), 7.13-7.21 (5H, m, ArH), 8.53 (1H, d, J=5.2Hz, ArH), 8.69(1H, s, ArH); MS(m/z): 368(M+).
4-Methoxy-α-[(4H-pyrrolo-3-pyridyl)thio]phenyl acetic acid (9)
The ester 8, (500 mg, 1.36 mmol) was dissolved in a 1:1 ethanol and tetrahydrofuran mixture (50 mL), and 5 % aqueous NaOH (100 mL) was slowly added. The reaction mixture was then stirred at room temperature for 1 hour, concentrated, and acidified to pH 3∼4 with dilute HCl. The brown oil was extracted with CH2Cl2, and the organic phase was diluted, filtered, and concentrated to give the crude acid. Recrystallization from methanol and n-hexane (1:4) gave the purified white solid, yield: 380 mg (82 %); mp :216-218 ˚C; IR (KBr) cm-1: 3410, 1720; 1H-NMR (D2O) δ: 3.72 (3H, s, OCH3), 4.91 (1H, s, CH), 6.34 (2H, t, pyrrole-H), 6.85 (2H, t, pyrrole-H), 7.16-7.23 (4H, m, ArH), 7.35 (1H, d, ArH), 8.48 (1H, d, J=7.2Hz, ArH), 8.63 (1H, s, ArH); MS(m/z): 340(M+).
2-(4-Methoxyphenyl)pyrrolo[2,1-d]pyrido[2,3-c][1,5]thiazepine-3(2H)-one (1)
A suspension of PCl5 (276 mg, 1.32 mmol) in CH2Cl2 (20 mL) was carefully added to a well-stirred solution of compound 9 (300 mg, 0.88 mmol) in anhydrous CH2Cl2 (40 mL). The mixture was heated at 60˚C for 12 hours and then further reacted at the room temperature for 12 hours. The solvent was removed under reduced pressure and the dark oily residue was treated with ethyl acetate. The organic layer was washed with 5% aqueous NaOH and water, then dried, filtered and concentrated to give a brown pasty residue which was purified by column chromatography (ethyl acetate/n-hexane=1:3). Yield: 200 mg (72%); mp: 145-147˚C; IR (KBr) cm-1: 1650; 1H-NMR (CDCl3) δ: 3.76 (3H, s, OCH3), 4.30 (1H, s, CH), 6.41 (1H, t, pyrrole-H), 6.76 (2H, d, J=8.4Hz, pyrrole-H), 7.12∼7.21 (5H, m, ArH), 8.50 (1H, d, J=7.2Hz, ArH), 8.68 (1H, s, ArH); MS(m/z): 322(M+).