Epoxidation of Diosgenin, 25(R)-1,4,6-Spirostatrien-3-one and 25(R)-4,6-Spirostadien-3β-ol
Abstract
:Introduction
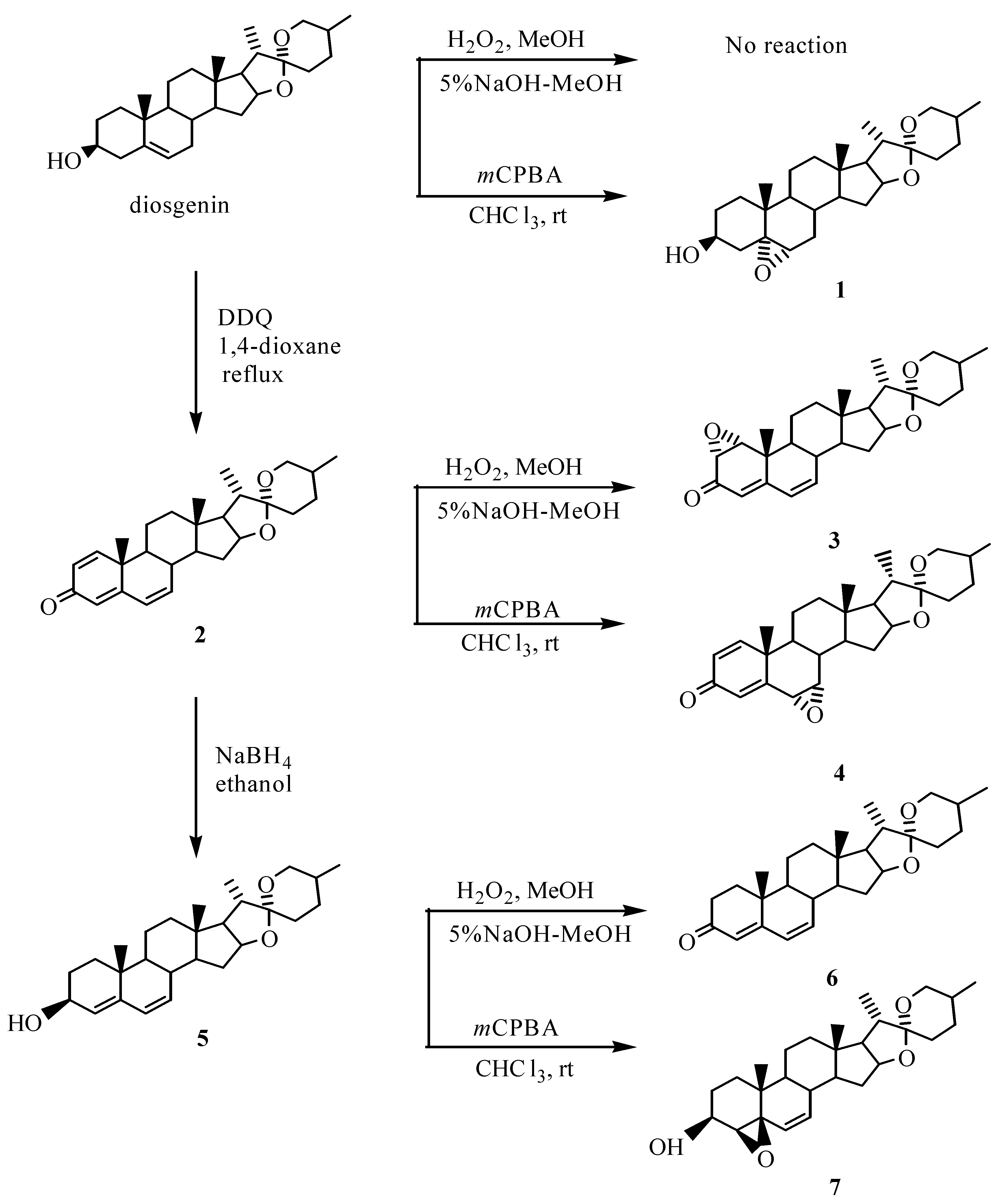
Results and discussion
Acknowledgements
Experimental
General
References
- Dewick, P. M. Medicinal Natural Products, 2nd ed.; John Wiley & Sons: Chichester, U.K., 2002; pp. 237–289. [Google Scholar]
- Laguna, J.; Gomez-Puyoy, A.; Pena, A.; Guzman-Garcia, J. Effect of diosgenin on cholesterol metabolism. J. Atheroscler. Res. 1962, 2, 459–470. [Google Scholar]
- Peifer, J. J.; Guzman, J. Hypocholesterolemic effects of diosgenin in the rat. Circulation 1966, 34, III-25. [Google Scholar]
- Accatino, L.; Pizarro, M.; Solis, N.; Koenig, C. S. Effects of diosgenin a plant-derived steroid, on bile secretion and hepatocellular cholestasis induced by estrogens in the rat. Hepatology 1998, 28, 129–140. [Google Scholar]
- Cayen, M. N.; Dvornik, D. Effect of diosgenin on lipid metabolism in rats. J. Lipid Res. 1979, 20, 162–174. [Google Scholar]
- Uchida, K.; Takase, H.; Nomura, Y.; Takeda, K.; Takeuchi, N.; Ishikawa, Y. Changes in biliary and fecal bile acids in mice after treatments with diosgenin and β-sitosterol. J. Lipid Res. 1984, 25, 236–245. [Google Scholar]
- Datt, K.; Datta, S. K.; Datta, P. C. Pharmacognostic evaluation of potential yams Dioscorea. J. Econ.Taxon. Bot. 1984, 5, 181–196. [Google Scholar]
- Noam, M.; Tamir, I.; Breuer, E.; Mechoulam, R. Conversion of ruscogenin into 1α- and 1β-hydroxycholesterol. Tetrahedron 1981, 37, 597–604. [Google Scholar]
- Liu, M.; Yu, B.; Hui, Y. First total synthesis of 25(R)-ruscogenin-1-yl β-D-xylopyranosyl-(1→3)-(β-D-glucopyranosyl-(1→2)-β-D-fucopyranoside, an Ophiopogonis saponin from the tuber of Liliope muscari (Decne.). Tetrahedron Lett. 1998, 39, 415–418. [Google Scholar]
- Furst, A.; Labler, L.; Meier, W. An effect procedure for preparing 1α,3β-dihydroxy-Δ5-steroids by reduction of 1α,2α-epoxy-4,6-dien-3-ones with lithium in ammonia. Helv. Chim. Acta 1981, 64, 1870–1892. [Google Scholar]
- Kobayashi, Y.; Taguchi, T.; Mitsuhashi, S.; Eguchi, T.; Ohshima, E.; Ikekawa, N. Studies on organic fluorine compounds. XXXIX. Studies on steroids. LXXIX. Synthesis of 1α,25-dihydroxy-26,26,26,27,27,27-hexafluorovitamin D. Chem. Pharm. Bull. 1982, 30, 4297–4303. [Google Scholar]
- Araghiniknam, M.; Chung, S.; Tresa, N.-W.; Eskelson, C.; Watson, R. R. Antioxidant activity of dioscorea and dehydroepiandrosterone (DHEA) in older humans. Life Sci. 1996, 59, 147–157. [Google Scholar]
- Schulz, S.; Klann, R. C.; Schonfeld, S.; Nyce, J. W. Mechanisms of cell growth inhibition and cell cycle arrest in human colonic adenocarcinoma cells by dehydroepiandrosterone: role of isoprenoid biosynthesis. Cancer Res. 1992, 52, 1372–1376. [Google Scholar]
- Boros, L. G.; Puigjaner, J.; Cascante, M; Lee, W. N.; Brandes, J. L.; Brassilian, S. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res. 1997, 57, 4242–4248. [Google Scholar]
- Gao, H.; Dias, J. R. Synthetic studies toward 1α-hydroxytestosterone from testosterone. In Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), 2000, September, 1-30.
- Gamoh, K.; Hirayama, M.; Ikekawa, N. Stereocontrolled synthesis of Withanolide D and related compounds. J. Chem. Soc., Perkin Trans. I 1984, 440–454. [Google Scholar]
- Sharpless, K. B.; Verhoeven, T. R. Metal-catalysed, highly selective oxygenations of olefins and acetylenes with tert-butyl hydroperoxide. Practical considerations and mechanisms. Aldrichim. Acta. 1979, 12, 63–75. [Google Scholar]
- Sample Avalability: Samples of compounds 1, 2, 3, 4, 5 and 7 are available from the author.
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ma, E.; Kim, J. Epoxidation of Diosgenin, 25(R)-1,4,6-Spirostatrien-3-one and 25(R)-4,6-Spirostadien-3β-ol. Molecules 2003, 8, 886-893. https://doi.org/10.3390/81200886
Ma E, Kim J. Epoxidation of Diosgenin, 25(R)-1,4,6-Spirostatrien-3-one and 25(R)-4,6-Spirostadien-3β-ol. Molecules. 2003; 8(12):886-893. https://doi.org/10.3390/81200886
Chicago/Turabian StyleMa, Eunsook, and Jongwon Kim. 2003. "Epoxidation of Diosgenin, 25(R)-1,4,6-Spirostatrien-3-one and 25(R)-4,6-Spirostadien-3β-ol" Molecules 8, no. 12: 886-893. https://doi.org/10.3390/81200886



