Michael Reactions of Arylidenesulfonylacetonitriles. A New Route to Polyfunctional Benzo[a]quinolizines
Abstract
:Introduction
Results and Discussion
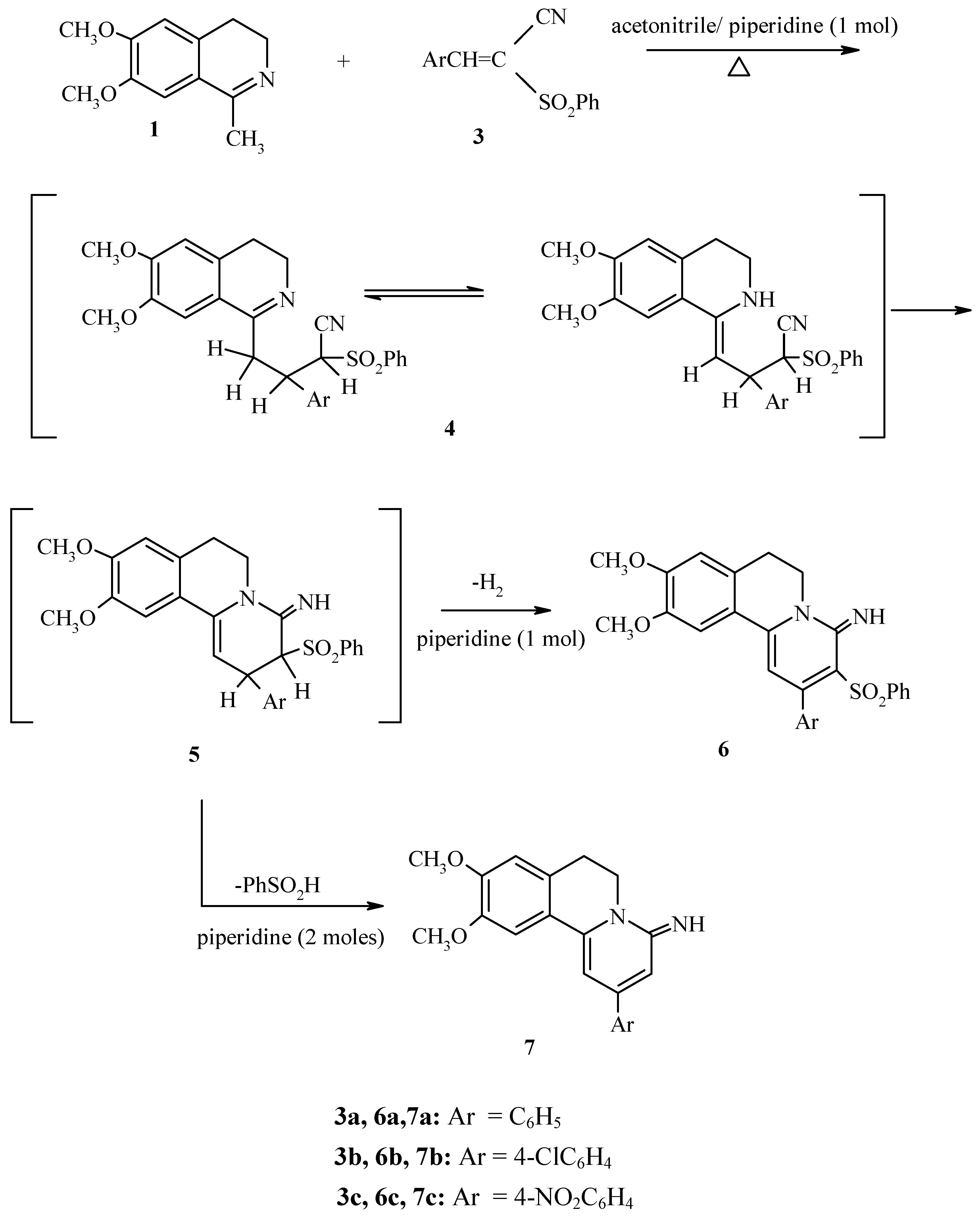

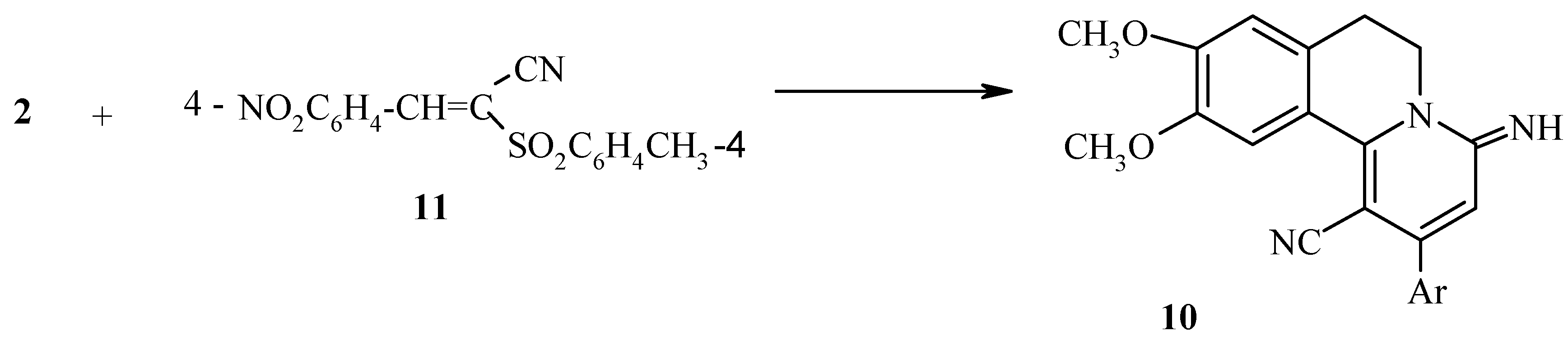
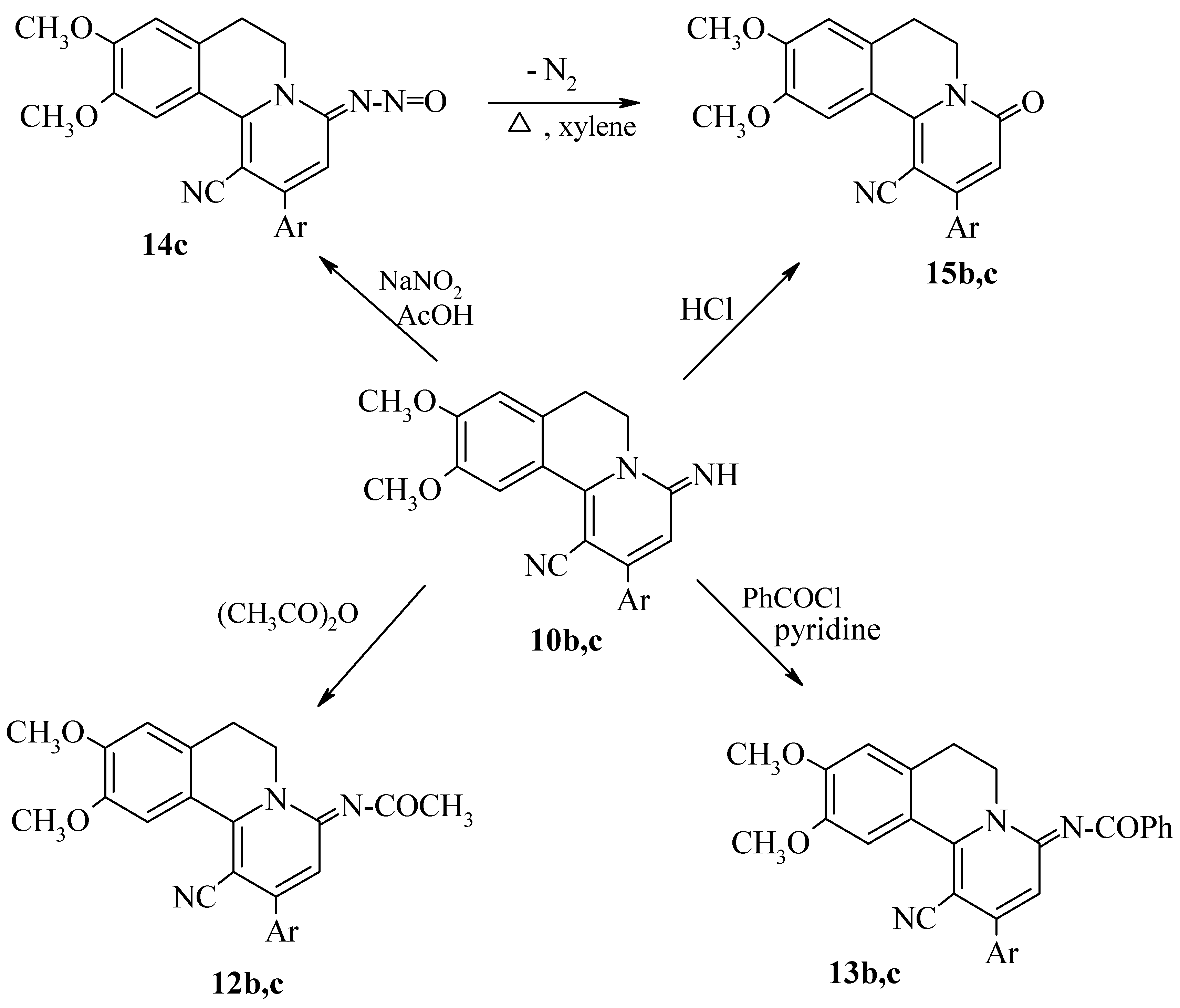
Experimental
General
Synthesis of 2-aryl-6,7-dihydro-9,10-dimethoxy-4-imino-2-phenylsulphonyl-benzo[a]quinolizines 6 and 9.
Synthesis of 2-aryl-6,7-dihydro-9,10-dimethoxy-4-iminobenzo[a]-quinolizines 7 and 10
Nitrosation of 10c.
Thermolysis of 14c.
Acylations of 10b,c.
Hydrolysis of 10 b,c.
| Compd. no. | Color | Yield % | m.p.°C solvent | Mol. formula Mol. Wt. | % Analysis Calcd. (Found) | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | |||||
| 6a | yellow | 80 | 225-226 | C27H24N2O4S | 68.64 | 5.08 | 5.93 | 6.78 |
| DMF | 472.23 | (68.72) | (5.02) | (5.83) | (6.66) | |||
| 6b | dark | 82 | 264-266 | C27H23N2O4SCl | 63.96 | 4.54 | 5.53 | 6.32 |
| yellow | DMF | 506.72 | (64.23) | (4.44) | (5.52) | (6.38) | ||
| 6c | orange | 78 | 276-277 | C27H23N3O6S | 62.67 | 4.45 | 8.12 | 6.19 |
| DMF | 517.23 | (62.52) | (4.24) | (8.03) | (6.08) | |||
| 9a | dark | 84 | 258-259 | C28H23N3O4S | 67.61 | 4.63 | 8.45 | 6.44 |
| yellow | DMF | 497.23 | (67.43) | (4.52) | (8.62) | (6.27) | ||
| 9b | bright | 77 | 320-322 | C28H22N3O4SCl | 63.22 | 4.14 | 7.90 | 6.02 |
| brown | DMF | 531..72 | (63.04) | (4.03) | (7.84) | (6.14) | ||
| 7a | yellow | 81 | 329-331 | C21H20N2O2 | 75.90 | 6.02 | 8.43 | - |
| DMF | 332.19 | (75.63) | (6.14) | (8.63) | - | |||
| 7b | yellow | 85 | 206-207 | C21H19N2O2Cl | 68.76 | 5.18 | 7.64 | - |
| DMF | 366.68 | (68.64) | (5.02) | (7.83) | - | |||
| 7c | yellow | 88 | 214-215 | C21H19N3O4 | 66.84 | 5.04 | 11.14 | - |
| DMF | 377.19 | (66.90) | (5.13) | (11.24) | - | |||
| 10a | dark | 86 | 214-216 | C22H19N3O2 | 73.95 | 5.32 | 11.76 | - |
| yellow | DMF | 357.19 | (73.63) | (5.21) | (11.54) | - | ||
| 10b | bright | 79 | 223-224 | C22H18N3O2Cl | 67.43 | 4.60 | 10.73 | - |
| brown | DMF | 391.68 | (67.13) | (4.73) | (10.94) | - | ||
| 10c | dark | 89 | 275-277 | C22H18N4O4 | 65.67 | 4.48 | 13.93 | - |
| yellow | DMF | 402.19 | (65.51) | (4.32) | (13.83) | - | ||
| 12b | dark | 84 | 153-155 | C24H20N3O3Cl | 66.44 | 4.61 | 9.69 | - |
| yellow | EtOH | 433..70 | (66.12) | (4.51) | (9.82) | - | ||
| 12c | dark | 78 | 150-151 | C24H20N4O5 | 64.86 | 4.50 | 12.61 | - |
| yellow | EtOH | 444.21 | (64.84) | (4.32) | (12.41) | - | ||
| 13b | dark | 77 | 241-242 | C29H22N3O3Cl | 70.23 | 4.44 | 8.48 | - |
| yellow | DMF | 495.72 | (70.13) | (4.24) | (8.21) | - | ||
| 13c | brown | 79 | 260-262 | C29H22N4O5 | 68.77 | 4.35 | 11.07 | - |
| DMF | 506.23 | (68.63) | (4.11) | (10.90) | - | |||
| 14c | red | 81 | 250-251 | C22H17N5O5 | 61.25 | 3.94 | 16.24 | - |
| DMF | 431.19 | (61.21) | (3.67) | (16.42) | - | |||
| 15b | yellow | 78 | 294-295 | C22H17N2O3Cl | 67.26 | 4.33 | 7.13 | - |
| DMF | 392.67 | (67.13) | (4.12) | (7.34) | - | |||
| 15c | yellow | 83 | 244-246 | C22H17N3O5 | 65.51 | 4.22 | 10.42 | - |
| DMF | 403.17 | (65.23) | (4.12) | (10.35) | - | |||
| Compd. no. | IR (cm-1) | 1H NMR (δ ppm) | M+ |
|---|---|---|---|
| 6a | 3350 | 2.6 (m, 2H); 3.8 (s, 3H); 3.9 (s, 3H); 4.1 (m, 2H); 6.9 (s, 1H); 7.0- | 472 |
| (NH) | 7.7 (m, 10H); 7.8 (s, 1H); 7.9 (s, 1H), 8.8 (s, 1H) | ||
| 6b | 3380 | 3.0 (m, 2H); 3.8 (s, 6H); 4.1 (m, 2H); 7.0 (s, 1H); | 507 |
| (NH) | 7.2-7.6 (m, 10H); 7.9 (s, 2H). | ||
| 6c | 3446 | 3.1 (m, 2H); 3.8 (s, 6H); 4.1 (m, 2H); 6.9 (s, 1H); 7.1-8.5 (m, | 517 |
| (NH) | 12H). | ||
| 9a | 2216 (CN), | 3.0 (m, 2H); 3.8 (s, 6H); 4.1 (m, 2H); 6.9 (s, 1H); 7.0-7.6 (m, | 497 |
| 3417 (NH) | 11H); 7.7 (s, 1H) | ||
| 9b | 2219 (CN), | 2.8 (m, 2H); 3.8 (s, 3H); 3.9 (s, 3H); 4.1 (m, 2H); 7.2 (s, 1H); 7.3- | 532 |
| 3415 (NH) | 7.7 (m, 10H); 7.9 (s, 1H) | ||
| 7a | 3386 | 2.9 (m, 2H); 3.3 (s, 3H); 3.4 (s, 3H); 3.8 (m, 2H); 6.7 (s, 1H); 6.8 | 332 |
| (NH) | (s, 1H); 6.9 (s, 1H), 7.1 (s, 1H) 7.2-7.6 (m, 6H) | ||
| 7b | 3252 | 2.9 (m, 2H); 3.8 (s, 3H); 3.9 (s, 3H); 4.1 (m, 2H); 6.3 (s, 1H); 6.4 | 367 |
| (NH) | (s, 1H); 6.7 (s,1H); 7.1 (s, 1H); 7.4-7.8 (m, 5H) | ||
| 7c | 3323 | 2.9 (m, 2H); 3.8 (s, 3H); 3.9 (s, 3H); 4.2 (m, 2H); 6.4 (s, 1H); 6.5 | 377 |
| (NH) | (s, 1H); 6.7 (s, 1H), 6.9 (s, 1H) 7.1-7.6 (m, 5H) | ||
| 10a | 2221 (CN), | 2.9 (m, 2H); 3.8 (s, 3H); 3.9 (s, 3H); 4.0 (m, 2H); 6.3 (s, 1H); 6.4 | 357 |
| 3316 (NH) | (s, 1H); 6.7 (s, 1H); 7.2-7.6 (m, 5H) | ||
| 10b | 2225 (CN), | 2.9 (m, 2H); 3.8 (s, 3H); 3.9 (s, 3H); 4.0 (m, 2H); 6.8 (s, 1H); 6.9 | 392 |
| 3420(NH) | (s, 1H); 7.1 (s, 1H); 7.4-8.2 (m, 5H) | ||
| 10c | 2200 (CN), | 2.8 (m, 2H); 3.6 (s, 3H); 3.7 (s, 3H); 4.0 (m, 2H); 6.4 (s, 1H); 6.9 | 402 |
| 3307(NH) | (s, 1H); 7.1 (s, 1H); 7.4-8.2 (m, 5H) | ||
| 12b | 1656(CO), | 2.8 (m, 2H); 3.7 (s, 3H); 3.9 (s, 6H); 4.0 (m, 2H); 6.7 (s, 1H); 7.0 | 434 |
| 2217 (CN) | (s, 1H); 7.4-8.2 (m, 5H) | ||
| 12c | 1658(CO), | 2.0 (s, 3H); 2.9 (m, 2H); 3.9 (s, 6H); 4.0 (m, 2H); | 444 |
| 2210(CN) | 6,5 (s, 1H); 6.8 (s, 1H); 7.4-7.6 (m, 4H); 7.9 (s, 1H) | ||
| 13b | 1654(CO), | 2.9 (m, 2H); 3.8 (s, 3H); 3.9 (s, 3H); 4.1 (m, 2H); 6.7 (s, 1H); 7.3- | 496 |
| 2211(CN) | 8.2 (m, 11H) | ||
| 13c | 1672 (CO), | 3.0 (m, 2H); 3.9 (s, 6H); 4.6 (m, 2H); 6.8 (s, 1H), 7.3 (s, 1H); 7.4- | 506 |
| 2210(CN) | 8.2 (m, 10H) | ||
| 14c | 2218(CN) | 2.7 (m, 2H); 3.8 (s, 6H); 4.0 (m, 2H); 6.7 (s, 1H); 6.8 (s, 1H); 7.4- | 431 |
| 8.2 (m, 5H) | |||
| 15b | 1659 (CO), | 2.8 (m, 2H); 3.7 (s, 3H); 3.9 (s, 3H); 4.0 (m, 2H); 6.7 (s, 1H); 6.8 | 393 |
| 2216 (CN) | (s, 1H); 7.4-8.2 (m, 5H) | ||
| 15c | 1666(CO), | 2.7 (m, 2H); 3.8 (s ,3H); 4.0 (s, 3H); 4.1 (m, 2H); 6.7 (s, 1H); 6.9 | 403 |
| 2218 (CN) | (s, 1H); 7.4-8.2 (m, 5H) |
- Compound 10c: 13C-NMR 27.54, 41.51, 56.78, 56.92, 100.73, 112.06, 113.92, 118.62, 119.82, 120.05, 123.40, 124.31, 130.70, 133.72, 135.31, 139.32, 142.12, 147.82, 148.65, 157.69.
- Compound 9a: 13C-NMR 28.95, 47.44, 58.31, 58.39, 95.67, 108.45, 112.09, 114.03, 115.60, 119.22, 119.72, 126.32, 130.29, 130.79, 130.96, 132.51, 133.62, 136.76, 150.08, 152.21, 155.38, 155.94, 157.48, 158.58.
References
- Saraf, S. Heterocycles 1981, 16, 803.
- Gootjes, J.; Nauta, W.Th. Rec. Trav. Chim. 1965, 84, 1183. [CrossRef]
- Saito, S.; Tanaka, T.; Kotera, K.; Nakai, H.; Sugimoto, N.; Horii, Z.; Ikeda, M.; Tamura, Y. Chem. Parm. Bull. (Tokyo) 1965, 13, 614.
- Pecherer, B.; Humiec, F.; Brossi, A. Syn. Commun. 1972, 2, 315.
- Kametani, T.; Surgenor, S.; Fukumoto, K. Heterocycles 1980, 14, 303.
- Meredith, R.F.K.; Ritchi, A.C.; Walker, T.; Whiting, K.D.E. J. Chem. Soc. 1963, 2672. [CrossRef]
- Kappe, T.; Linnau, Y. Monatshefte Chem. 1963, 100, 1726.
- Akiba, K.; Nakatani, M.; Wada, M.; Yamamoto, Y. J. Org. Chem. 1985, 50, 63. [CrossRef]
- Benevsky, P.; Stille, JR. Tetrahedron Lett. 1997, 38, 8475.
- Elwan, N.M.; Abdelhadi, H.A.; Abdallah, T.A.; Hassaneen, H.M. Tetrahedron 1996, 52, 3541.
- Abdelhadi, H.A.; Elwan, N.M.; Abdallah, T.A.; Hassaneen, H.M. J. Chem. Res. (S) 1996, 292.
- Spath, E.; Polgar, N. Monatshefte Chem. 1929, 51, 190.
- Balasubramanian, M.; Baliah, V. J. Indian. Chem. Soc. 1955, 32, 493.
- Openshaw, H.T.; Whittaker, N. J. Chem. Soc. 1961, 4939. [CrossRef]
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Abdallah, T.A.; Abdelhadi, H.A.; Hassaneen, H.M.; Hassaneen, H.M. Michael Reactions of Arylidenesulfonylacetonitriles. A New Route to Polyfunctional Benzo[a]quinolizines. Molecules 2002, 7, 540-548. https://doi.org/10.3390/70700540
Abdallah TA, Abdelhadi HA, Hassaneen HM, Hassaneen HM. Michael Reactions of Arylidenesulfonylacetonitriles. A New Route to Polyfunctional Benzo[a]quinolizines. Molecules. 2002; 7(7):540-548. https://doi.org/10.3390/70700540
Chicago/Turabian StyleAbdallah, Tayseer. A., Hyam. A. Abdelhadi, Huwida M. Hassaneen, and Hamdi M. Hassaneen. 2002. "Michael Reactions of Arylidenesulfonylacetonitriles. A New Route to Polyfunctional Benzo[a]quinolizines" Molecules 7, no. 7: 540-548. https://doi.org/10.3390/70700540




