Selective Nitration of Aromatic Compounds with Bismuth Subnitrate and Thionyl Chloride
Abstract
:Introduction
Results and Discussion

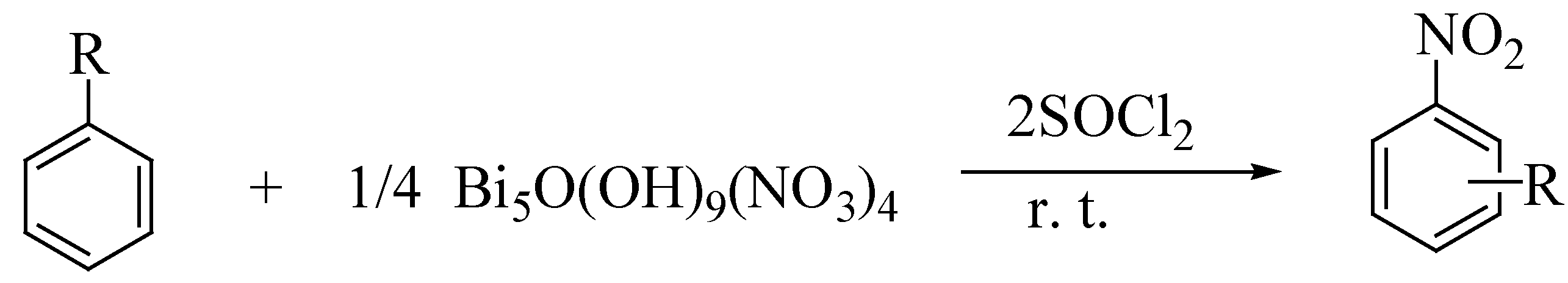
| Entry | Substrate | Product | Sub:NO3 ratio | Time h | Yield% |
| 1. | benzene | nitrobenzene | 1 : 1 | 6 | 87 |
| 2. | toluene | 2-nitrotoluene 4- nitrotoluene | 1 : 1 | 3 | 62 27 |
| 3. | m-xylene | 4- nitro-m-xylene | 1 : 1 | 3 | 74 |
| 4. | mesitylene | 2-nitromesitylene | 1 : 1 | 2 | 84 |
| 5. | acetanilide | 2-nitroacetanilide 4-nitroacetanilide | 1 : 1 | 3 | 16 52 |
| 6. | anisole | 2-nitroanisole 4-nitroanisole | 1 : 1 | 2 | 12 76 |
| 7. | 4-bromoanisole | 4-bromo-2-nitroanisole | 1 : 1 | 6 | 75 |
| 8. | 2-chloroanisole | 2-chloro-4-nitroanisole | 1 : 1 | 5 | 74 |
| 9. | bromobenzene | 2-bromonitrobenzene 4- bromonitrobenzene | 1 : 1 | 9 | 18 60 |
| 10. | chlorobenzene | 2-chloronitrobenzene 4- chloronitrobenzene | 1 : 1 | 9 | 27 49 |
| Entry | Substrate | Product | Sub:NO3 ratio | Time h | Yield % |
| 1. | naphthalene | 1-nitronaphthalene | 1 : 1 | 2 | 68 |
| 2. | 2-methylnaphthalene | 1-nitro-2-methyl naphthalene | 1 : 1 | 2 | 76 |
| 3. | acenaphthene | 5-nitroacenaphthene | 1 : 1 | 2 | 72 |
| 4. | biphenylene | 2-nitrobiphenylene | 1 : 1 | 1 | 81 |
| 5. | fluorene | 2-nitrofluorene | 1 : 1 | 2 | 74 |
| 6. | phenanthrene | 9-nitrophenanthrene | 1 : 1 | 2 | 64 |
| 7. | pyrene | 1-nitropyrene | 1 : 1 | 1 | 75 |
| 8. | biphenyl | 4-nitrobiphenyl | 1 : 1 | 1 | 73 |
| Entry | Substrate | Product | Sub:NO3 ratio | Time h | Yield% |
| 1. | phenol | 2-nitrophenol 4- nitrophenol | 1 : 1 | 0.5 | 42 46 |
| 2. | phenol | 2,4-dinitrophenol 2,6- dinitrophenol | 1 : 2 | 2 | 72 14 |
| 3. | o-cresol | 2-methyl-4-nitrophenol 2-methyl-6-nitrophenol | 1 : 1 | 0.5 | 56 28 |
| 4. | o-cresol | 4,6-dinitro-2-methylphenol | 1 : 2 | 2 | 83 |
| 5. | p- cresol | 4-methyl-2-nitrophenol | 1 : 1 | 0.5 | 86 |
| 6. | p- cresol | 2,6-dinitro-4-methylphenol | 1 : 2 | 1 | 82 |
| 7. | 2-bromophenol | 2-bromo-4-nitrophenol 2-bromo-6-nitrophenol | 1 : 1 | 1 | 52 22 |
| 8. | 2- bromophenol | 2-bromo-4,6-dinitrophenol | 1 : 2 | 3 | 70 |
| 9. | 4-chlorophenol | 4-chloro-2-nitrophenol | 1 : 1 | 1 | 78 |
| 10. | 4- chlorophenol | 4-chloro-2,6-dinitrophenol | 1 : 2 | 3 | 72 |
| 11. | salicyldehyde | 4-nitrosalicyldehyde | 1 : 1 | 3 | 63 |
| 12. | 4-nitrophenol | 2,4-dinitrophenol | 1 : 1 | 3 | 78 |
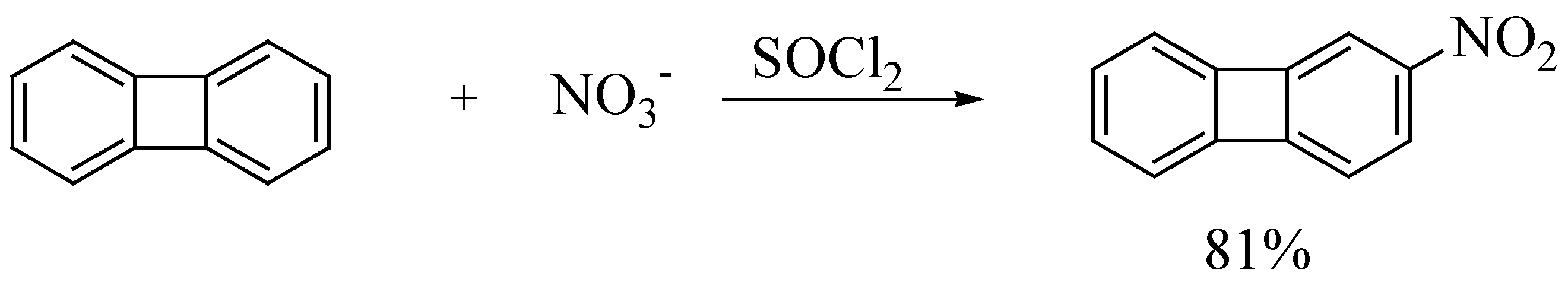

Experimental
General
Typical procedure
References
- Olah, G.A.; Malhorta, R.; Narang, S.C. Nitration Methods and Mechanisms; VCH: New York, 1989. [Google Scholar]
- Becker, F.F.; Banik, B.K. Bioorg. Med. Chem. 1998, 50, 2877. [CrossRef]
- Zollinger, H. Color Chemistry: Properties and Applications of Organic Dyes, 2nd Edition; John Wiley: New York, 1991. [Google Scholar]
- Meyer, R.; Kholar, J.; Homburg, A. Explosives, 5th Edition; John Wiley: New York, 2002. [Google Scholar]
- Ward, E.R. Chem. Ber 1979, 15, 297.
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry, 5th Edition; John Wiley: New York, 2001; p. 696. [Google Scholar]
- Ridd, J.H. Acta Chem. Scand 1998, 52, 11. [CrossRef]
- Olah, G.A.; Fung, A.P.; Narang, S.C.; Olah, J.A. J. Org. Chem. 1981, 46, 3533. [CrossRef]
- Gigantee, B.; Prazeres, A.O.; Marcelo-Curto, M.J.; Cornelis, A.; Laszlo, P. J. Org. Chem. 1995, 60, 3445. [CrossRef]
- Mellor, J.M.; Mittoo, S.; Parkes, R; Millar, R.W. Tetrahedron 2000, 56, 8019. [CrossRef]
- Cornelis, A.; Laszlo, P. J. Org. Chem. 1983, 48, 4771. [CrossRef]
- Dove, M.F.A.; Manz, B.; Montgomery, G.; Pattenden, G.; Wood, S.A. J. Chem. Soc., Perkin Trans. 1 1998, 1589.
- Cornelis, A.; Delaude, L.; Gerstmans, A.; Laszlo, P. Tetrahedron Lett 1988, 29, 5909.
- Bisarya, S.C.; Joshi, S.K.; Holkar, A.G. Synth. Commun. 1993, 23, 1125. [CrossRef]
- Zolfigol, M.A.; Ghaemi, E.; Madrakian, E. Molecules 2001, 6, 614.
- Leonard, N.M.; Wieland, L.C.; Mohan, R.S. Tetrahedron 2002, 58, 8373.
- Suzuki, H.; Ikegami, T.; Matano, Y. Synthesis 1997, 3, 249. [CrossRef]
- Mellor, J.W. Compr. Treat. Inorg. Theor. Chem. 1929, 9, 705.
- Rajah, C.A. Dtsch. Apoth. Ztg. 1935, 1714.
- Samajdar, S.; Becker, F.F.; Banic, B.K. Tetrahedron Lett. 2000, 41, 8017.
- Sundvall, B. Acta Chem. Scand (A) 1979, 33, 219. [CrossRef]
- Hejazi, R. Int. J. Pharm. 2002, 235, 87.
- Hakimelahi, G.H.; Sharghi, H.; Zarrinmayeh, H.; Khalafi-Nezhad, A. Helv. Chim. Acta 1984, 67, 906–1984.
- Barton, J.W.; Whitaker, K.E. J. Chem. Soc. (C) 1968, 1663.
- Baker, W.; Barton, J.W.; McOmie, J.F. J. Chem.Soc. 1958, 2666.
- Zolfigol, M.A.; Madrakian, E.; Ghaemi, E. Molecules 2002, 7, 734.
- Sample availability: Not available
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Muathen, H.A. Selective Nitration of Aromatic Compounds with Bismuth Subnitrate and Thionyl Chloride. Molecules 2003, 8, 593-598. https://doi.org/10.3390/80700593
Muathen HA. Selective Nitration of Aromatic Compounds with Bismuth Subnitrate and Thionyl Chloride. Molecules. 2003; 8(7):593-598. https://doi.org/10.3390/80700593
Chicago/Turabian StyleMuathen, Hussni A. 2003. "Selective Nitration of Aromatic Compounds with Bismuth Subnitrate and Thionyl Chloride" Molecules 8, no. 7: 593-598. https://doi.org/10.3390/80700593




