Experimental
General
Melting points were determined on a Boëtius apparatus and are uncorrected. IR spectra were obtained on a Nicolet 205 FT-IR spectrometer. 1H-NMR and 13C-NMR were recorded on a Bruker ACF-250 spectrometer at 250.13 MHz using TMS as internal standard. Chemical shifts are given in ppm and coupling constant (J) values are in Hz. The multiplicity of the signals on the 13C-NMR spectra was determined using the Distorsionless Enhancement Polarization Transfer (DEPT) sequence. Elemental analyses were performed in a Leco CHNS-932 instrument. The mass spectra were recorded in a AMD 402/3 spectrometer AMD Intectra GmbH. Reactions were monitored by thin-layer chromatography (TLC) on precoated plates with silica gel 60 GF254 (Merck) and spots were visualized with UV light or a spray of vanillin in perchloric acid 1 % w/v and subsequent heating. Flash column chromatography was performed on silica gel 60 (Merck, 70-230 mesh). “Usual work-up” refers to dilution with water, extraction with an organic solvent, washing the extract with HCl (5%) and/or KHCO3 (5%) and water, drying over anhydrous MgSO4 and removal of the solvent under reduced pressure. The solvents were purified and dried according to recommended procedures.
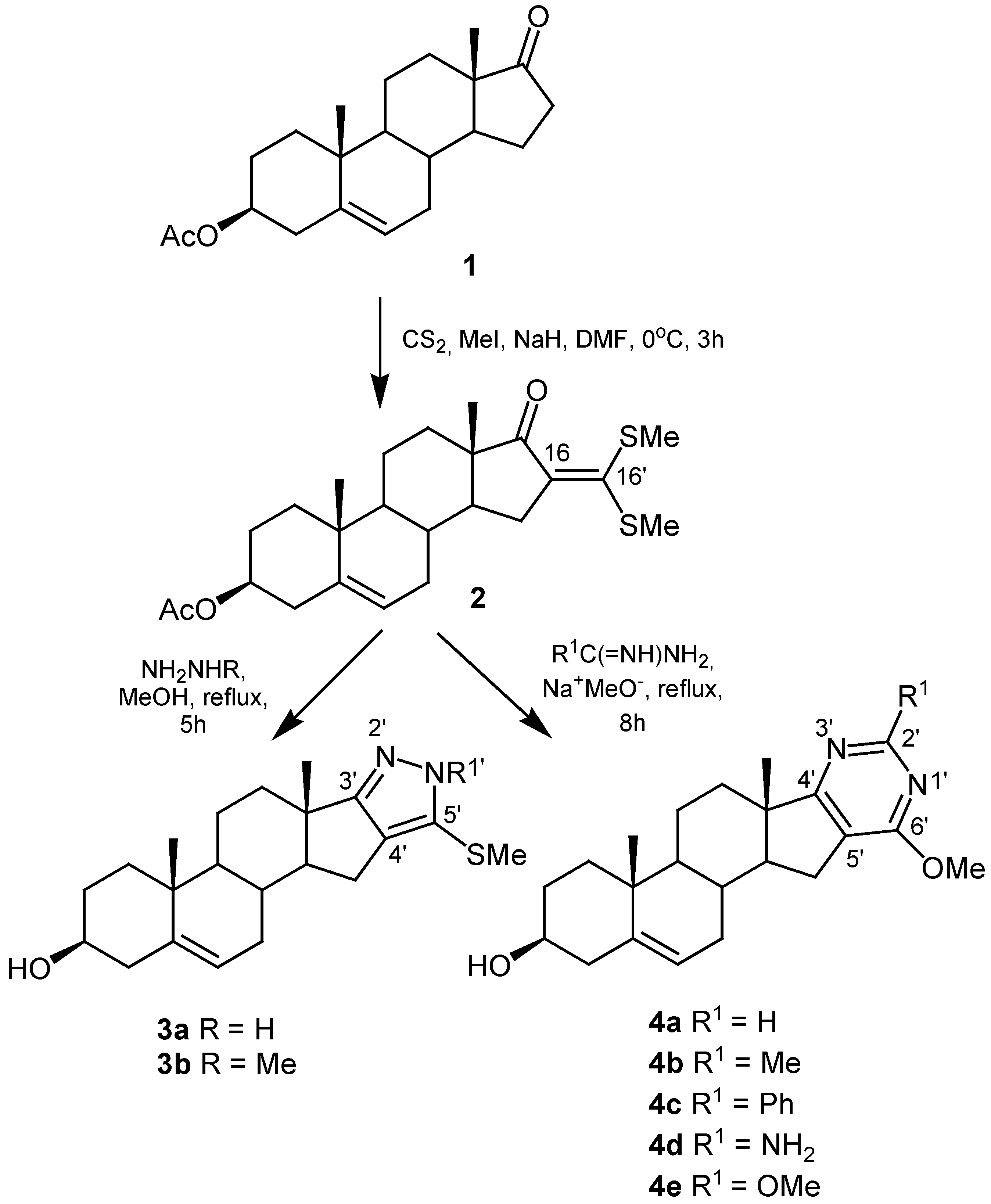
3β-acetoxy-16-[bis(methylthio)methylene]androst-5-en-17-one (2)
Sodium hydride (60%, 303 mg, 7.6 mmol), carbon disulfide (0.46 mL, 7.6 mmol) and methyl iodide (0.76 mL, 12.1 mmol) were added to a stirred solution of androstenolone acetate 1 (1.000 g, 3.03 mmol) in anhydrous DMF (60 mL). The mixture was stirred for 3 hours at 0 °C, poured into ice-water (200 mL) and extracted with chloroform (3 x 100 mL). The combined extracts were washed with water (3 x 20 mL), dried over anhydrous MgSO4 and the solvent was evaporated. The crude product was purified by column chromatography with 10:1 toluene/ethyl acetate elution and crystallized from acetone to furnish 2 (0.8165 g, 62 %) as yellow needles, mp: 165.7–168.0 °C; IR (KBr, cm–1): 1739 (C=O, Ac), 1682 (C=O), 1479 (C=C); 1H-NMR (CDCl3): δ = 0.91 (s, 3H, Me-19), 1.04 (s, 3H, Me-18), 2.03 (s, 3H, Ac), 2.44 (s, 3H, SMe), 2.45 (s, 3H, SMe), 4.59 (m, 1H, H-3α), 5.40 (d, 1H, J = 4.9 Hz, H-6); 13C-NMR (CDCl3): δ = 14.3 (Me-18), 17.6 (SMe), 18.5 (SMe), 19.3 (Me-19), 20.3 (C-11), 21.4 (CH3CO), 27.7 (C-2), 30.8 (C-12), 30.9 (C-8), 31.8 (C-7), 32.9 (C-15), 36.7 (C-10), 36.8 (C-1), 38.0 (C-4), 49.7 (C-13), 50.1 (C-14), 50.1 (C-9), 73.7 (C-3), 121.8 (C-6), 135.4 (C-16), 140.0 (C-5), 149.9 (C-16’), 170.5 (CH3CO), 205.0 (C-17); MS (EI): m/z (%): 434 (65.8, M+.), 374 (100); Calc for C24H34O3S2: C 66.32, H 7.88, S 14.75; Found: C 66.32, H 7.99, S 14.67.
5’-Methylthio- pyrazolo[4’,3’:16,17]androst-5-en-3β-ol (3a)
Compound 2 (200 mg, 0.46 mmol) was dissolved in anhydrous methanol (50 mL), hydrazine hydrate (80%, 0.8 mL) was added and the mixture was stirred at reflux for 4 hours until all the starting material had disappeared as indicated by TLC (disappearance of yellow color). Then the reaction mixture was concentrated and usual work-up (CHCl3) yielded a crude product, which was purified by column chromatography with 3:1 chloroform/ethyl acetate elution to give 117.8 mg (66 %) of 3a, mp (ethanol): 176.2–178.0 °C; IR (KBr, cm–1): 3354 (OH), 3168 (NH), 1454, 1436, 1408, 1373 (C=C, C=N); 1H-NMR (CDCl3): δ = 1.02 (s, 3H, Me-19), 1.07 (s, 3H, Me-18), 2.42 (s, 3H, SMe), 3.53 (m, 1H, H-3α), 4.27 (broad signal, NH), 5.38 (d, 1H, J = 5.20 Hz, H-6); 13C-NMR (CDCl3): δ = 17.8 (SMe), 17.9 (Me-18), 19.4 (Me-19), 20.4 (C-11), 30.3 (C-12), 30.8 (C-8), 31.5 (C-7), 32.1 (C-2), 33.7 (C-15), 36.7 (C-10), 37.1 (C-1), 40.9 (C-13), 42.2 (C-4), 50.5 (C-9), 62.1 (C-14), 71.5 (C-3), 121.0 (C-6), 123.9 (C-4’), 131.3 (C-5’), 141.1 (C-5), 168.1 (C-3’); MS (EI): m/z (%): 358 (21.7, M+.), 287 (100); Calc. for C21H30N2OS: C 70.35, H 8.43, N 7.81, S 8.94; Found: C 70.08, H 8.69, N 8.02, S 8.78.
1’-Methyl-5’-methylthio- pyrazolo[4’,3’:16,17]androst-5-en-3β-ol (3b)
Compound 2 (200 mg, 0.46 mmol) was dissolved in anhydrous methanol (50 mL), methyl hydrazine (1 mL) was added and the mixture was stirred at reflux for 5 hours until all the starting material had disappeared as indicated by TLC. Then the reaction mixture was concentrated and usual work-up (CHCl3) yielded a crude product, which was purified by column chromatography with 4:1 chloroform/ethyl acetate elution to afford 129.4 mg (70 %) of 3b, mp (ethanol): 181–183 °C; IR (KBr, cm–1): 3349 (OH), 1455, 1371, 1356, 1349 (C=C, C=N); 1H-NMR (CDCl3): δ = 1.00 (s, Me-19), 1.06 (s, Me-18), 2.36 (s, 3H, SMe), 3.53 (m, 1H, H-3α), 3.80 (s, 3H, NMe), 5.38 (d, 1H, J = 4.9 Hz, H-6); 13C-NMR (CDCl3): δ = 17.9 (SMe), 18.1 (Me-18), 19.3 (Me-19), 20.5 (C-11), 30.3 (C-12), 30.8 (C-8), 31.5 (C-7), 32.1 (C-2), 33.7 (C-15), 36.3 (NMe), 36.7 (C-10), 37.1 (C-1), 40.8 (C-13), 42.2 (C-4), 50.5 (C-9), 61.8 (C-14), 71.5 (C-3), 121.0 (C-6), 124.9 (C-4’), 129.3 (C-5’), 141.2 (C-5), 167.3 (C-3’); MS (EI): m/z (%): 372 (38.6, M+.), 327 (100); Calc. for C22H32N2OS: C 70.92, H 8.66, N 7.52, S 8.61; Found: C 70.5 8, H 8.74, N 7.53, S 8.49.
6’-Methoxy- pyrimido[5’,4’:16,17]androst-5-en-3β-ol (4a)
Compound 2 (100 mg, 0.23 mmol) was dissolved in a solution of sodium (26.5 mg, 1.15 mmol) in anhydrous methanol (50 mL), formamidinium acetate (120 mg, 1.15 mmol) was added and the mixture was stirred at reflux for 8 hours. Usual work-up (CHCl3) yielded a crude product, which was purified by column chromatography to furnish 50.74 mg (68 %) of 4a, mp (acetone): 178–180 °C; IR (KBr, cm–1): 3450 (OH), 1593, 1558 (C=C, C=N); 1H-NMR (CDCl3): δ = 0.98 (s, 3H, Me-19), 1.08 (s, 3H, Me-18), 3.53 (m, 1H, H-3α), 3.98 (s, 3H, OMe), 5.38 (d, 1H, J = 5.20 Hz, H-6,), 8.60 (s, 1H, H-2’); 13C-NMR (CDCl3): δ = 16.8 (Me-18), 19.4 (Me-19), 20.5 (C-11), 26.5 (C-15), 30.6 (C-8), 31.6 (C-7), 31.3 (C-12), 32.9 (C-2), 36.8 (C-10), 37.1 (C-1), 42.2 (C-4), 46.0 (C-13), 50.6 (C-9), 53.5 (MeO), 55.6 (C-14), 71.6 (C-3), 118.8 (C-5’), 121.0 (C-6), 141.2 (C-5), 159.9 (C-6’), 165.7 (C-2’), 181.6 (C-4’); MS (EI): m/z (%): 354 (74.22, M+.), 243 (100); Calc. for C22H30N2O2: C 74.54, H 8.53, N 7.90; Found: C 74.28, H 8.31, N 7.68.
6’-Methoxy-2’-methyl- pyrimido[5’,4’:16,17]androst-5-en-3β-ol (4b)
Compound 2 (100 mg, 0.23 mmol), sodium (26.5 mg, 1.15 mmol) in anhydrous methanol (50 mL) and acetamidinium hydrogen chloride (110 mg, 1.15 mmol) was reacted as described for preparation of 4a to furnish 58.4 mg (75 %) of 4b, mp (acetone): 210–212 °C; IR (KBr, cm–1): 3363 (OH), 1591, 1565 (C=C, C=N); 1H-NMR (CDCl3): δ = 0.96 (s, Me-19), 1.07 (s, Me-18), 2.60 (s, Me-2’), 3.53 (m, 1H, H-3α), 3.95 (s, OMe), 5.38 (d, 1H, J = 5.20 Hz, H-6,); 13C-NMR (CDCl3): δ = 16.8 (Me-18), 19.4 (Me-19), 20.5 (C-11), 25.8 (Me-2’), 26.1 (C-15), 30.6 (C-8), 31.2 (C-12), 31.6 (C-7), 32.9 (C-2), 36.7 (C-10), 37.1 (C-1), 42.2 (C-4), 46.0 (C-13), 50.6 (C-9), 53.2 (MeO), 55.7 (C-14), 71.5 (C-3), 115.1 (C-5’), 121.0 (C-6), 141.2 (C-5), 165.3 (C-6’), 166.5 (C-2’), 181.8 (C-4’); MS (EI): m/z (%): 368 (76.95, M+.), 353 (100); Calc. for C23H32N2O2: C 74.96, H 8.75, N 7.60; Found: C 74.45, H 8.62, N 7.33.
6’-Methoxy-2’-phenyl- pyrimido[5’,4’:16,17-c]androst-5-en-3β-ol (4c)
Compound 2 (100 mg, 0.23 mmol), sodium (26.5 mg, 1.15 mmol) in anhydrous methanol (50 mL) and benzamidinium hydrogen chloride (180 mg, 1.15 mmol) was reacted as described for preparation of 4a to furnish 57.12 mg (62 %) of 4c, mp (acetone): 241–243 °C; IR (KBr, cm–1): 3396 (OH), 1593, 1552 (C=C, C=N); 1H-NMR (CDCl3): δ = 1.02 (s, Me-19), 1.10 (s, Me-18), 3.54 (m, 1H, H-3α), 4.09 (s, OMe), 5.38 (d, 1H, J = 5.20 Hz, H-6), 7.43–7.46 (m, 3H, Ph), 8.43–8.47 (m, 2H, Ph); 13C-NMR (CDCl3): δ = 16.9 (C-18), 19.4 (C-19), 20.5 (C-11), 26.4 (C-15), 30.7 (C-8), 31.3 (C-12), 31.6 (C-7), 33.1 (C-2), 36.8 (C-10), 37.1 (C-1), 42.3 (C-4), 46.1 (C-13), 50.8 (C-9), 53.3 (MeO), 55.8 (C-14), 71.6 (C-3), 116.2 (C-5’), 121.1 (C-6), 128.2, 128.3, 130.0, 138.3 (Ph), 141.2 (C-5), 163.2 (C-6’), 165.5 (C-2’), 182.4 (C-4’); MS (EI): m/z (%): 430 (100, M+.); Calc. for C28H34N2O2: C 78.10, H 7.96, N 6.51; Found: C 77.93, H 7.82, N 6.34.
2’-Amino-6’-methoxy- pyrimido[5’,4’:16,17]androst-5-en-3β-ol (4d)
Compound 2 (100 mg, 0.23 mmol), sodium (26.5 mg, 1.15 mmol) in anhydrous methanol (50 mL) and guanidinium nitrate (140 mg, 1.15 mmol) was reacted as described for preparation of 4a to furnish 49.98 mg (64 %) of 4d, mp (acetone): 218–221 °C; IR (KBr, cm–1): 3472 (OH), 3318, 3209 (NH2), 1602, 1568 (C=C, C=N); 1H-NMR (CDCl3): δ = 1.00 (s, Me-19), 1.06 (s, Me-18), 3.06 (s, 2H, NH2), 3.53 (m, 1H, H-3α), 5.38 (d, 1H, H-6, J = 4.9 Hz); 13C-NMR (CDCl3): δ = 16.8 (C-18), 19.4 (C-19), 20.5 (C-11), 26.1 (C-15), 30.6 (C-8), 31.2 (C-12), 31.6 (C-7), 32.9 (C-2), 36.7 (C-10), 37.1 (C-1), 42.2 (C-4), 46.0 (C-13), 50.6 (C-9), 53.2 (MeO), 55.7 (C-14), 71.5 (C-3), 113.4 (C-5’), 121.0 (C-6), 141.2 (C-5), 162.8 (C-6’), 169.8 (C-2’), 181.8 (C-4’); MS (EI): m/z (%): 369 (100, M+.); Calc. for C22H31N3O2: C 71.51, H 8.46, N 11.37; Found: C 71.38, H 8.64, N 11.13.
2’,6’-dimethoxy- pyrimido[5’,4’:16,17]androst-5-en-3β-ol (4e)
Compound 2 (100 mg, 0.23 mmol), sodium (26.5 mg, 1.15 mmol) in anhydrous methanol (50 mL) and S-methylisothiuronium sulfate (320 mg, 1.15 mmol) was reacted as described for preparation of 4a, to furnish 52.11 mg (59 %) of 4e, mp (acetone): 214–216° C; IR (KBr, cm–1): 3463 (OH), 1591, 1559 (C=C, C=N); 1H-NMR (CDCl3): δ = 0.96 (s, Me-19), 1.07 (s, 3 Me-18), 3.53 (m, 1H, H-3α), 3.95 (s, OMe), 4.08 (s, OMe ), 5.38 (d, 1H, J = 5.20 Hz, H-6); 13C-NMR (CDCl3): δ = 16.8 (C-18), 19.4 (C-19), 20.5 (C-11), 26.4 (C-15), 30.7 (C-8), 31.3 (C-12), 31.6 (C-7), 33.1 (C-2), 36.8 (C-10), 37.1 (C-1), 42.3 (C-4), 46.1 (C-13), 50.8 (C-9), 53.2 (MeO), 53.6 (MeO), 55.8 (C-14), 71.6 (C-3), 113.4 (C-5’), 121.1 (C-6), 141.2 (C-5), 169.8 (C-2’), 181.8 (C-4’), 163.1 (C-6’); MS (EI): m/z (%): 384 (100 M+.); Calc. for C23H32N2O3: C 71.84, H 8.39, N 7.29; Found: C 71.67, H 8.44, N 7.18.