Crystal Structure of N,N-bis-(3-Carbomethoxy-5-methyl-pyrazol-1-ylmethyl)aniline
Abstract
:Introduction
Results and Discussion
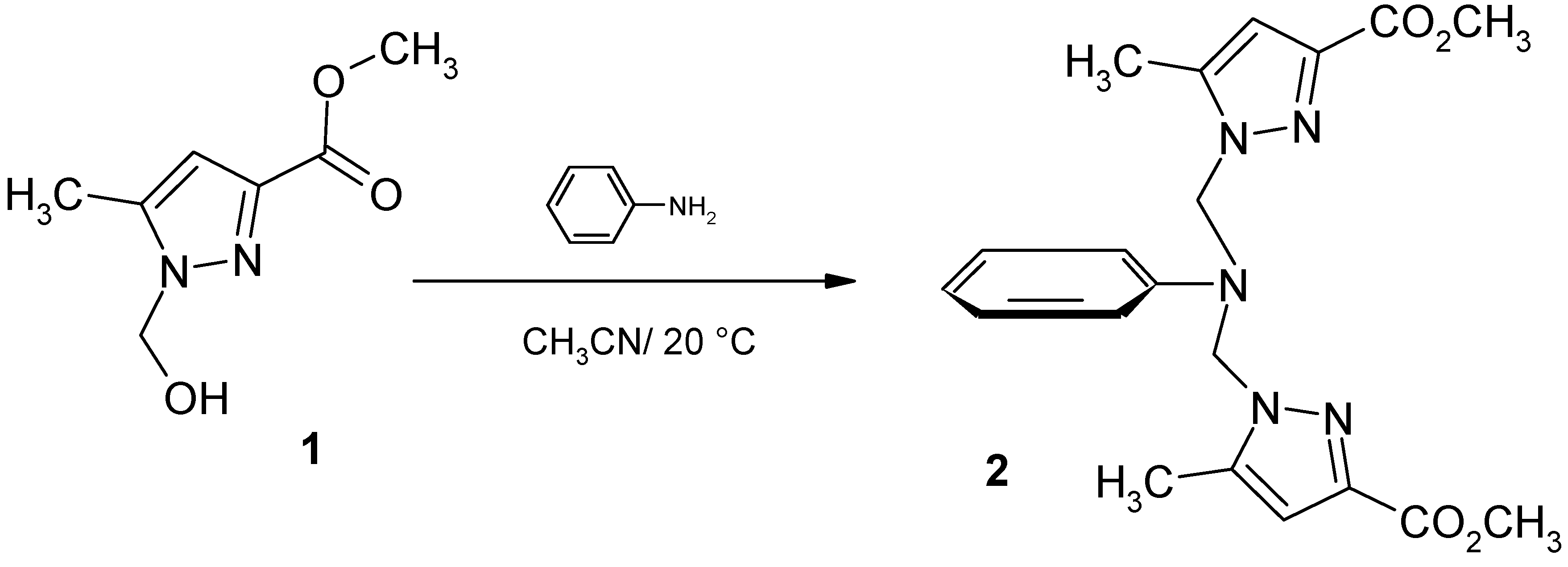
Synthesis of the tripodal ligand 2.
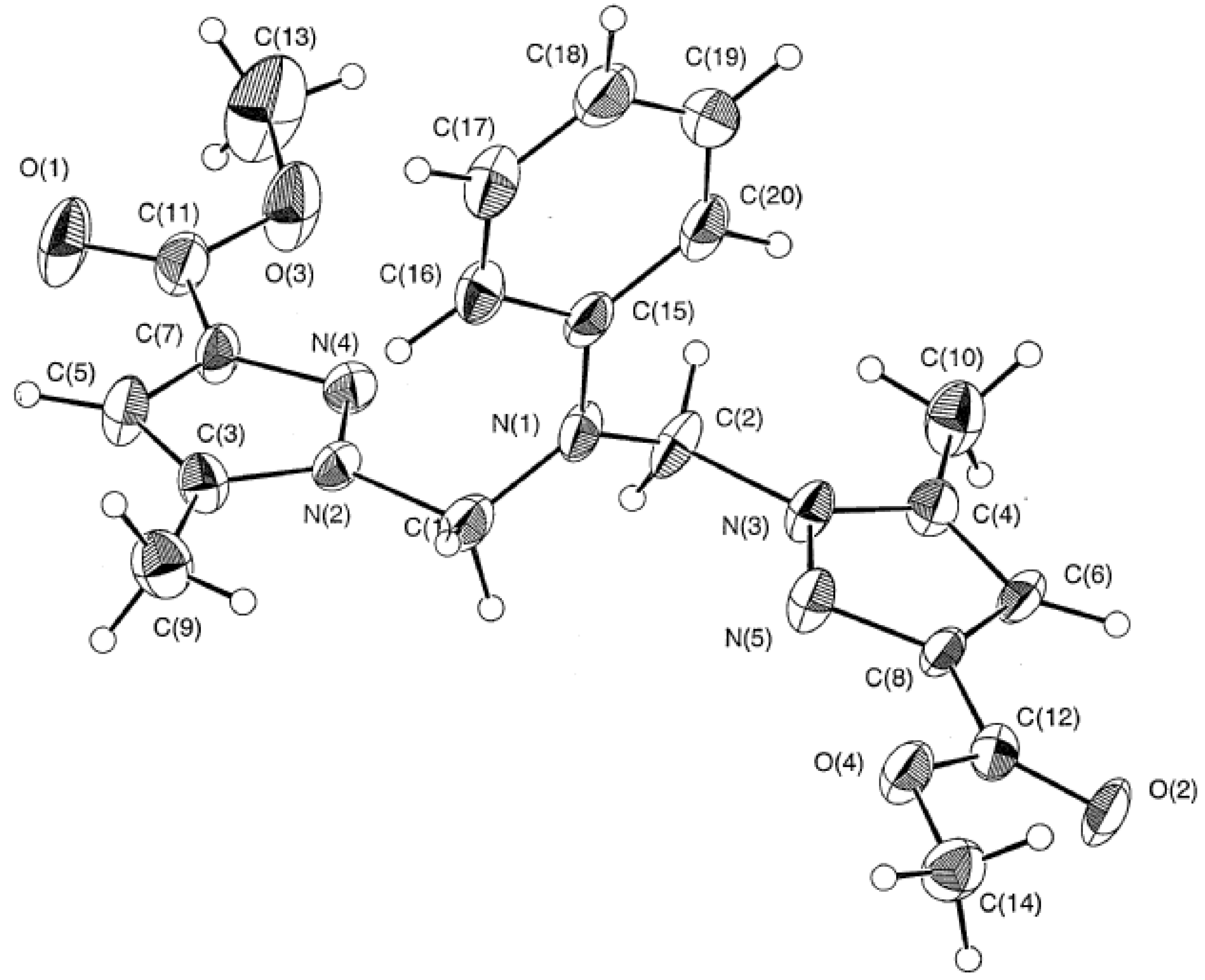
Crystallographic study of tripodal ligand 2
| Formula weight = 397.43 | λ = 0.71073 Å |
| Crystal system: triclinic | μ (Mo Kα) = 0.93 cm-1 |
| Space group: P 1 | 2θmax = 64 ° |
| a = 6.624(1) Å | T = 298K |
| b = 12.315(2) Å | Block of white crystals |
| c = 12.770(2) Å | near prismatic form 0.16, 0.17, 0.20 mm |
| α = 85.39(1)° | R = 0.041 (on F) |
| β = 83.09(2)° | Rw = 0.068 (on F2) |
| γ = 78.54(1)° | (ΔP)max = +0.81 |
| V = 1011.8(3)Å3 | (ΔP)min = -0.88 |
| Z = 2 | No. of refl. measured = 8258 |
| Dx = 1.305 g/cm3 | No. of refl. used = 1656 |
| Transmission min = 0.667 | No. of parameters = 265 |
| Transmission max = 0.986 | goodness-of-fit = 0.583 |
| F (000) = 420 | |
| Measurement- Kappa CCD – Nonius Diffractometer | |
| Program system: MaXus | |
| Absorption correction : Sortav | |
| Atom | x/a | y/b | z/c | U(iso) |
|---|---|---|---|---|
| O1 | -0.0007(4) | 0.2792(3) | 0.69695(19) | 0.079(2) |
| O3 | -0.1013(4) | 0.3459(2) | 0.5409(2) | 0.0840(18) |
| N1 | 0.4611(4) | 0.2487(2) | 0.23541(19) | 0.0482(16) |
| N2 | 0.4449(4) | 0.1756(2) | 0.41638(18) | 0.0401(14) |
| N4 | 0.2481(4) | 0.2309(2) | 0.43834(19) | 0.0431(15) |
| C1 | 0.5254(5) | 0.1555(3) | 0.3053(3) | 0.0500(19) |
| C3 | 0.5425(5) | 0.1409(3) | 0.5052(2) | 0.0466(18) |
| C5 | 0.4013(5) | 0.1760(3) | 0.5878(2) | 0.051(2) |
| C7 | 0.2231(5) | 0.2313(3) | 0.5435(2) | 0.0441(18) |
| C9 | 0.7600(5) | 0.0792(3) | 0.5014(3) | 0.062(2) |
| C11 | 0.0323(5) | 0.2866(3) | 0.6023(3) | 0.054(2) |
| C13 | -0.2885(8) | 0.4112(7) | 0.5927(4) | 0.132(4) |
| C15 | 0.5511(5) | 0.3428(3) | 0.2293(2) | 0.0426(18) |
| C16 | 0.7019(5) | 0.3506(3) | 0.2943(2) | 0.050(2) |
| C17 | 0.7921(5) | 0.4428(3) | 0.2861(3) | 0.063(2) |
| H5 | 0.418(4) | 0.172(3) | 0.659(3) | 0.054114 |
| H6 | 0.270(4) | 0.192(2) | -0.158(3) | 0.048953 |
| Atom | Atom | Distance | Atom | Atom | Distance |
|---|---|---|---|---|---|
| O1 | C11 | 1.201(5) | N2 | N4 | 1.353(4) |
| O3 | C11 | 1.323(5) | C15 | C20 | 1.399(6) |
| N1 | C1 | 1.424(5) | C15 | C16 | 1.395(6) |
| N1 | C15 | 1.398(5) | C4 | C6 | 1.359(6) |
| N2 | C3 | 1.370(5) | C3 | C5 | 1.360(6) |
| N5 | C8 | 1.335(5) | N4 | C7 | 1.334(5) |
| C3 | C9 | 1.487(6) | C5 | C7 | 1.399(5) |
| C17 | C18 | 1.376(7) | C7 | C11 | 1.462(6) |
| C19 | C20 | 1.365(7) | C4 | C6 | 1.359(6) |
| C15 | C20 | 1.399(6) | C3 | C5 | 1.360(6) |
| Atom | Atom | Atom | Angle |
|---|---|---|---|
| C1 | N1 | C2 | 116.8(4) |
| C1 | N1 | C15 | 121.3(4) |
| C2 | N1 | C15 | 121.3(4) |
| N4 | N2 | C3 | 112.9(3) |
| N5 | N3 | C4 | 112.8(3) |
| N2 | N4 | C7 | 103.8(3) |
| N3 | N5 | C8 | 104.3(3 |
| N2 | C3 | C5 | 105.6(4) |
| N2 | C3 | C9 | 122.9(4) |
| C5 | C3 | C9 | 131.5(4) |
| C3 | C5 | C7 | 106.0(4) |
| N4 | C7 | C5 | 111.7(4) |
| N4 | C7 | C11 | 122.7(4) |
| N5 | C8 | C6 | 111.3(4) |
| N5 | C8 | C12 | 123.0(4) |
| O1 | C11 | O3 | 123.1(4) |
| O1 | C11 | C7 | 123.6(4) |
Conclusions
Experimental
General
Synthesis of N,N-bis-(3-carbomethoxy-5-methylpyrazol-1-ylmethyl)aniline (2)
Acknowledgements
References
- Sorrell, T.N.; Vankai, V.A.; Garrity, M.L. Inorg. Chem. 1991, 30, 207.
- Togni, A.; Venanzi, L.M. Angew. Chem. Int. Ed. Engl. 1994, 33, 497.
- Touzani, R.; Ramdani, A.; Ben-Hadda, T.; El Kadiri, S.; Maury, O.; Le Bozec, H.; Dixneuf, P.H. Synth. Comm. 2001, 31, 1315.
- Launay, J.-P. Chem. Soc. Rev. 2001, 30, 386.
- Sondaz, E.; Jaud, J.; Launay, J.-P.; Bonvoisin, J. Eur. J. Inorg. Chem. 2002, 8, 1924.
- Bol, J.E.; Maase, B.; Gonesh, G.; Driessen, W.L.; Goubitz, K.; Reedijk, J. Heterocycles 1997, 45, 1477.
- Sheu, S.C.; Tien, M.J.; Cheng, M.C.; Ho, T.I.; Peng, S.M.; Lin, Y.C. J. Chem. Soc. Dalton Trans 1995, 3503.
- Driessen, W.L. Rec. J.R. Neth. Chem. Soc. 1982, 101, 441.Driessen, W.L.; Graaff, R.A.G.; Parlevliet, F.J.; Reedijk, J.; Vos, R.M. Inorg. Chim. Acta 1994, 216, 43.
- Dvoretzky, I.; Richter, G.H. J. Org. Chem. 1950, 15, 1285.
- Mackay, S.; Gilmore, C.J.; Edwards, C.; Stewart, N.; Shankland, K. maXus. In Computer Program for the Solution and Refinement of Crystal Structures; Bruker Nonius, The Netherlands, MacScience, Japan and The University of Glasgow; 1999. [Google Scholar]
- Johnson, C. K. ORTEP-II. In A Fortran Thermal-Ellipsoid Plot Program; Report ORNL-5138; Oak Ridge National Laboratory: Oak Ridge, Tennessee, USA, 1976. [Google Scholar]
- Otwinowski, Z.; Minor, W. Methods in Enzymology; Sweet, C.W., Jr., Sweet, R.M., Eds.; New York: Academic Press, 1997; Volume 276, p. 307. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. J. Appl. Cryst. 1994, 27, 435.
- Waasmaier, D.; Kirfel, A. Acta Cryst. 1995, A51, 416.
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Daoudi, M.; Larbi, N.B.; Benjelloun, D.; Kerbal, A.; Launay, J.P.; Bonvoisin, J.; Jaud, J.; Mimouni, M.; Ben-Hadda, T. Crystal Structure of N,N-bis-(3-Carbomethoxy-5-methyl-pyrazol-1-ylmethyl)aniline. Molecules 2002, 7, 690-696. https://doi.org/10.3390/70900690
Daoudi M, Larbi NB, Benjelloun D, Kerbal A, Launay JP, Bonvoisin J, Jaud J, Mimouni M, Ben-Hadda T. Crystal Structure of N,N-bis-(3-Carbomethoxy-5-methyl-pyrazol-1-ylmethyl)aniline. Molecules. 2002; 7(9):690-696. https://doi.org/10.3390/70900690
Chicago/Turabian StyleDaoudi, Maria, Najib Ben Larbi, Driss Benjelloun, Abdelali Kerbal, Jean Pierre Launay, Jacques Bonvoisin, Joël Jaud, Mostafa Mimouni, and Taibi Ben-Hadda. 2002. "Crystal Structure of N,N-bis-(3-Carbomethoxy-5-methyl-pyrazol-1-ylmethyl)aniline" Molecules 7, no. 9: 690-696. https://doi.org/10.3390/70900690




