Synthesis and Structure of bis(Dibutyldithiocarbamate)zinc(II): Zn2[(n-Bu)2NCSS]4
Abstract
:Introduction
Results and Discussion
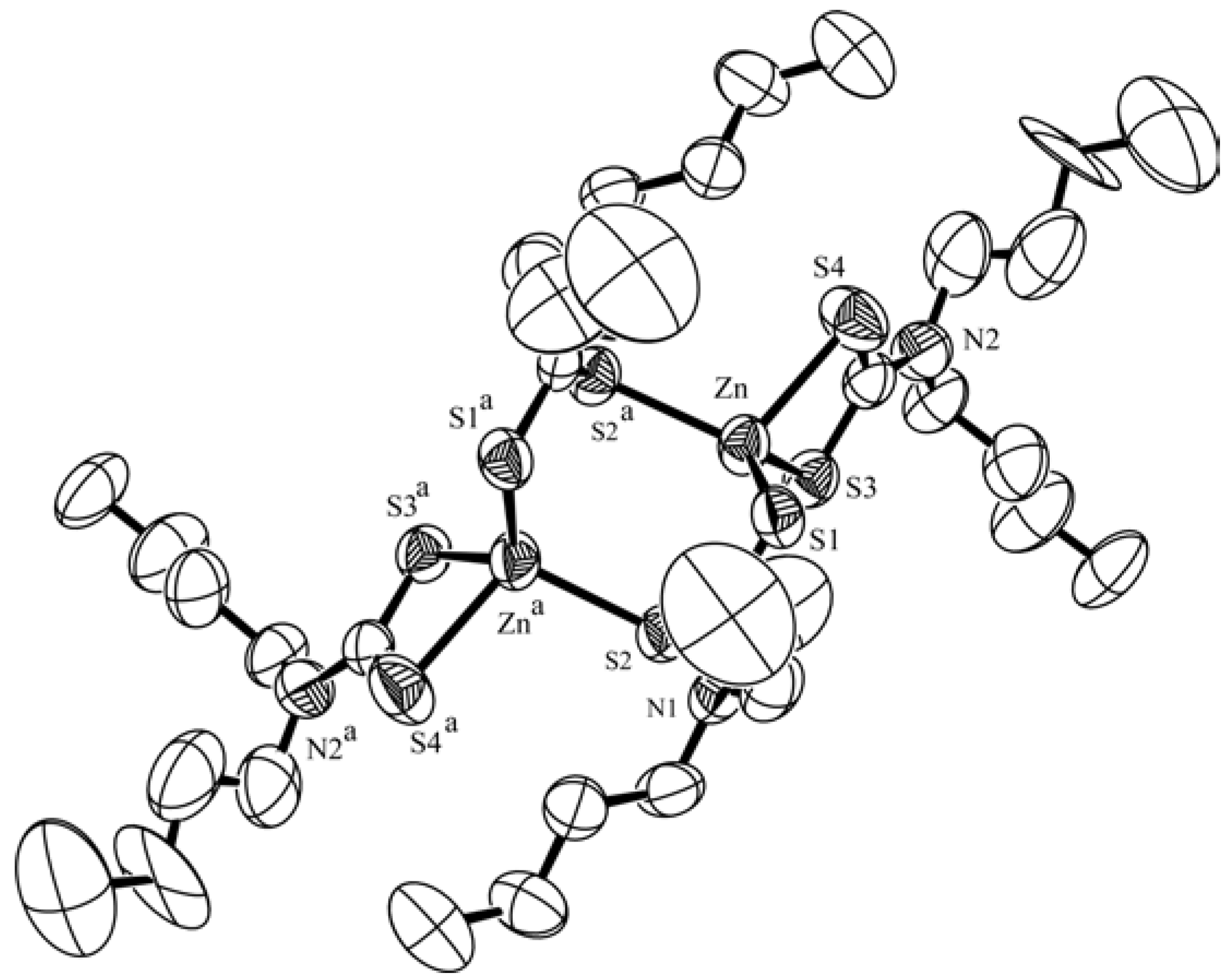
X-Ray Crystallography [11]



X-ray photoelectron spectroscopy (XPS)
| Compounds | Binding energies /eV | |||
|---|---|---|---|---|
| Zn(2p)3/2 | C(1s) | N(1s) | S(2p) | |
| ZnCl2 [12] | 1023.1 | |||
| Na[S2CN(n-C4H9)2] | 287.0, 284.9 | 398.9 | 164.2, 162.2 | |
| Zn2[S2CN(n-C4H9)2]4 | 1021.6 | 287.0, 284.6 | 399.7 | 162.0 |
IR spectra
Electronic Spectra
Conclusions
Experimental
General
Preparation of bis(dibutyldithiocarbamate) zinc(II)
X-Ray Crystallography
| a. Formula | Zn2C36H72N4S8 |
| b. Crystal system | Monoclinic |
| c. Space group | C2/c |
| d. Unit cell dimensions | a = 23.329(3) Å α = 90 deg. b = 17.090(2) Å β = 127.560(10) deg. c = 16.115(2) Å γ = 90 deg. |
| e. Volume, Z | 5093.1(11) Å 3, 8 |
| f. Density (calculated) | 1.237 mg/m3 |
| g. Absorption coefficient | 1.297 mm-1 |
| h. F(000) | 2016 |
| i. Crystal size | 0.54 x 0.50 x 0.42 mm |
| j. θ range for data collection | 1.62 to 25.00 deg. |
| k. Reflections collected | 5841 |
| l. Independent reflections | 4489 [R(int) = 0.0133] |
| m. Max. and min. transmission | 0.6437 and 0.5521 |
| n. Final R indices [I>2sigma(I)] | R1 = 0.0450, wR2 = 0.1192 |
| o. Extinction coefficient | 0.00028(10) |
| p. Largest diff. peak and hole | 0.472 and -0.351 e/Å 3 |
Acknowledgments
References and Notes
- Dance, I. G.; Garbutt, R. G.; Scudder, M. L. Inorg. Chem. 1990, 29, 1571.
- Bochmann, M.; Webb, K. J.; Hursthouse, M. B.; Mazid, M. J. Chem. Soc., Dalton Trans. 1991, 2317.
- Santos, R. A.; Gruff, E. S.; Koch, S.A. J. Am. Chem. Soc. 1991, 113, 469.
- Jian, F.; Wang, Z.; Bai, Z.; You, X.; Fun, H.K.; Chinnakali, K. J. Chem. Crystallogr. 1999, 29, 227.
- Zhang, C.; Chadha, R.; Reddy, H. K.; Schrauzer, G. N. Inorg. Chem. 1991, 30, 763.
- Casals, I.; Gonzalez-Duarte, P.; Lopez, C.; Solans, X. Polyhedron 1990, 9, 763.
- Mann, S.; Ozin, G. A. Nature 1996, 382, 313.
- Zhu, H.; Tang, N.; Gan, X.; Zhang, W.; Tan, M.; Wang, A. Polyhedron 1993, 8, 945.
- Su, C.; Tang, N.; Tan, M.; Liu, W.; Gan, X. Polyhedron 1996, 15, 73.
- Jiang, X.; Zhang, W.; Zhong, Y.; Wang, S. L. Coll. Czech. Chem. Commun. 2002, 11, 1629.
- CCDC 160049 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033; e-mail: [email protected])
- Shu, C. Y.; Tan, M. N.; Zhang, Z. F.; Tang, N.; Cai, L. P.; Xue, Q. J. Synth. React. Inorg. Met-Org. Chem. 1999, 29, 35.
- John, F. M.; William, F. S.; Peter, E. S.; Kenneth, D. B. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronica Inc.: Minnesota, 1995; p. 122. [Google Scholar]
- Yoshida, T.; Yamasaki, K.; Sawada, S. Bull. Chem. Soc. Jpn. 1979, 52, 2908.
- Nakamoto, K. IR and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: New York, 1978; p. 339. [Google Scholar]
- Janssen, M. Rec. Trav. Chim. 1960, 79, 1066.
- Sample Availability: Available from the authors.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Zhang, W.; Zhong, Y.; Tan, M.; Tang, N.; Yu, K. Synthesis and Structure of bis(Dibutyldithiocarbamate)zinc(II): Zn2[(n-Bu)2NCSS]4. Molecules 2003, 8, 411-417. https://doi.org/10.3390/80500411
Zhang W, Zhong Y, Tan M, Tang N, Yu K. Synthesis and Structure of bis(Dibutyldithiocarbamate)zinc(II): Zn2[(n-Bu)2NCSS]4. Molecules. 2003; 8(5):411-417. https://doi.org/10.3390/80500411
Chicago/Turabian StyleZhang, Weiguang, Yun Zhong, Minyu Tan, Ning Tang, and Kaibei Yu. 2003. "Synthesis and Structure of bis(Dibutyldithiocarbamate)zinc(II): Zn2[(n-Bu)2NCSS]4" Molecules 8, no. 5: 411-417. https://doi.org/10.3390/80500411




