Concept Definition
Molecules are, in general, localized entities. In a typical molecular solid, the intermolecular interactions are much weaker than the intramolecular bonding energies so the bulk properties of a molecular solid can usually be analyzed as the sum of individual molecular contributions, with small perturbations from the intramolecular forces. Such weak intermolecular interactions rarely extend beyond the nearest neighbors and the electronic structures of a molecular crystal are usually independent of the size of the crystal. Inorganic semiconductors and metals, on the other hand, consist of a network of ordered atoms with no discernible molecular unit. For a semiconductor crystal, electronic excitation consists of a loosely bounded electron-hole pair (the Mott-Wannier exciton), usually delocalized over a length much longer than the lattice constant [
10]. As the diameter of the semiconductor crystalline approaches this exciton Bohr diameter, its electronic properties start to change. This is the so-called
quantum confinement effect, which can be observed as a blue shift in the band gap or exciton energy [
11,
12].
Typically, bulk sample of CdS, irrespective of their size once >
ca. 20 nm, will absorb all electromagnetic radiation with an energy greater than the band gap (
hν > 2.42 eV); which is classified as direct in this case. However, as particles become smaller, their electronic structure changes. Eventually, continuous bands breaks down and there are discrete bonding and antibonding orbitals in this material [
13]. The electronic properties of such small particles are more like those of a molecule than an extended solid [
14]. A spatial electronic state diagram showing the quantum confinement effect is shown in
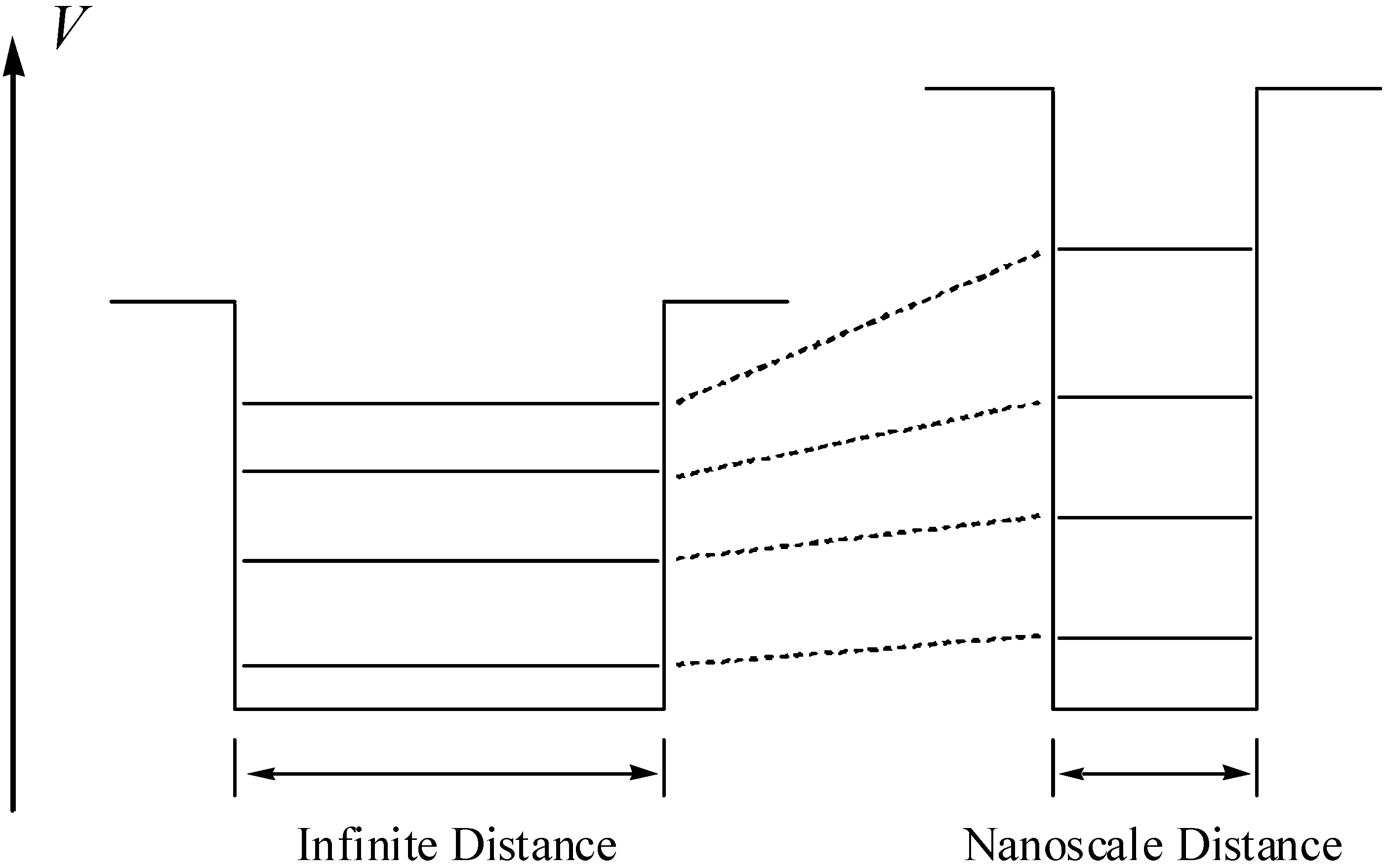
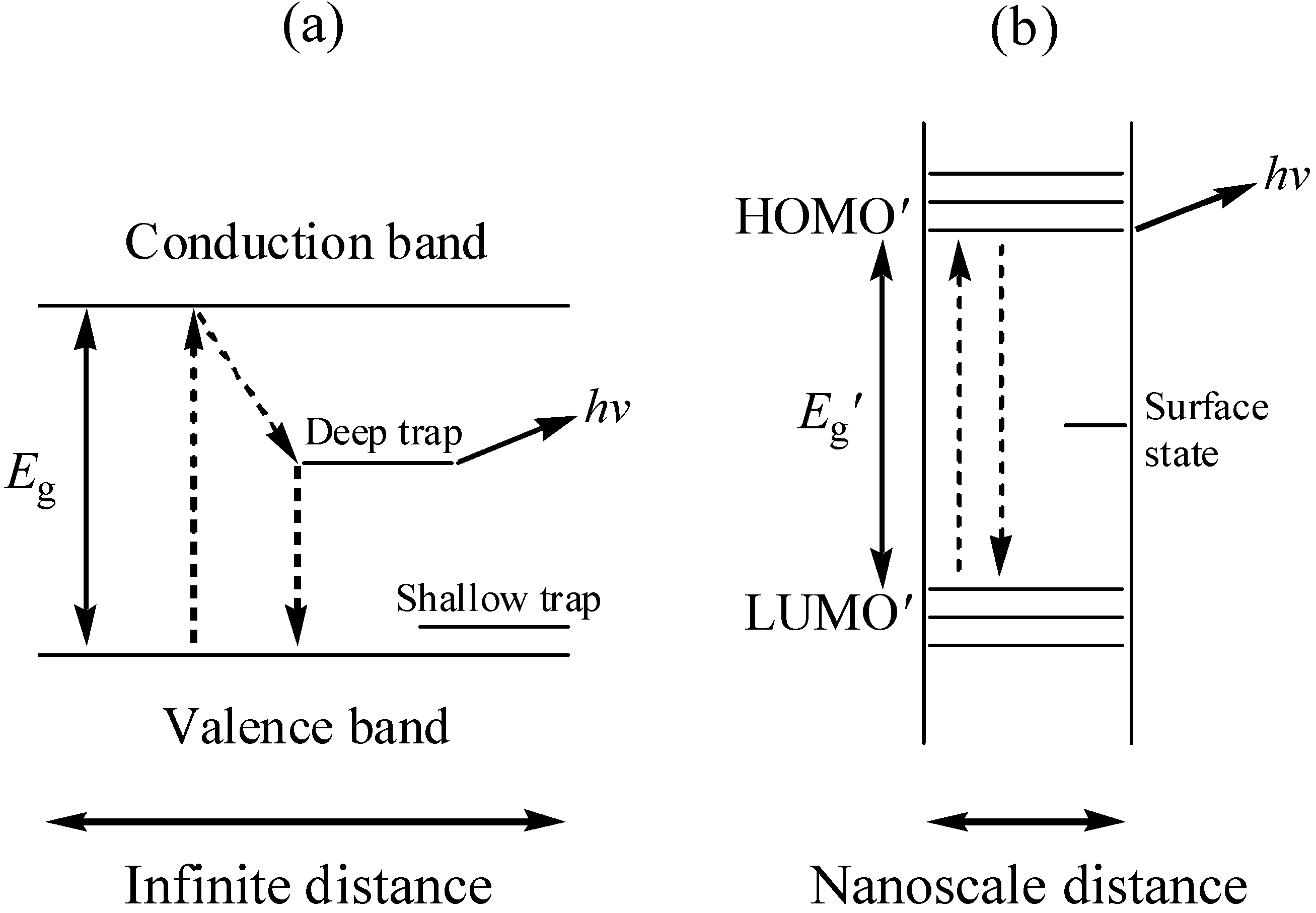
Figure 1. As the cluster properties are intermediate between molecules and bulk semiconductors, the quantum size effect can best be explained with hybrid molecular and semiconductor languages.
Figure 1.
The spatial electronic state diagram showing the quantum confinement effect in bulk semiconductors (a) and nanoparticles (b).
Figure 1.
The spatial electronic state diagram showing the quantum confinement effect in bulk semiconductors (a) and nanoparticles (b).
Model Design
The linear combination of atomic orbitals-molecular orbitals (LCAO-MO) method [
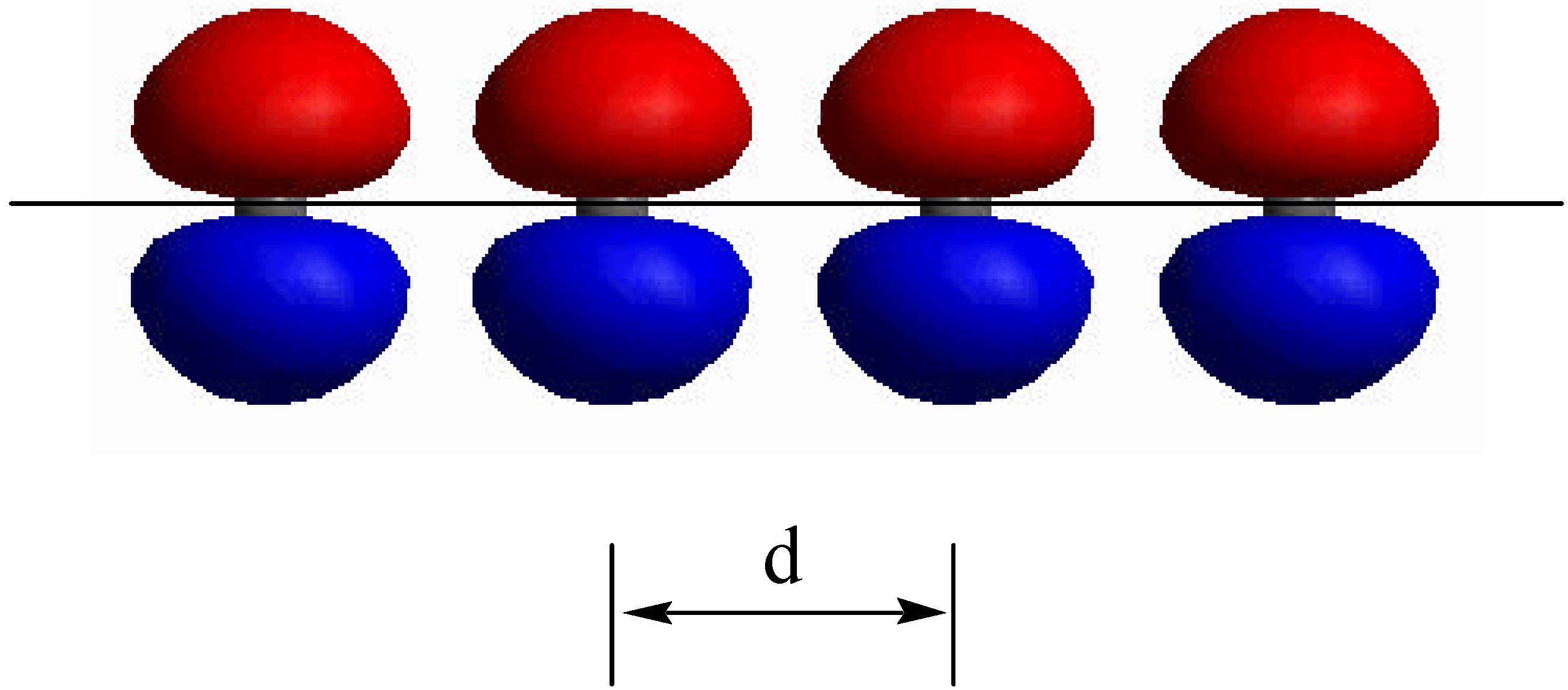
15] provides a natural framework to understand the evaluation of clusters from molecules to bulk and the size dependence of the lowest excited-state energy (band gap). An infinite chain of carbon atoms, separated by distance
d, each carrying one
pπ orbital (polyene, an idealized polyacetylene chain), provides a simple one-dimensional analogue of the bulk solid (
Figure 2). The infinite chain is equivalent to a
N-annulene where
N is infinite. The essential concepts behind the quantum confinement effects of semiconductor clusters can be understood by studying the length dependence of the
N-polyene. In the absence of any interactions, an
N-atom polyene has
N degenerate orbitals,
ϕi, each with on-site energy (Coulomb integral)
α, <
ϕi|
H|
ϕi>, where
H is the electronic Hamiltonian. By turning on the interaction between
ϕi and
ϕj, represented by the resonance integral
β, <
ϕi|
H|
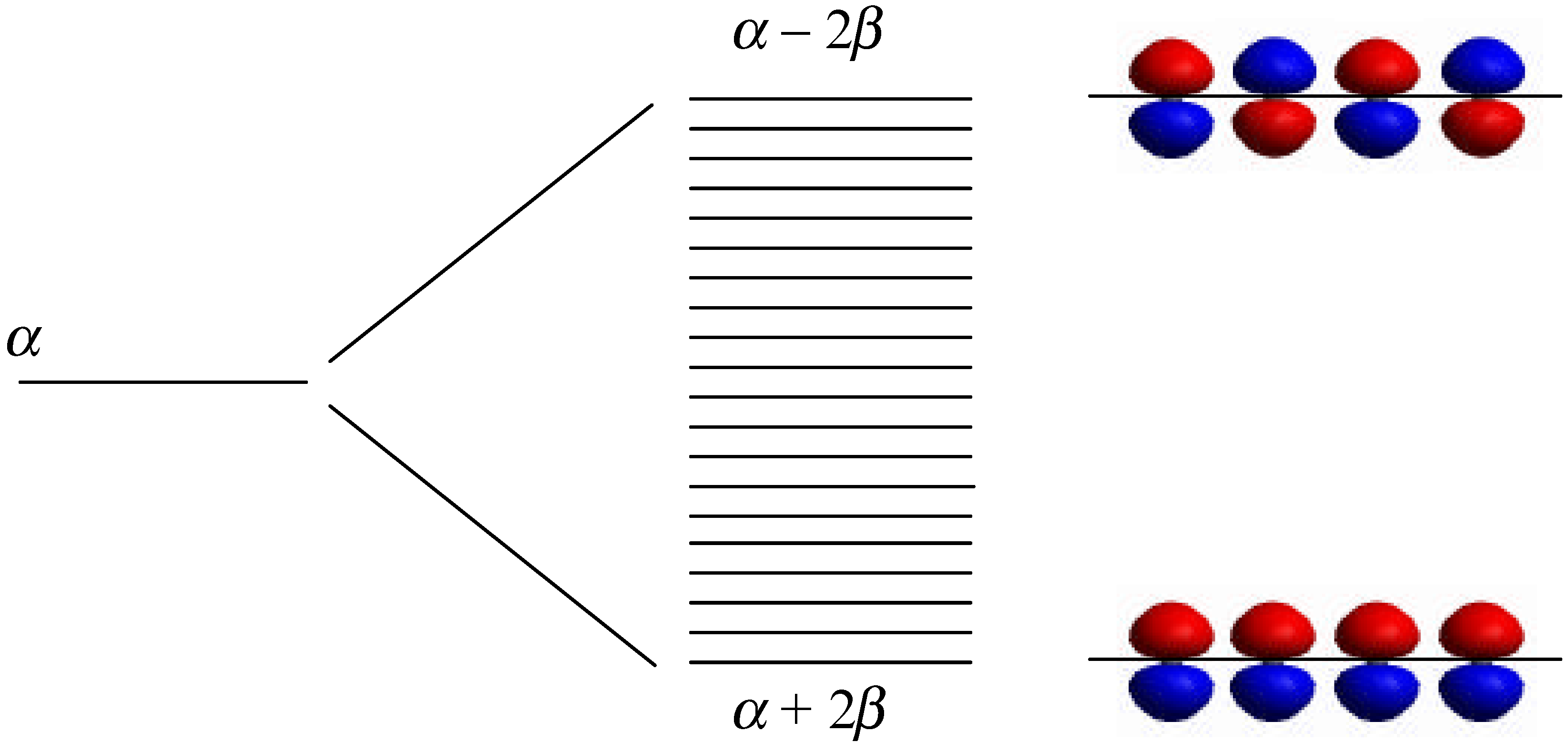
ϕj>, the degeneracy is removed (
Figure 3). The lowest energy orbital is at
α + 2
β and is bonding all neighboring atoms. The highest energy orbital is at
α − 2
β and is antibonding between all neighboring all neighbor ing atoms. In the middle at energy
α, there is a nonbonding orbital.
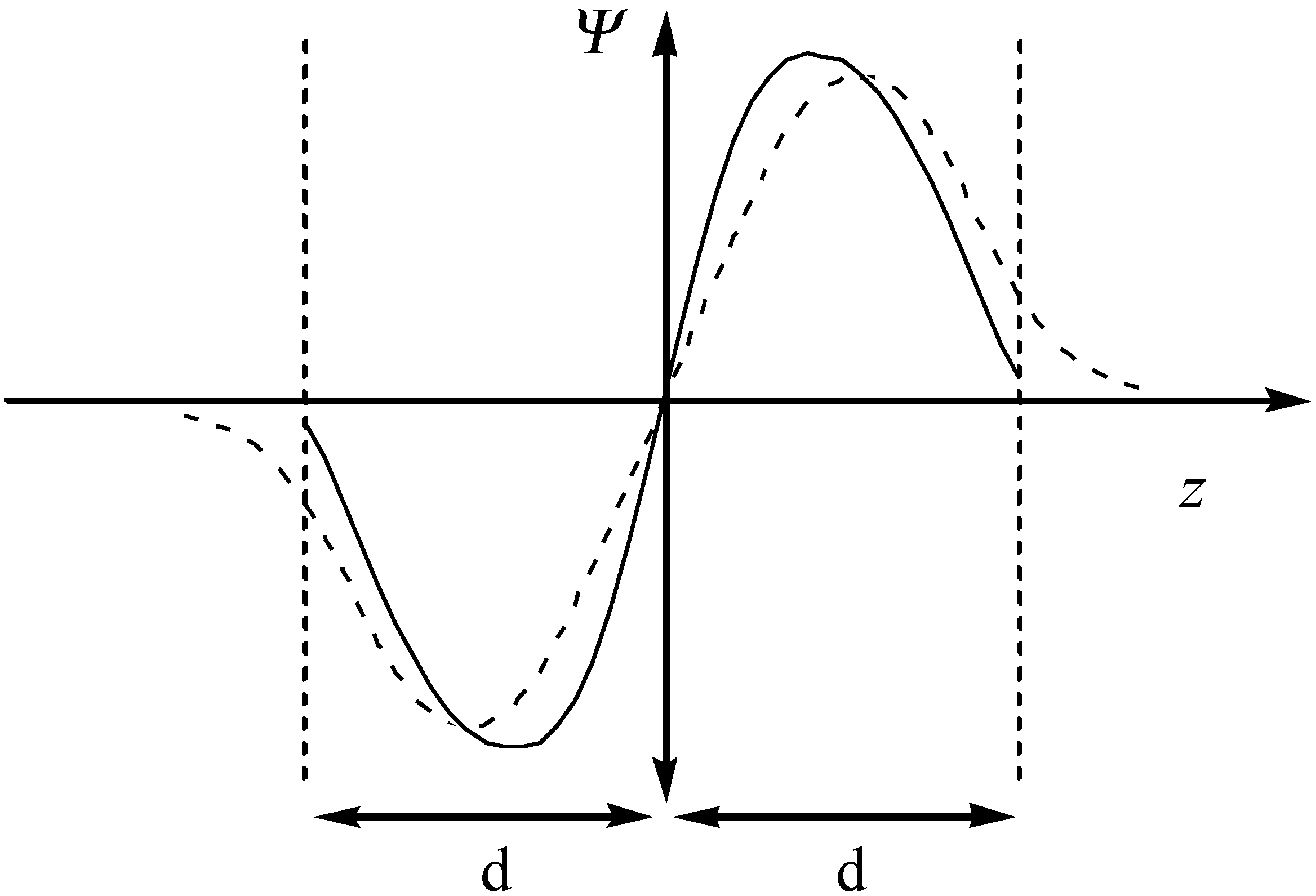
Figure 2.
N-polyene, a simple one-dimensional analogue of the bulk solid as the designed model.
Figure 2.
N-polyene, a simple one-dimensional analogue of the bulk solid as the designed model.
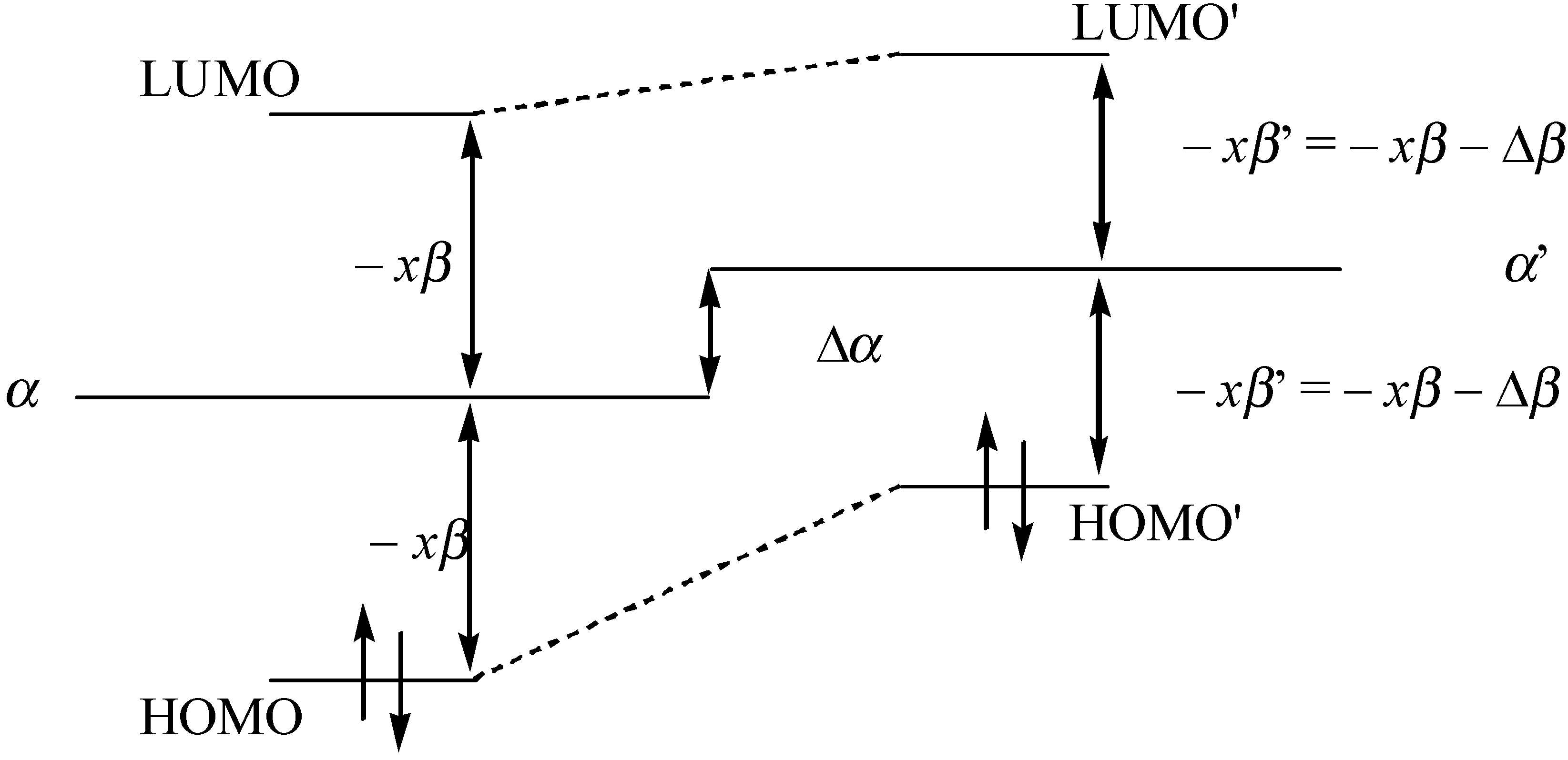
Figure 3.
Molecular orbital diagram for the N-polyene.
Figure 3.
Molecular orbital diagram for the N-polyene.
The energy levels of these orbitals,
Ej, can be easily obtained on the Hückel level as
For an infinite chain, the energy band is continuous from α + 2β to α − 2β with a bandwidth of 4β. For a chain of finite atoms, the eigenvalues are discrete. In the case of a linear polymer, there is no degeneracy. So, one can easily prove, by filling electrons into these orbitals, that the HOMO-LUMO gap increases with decreasing chain length, N.
The LCAO-MO method outlined above provides a simple framework to understand many important solid-state concepts. The premier thing here is that as the size of the N-polyene decreases, its energy band becomes discrete and separation between the eigenvalues, and the HOMO-LUMO gaps increase. This is in essence the quantum confinement effect, i.e., the increase of the excited-state energy with decreasing cluster size.
In a bulk semiconductor, the electron and hole are bound together by a screened Coulomb interaction to form a so-called Mott-Wannier exciton [
10]. This electron-hole interaction has to be induced for a more qualitative treatment of the quantum confinement effects. By assuming the energy band to be parabolic near the band gap (i.e., the effective mass approximation), the size-dependent shift (with respect to the bulk band gap) in the exciton energy of a small cluster (cluster radius ~ exciton radius) can be derived as
where
R is cluster radius, 1/
μ = 1/
me* + 1/
mh*,
me* is the electron effective mass,
mh* is the hole effective mass,
ε is the dielectric constant, and
![Molecules 08 00207 i002]()
is the effective Rydberg energy,
e4/2ε
2ℏ
2(
me*
-1 +
mh*
-1). The first term in the above equation is the band gap of the bulk materials, the second represents the particle-in-a-box quantum localization energy and has a simple 1 /
R2 dependence, the third term the Coulomb energy with a 1 /
R dependence, and the last term is the result of the spatial correlation effect. This last size-independent term is usually small but can become significant for semiconductors with small dielectric constant. Therefore, the cluster radius can be easily determined according to the above formula based on the absorption spectra.
Equation (2), although containing the basic physics of the quantum confinement effect, cannot be expected to be quantitatively correct, especially for very small clusters. This is because for small clusters the eigenvalues of the lowest excited states are located in a region of the energy band that is no longer parabolic (the breakdown of the effective mass approximation). It may be mentioned here that the variations of molecular properties of very small clusters can be interpreted by the electronic confinement effect, which will be discussed in the next section.
Case Study
The search of functional nanostructured materials for information storage has stimulated great interest in nowadays nano-science and technology [
16]. The study of nanosized metal sulfide semiconductor materials doped with impurities has been an area of intense activity in recent years [
17,
18]. Zinc sulfide is the best host for luminescent materials with the band gap of 3.7 eV. Since the rare-earth ions are excellent luminescence centers, nanosized ZnS doped with them is becoming a new kind of solid-state luminescent material though the radii of rare-earth ions is larger than that of zinc ions. We present here the synthesis of Eu-doped ZnS semiconductor nanoparticles, together with their photoluminescence behavior [
19].
ZnS:Eu nanoparticles
1 and
2 were prepared by two methods, A and B, respectively. In method A, Na
2S serves as the inorganic sulfur donor and S
2- ions from Na
2S react with Zn
2+ ions immediately. In method B, Zn
2+ ions react with S
2- ions from the organic sulfur donor CH
3CSNH
2 gradually. In order to characterize the particle size, the diffuse reflectance absorption spectrum of ZnS:Eu nanoparticles
1 was measured. Comparin g to the absorption maximum of ZnS bulk materials (3.7 eV), the absorption maximum of ZnS nanoparticles blueshifted to 320 nm (3.9 eV). Estimated from the Eq. (2), it is obtained that the grain size of the ZnS:Eu nanoparticle (sample
1) is 6.8 nm. The powder X-ray diffraction of ZnS:Eu semiconductor nanoparticles
1 is also measured. All peaks can be ascribed to a zinc blende crystal structure without extra phases and non-crystalline state. The peaks are markedly broadened, which is characteristic of the nanoparticles. The average grain size of ZnS:Eu nanoparticles can be estimated by Scherrer equation
where the symbols have their usual meanings. The average grain size of the nanoparticles is 6.9 nm estimating from Eq. (3), which agrees well with the result of the diffuse reflectance spectrum.
Figure 4.
The photoluminescence spectra of the sample 1 (solid line) and 2 (dashed line). λexc = 396 nm.
Figure 4.
The photoluminescence spectra of the sample 1 (solid line) and 2 (dashed line). λexc = 396 nm.
The photoluminescence spectra of
1 and
2 excited at 396 nm are shown in
Figure 4. No further splitting of the emission peaks is observed indicating that the site symmetry of Eu
3+ belongs to O
h group (zinc blende crystal belongs to O
h group). If the site symmetry of Eu
3+ belongs to O
h group, the emission at 590 nm originated from magneto-dipole allowed
5D
0 →
7F
1 is dominant. When the site symmetry of Eu
3+ is reduced, the intensity of the emission band at 616 nm, which is the electro-dipole allowed
5D
0 →
7F
2 transition, should increase. So it is evident that two emission peaks of Eu
3+ centered at 590 and 616 nm are sensitive to site symmetry and can be used to study site symmetry, i.e., to study the microstructure around Eu
3+ and the defects caused by doping of Eu
3+ in the lattice of ZnS. As the ion size of Eu
3+ is much larger than that of Zn
2+, the defects and the deformation of the lattice must appear when Eu
3+ gets into the lattice of ZnS, and the site symmetry of doping Eu
3+ ion must be decreased also. Therefore, it seems plausible to deduce that ZnS:Eu nanoparticles are doped by higher dopant concentration of Eu
3+ in
2 than in
1, which is related to the difference in the preparation procedures.

 is the effective Rydberg energy, e4/2ε2ℏ2(me*-1 + mh*-1). The first term in the above equation is the band gap of the bulk materials, the second represents the particle-in-a-box quantum localization energy and has a simple 1 / R2 dependence, the third term the Coulomb energy with a 1 / R dependence, and the last term is the result of the spatial correlation effect. This last size-independent term is usually small but can become significant for semiconductors with small dielectric constant. Therefore, the cluster radius can be easily determined according to the above formula based on the absorption spectra.
is the effective Rydberg energy, e4/2ε2ℏ2(me*-1 + mh*-1). The first term in the above equation is the band gap of the bulk materials, the second represents the particle-in-a-box quantum localization energy and has a simple 1 / R2 dependence, the third term the Coulomb energy with a 1 / R dependence, and the last term is the result of the spatial correlation effect. This last size-independent term is usually small but can become significant for semiconductors with small dielectric constant. Therefore, the cluster radius can be easily determined according to the above formula based on the absorption spectra.