Synthesis of New Lipophilic Phosphonate and Phosphonamidate Analogues of N-Acetylmuramyl-L-alanyl-D-isoglutamine Related to LK 423
Abstract
:Introduction
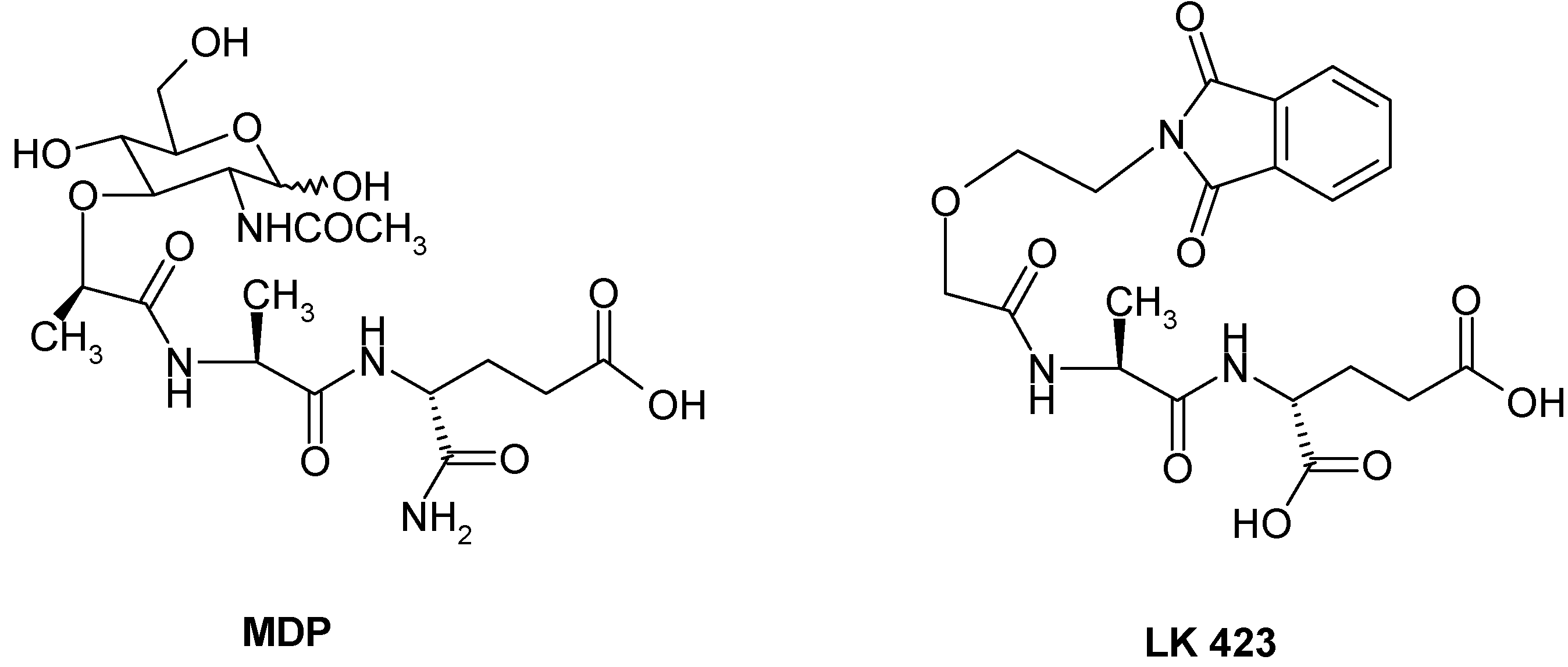
Results and Discussion
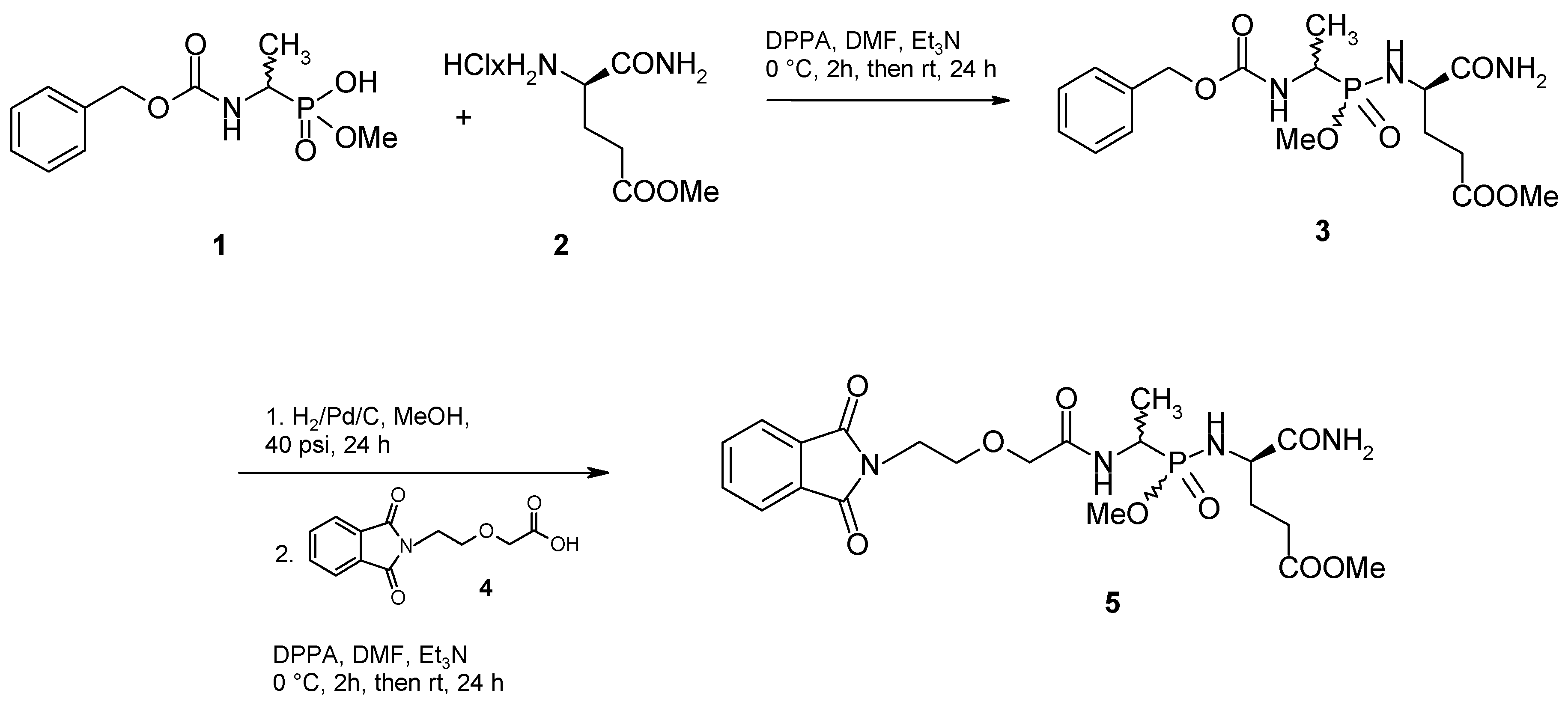
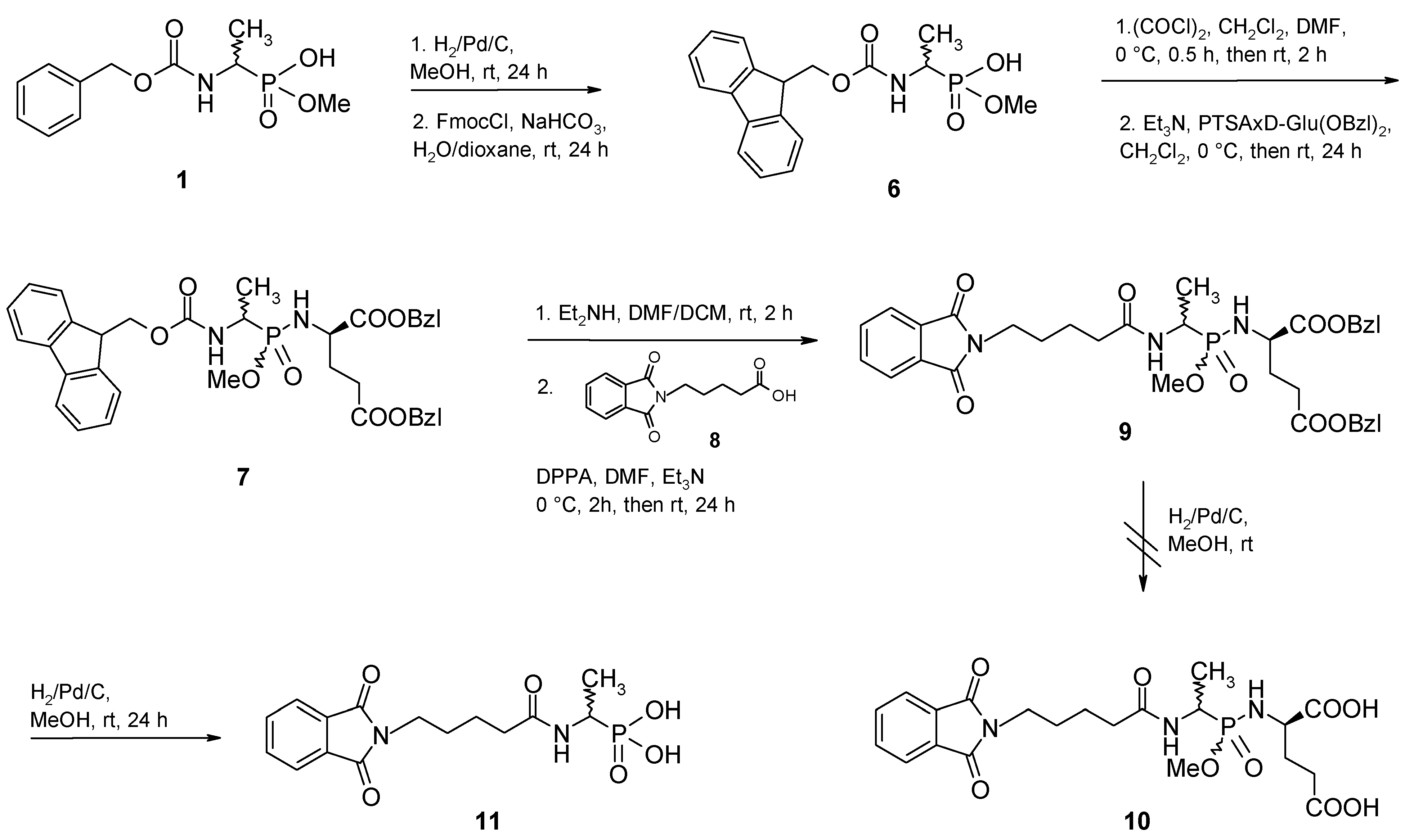
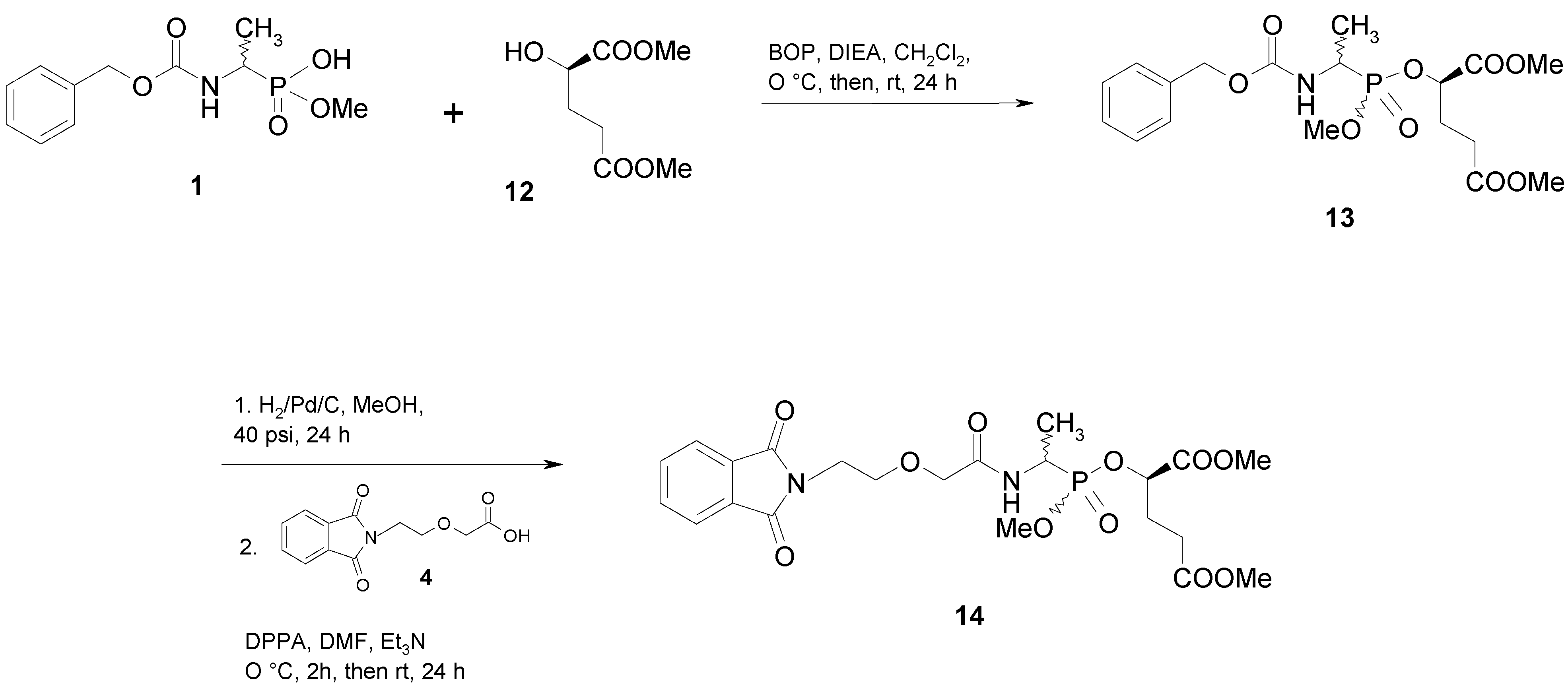

Conclusions
Experimental
General
References
- Ellouz, F.; Adam, A.; Cirobaru, R.; Lederer, E. Minimal Structural Requirements for Adjuvant Activity of Bacterial Peptidoglycan Derivatives. Biochem. Biophys. Res. Commun. 1974, 59, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Azuma, I.; Otani, T. Potentiation of Host Defense Mechanism against Infection by a Cytokine Inducer, an acyl-MDP Derivative, MDP-Lys(L18) (Romurtide) in Mice and Humans. Med. Res. Rev. 1994, 14, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Tsubura, E.; Azuma, I.; Une, T. Muroctasin, a Muramyl Dipeptide Derivative. Arzneim.-Forsch./Drug Res. 1988, 38, 951–952. [Google Scholar]
- Adam, A.; Lederer, E. Muramyl Peptides: Immunomodulators, Sleep Factors and Vitamins. Med. Res. Rev. 1984, 4, 111–152. [Google Scholar] [CrossRef] [PubMed]
- Baschang, G. Muramylpeptides and Lipopeptides: Studies toward Immunostimulants. Tetrahedron 1989, 45, 6331–6360. [Google Scholar] [CrossRef]
- Lefrancier, P.; Lederer, E. Muramyl-peptides. Pure Appl. Chem. 1987, 59, 449–454. [Google Scholar] [CrossRef]
- Hemmi, K.; Takeno, H.; Okada, S.; Nakaguchi, O.; Kitaura, Y.; Hashimoto, M. Total Synthesis of FK-156 Isolated from a Streptomyces as an Immunostimulating Peptide: Application of a Novel Copper Chelate Amino Protection. J. Am. Chem. Soc. 1981, 103, 7026–7028. [Google Scholar] [CrossRef]
- Migliore-Samour, D.; Bouchaudon, J.; Floc'h, F.; Zerial, A.; Ninet, L.; Werner, G.H.; Jolles, P.A. A Short Lipopeptide, Representative of a New Family of Immunological Ajuvans Devoid of Sugar. Life Sci. 1980, 26, 883–888. [Google Scholar]
- Sollner, M.; Pečar, S.; Štalc, A. The Influence of the Lipophilicity of the 7-Oxoacyl-L-alanyl-D-isoglutamines on Their Immunorestoration Activity in vivo. Eur. J. Med. Chem. 1996, 31, 927–933. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Camara, J.; Dalko, P.; Gero, S.D. Synthesis of Biologically Active Carbocyclic Analogues of N-Acetylmuramyl-L-Alanyl-D-isoglutamine (MDP). J. Org. Chem. 1989, 54, 3764–3766. [Google Scholar] [CrossRef]
- Kikelj, D.; Pečar, S.; Kotnik, V.; Štalc, A.; Wraber-Herzog, B.; Simčič, S.; Ihan, A.; Klamfer, L.; Povšič, L.; Grahek, R.; Suhadolc, E.; Hočevar, M.; Hönig, H.; Rogi-Kohlenprath, R. N-{trans-2-[[2'-(Acetylamino)cyclohexyl]oxy]acetyl}-L-alanyl-D-glutamic Acid: A Novel Immunologically Active Carbocyclic Muramyl Dipepeptide Analogue. J. Med. Chem. 1998, 41, 530–539. [Google Scholar]
- Gobec, S.; Urleb, U.; Simčič, S.; Wraber, B. Synthesis and Modulation of Cytokine Production by Two New Adamantane Substituted Acyclic Desmuramyldipeptide Analogs. Pharmazie 2001, 56, 523–526. [Google Scholar]
- Urleb, U.; Krbavčič, A.; Sollner, M.; Kikelj, D.; Pečar, S. Synthesis of Phthalimido-Desmuramylpeptide Analogues as Potential Immunomodulating Agents. Arch. Pharm. (Weinheim) 1995, 328, 113–117. [Google Scholar] [CrossRef]
- Danklmaier, J.; Hönig, H. Synthesis of Acyclic Analogs of N-Acetylmuramyl-L-alanyl-D-isoglutamine (MDP). Liebigs Ann. Chem. 1990, 145–150. [Google Scholar]
- Ochi, C.; Norisada, N.; Moriguchi, M.; Štalc, A.; Urleb, U.; Muraoka, S. Interleukin-10 Inducing Activity of LK423, a Phthalimido-desmuramyldipeptide Compound. Arzneim.-Forsch./Drug Res. 1999, 49, 72–79. [Google Scholar]
- Moriguchi, M.; Urabe, K.; Norisada, N.; Ochi, C.; Štalc, A.; Urleb, U.; Muraoka, S. Therapeutic Effects of LK 423, a Phthalimido-desmuramyldipeptide Compound, on Dextran Sulphate Sodium-Induced Colitis in Rodents Through Restoring Their Interleukin-10 Producing Capacity. Arzneim.-Forsch./Drug Res. 1999, 49, 184–192. [Google Scholar]
- Simčič, S.; Wraber, B.; Sollner, M.; Urleb, U.; Gobec, S. Modulation of Tumor Necrosis Factor Production With Desmuramyldipeptide Analogues. Pflug. Arch. Eur. J. Phy. 2000, 440, R64–R66. [Google Scholar] [CrossRef]
- Urleb, U.; Gobec, S.; Prelog, D. Synthesis and Activity of Phosphono Desmuramyldipeptide Analogs. Lett. Pept. Sci. 1995, 2, 193–197. [Google Scholar] [CrossRef]
- Gobec, S.; Urleb, U. Synthesis of New Phosphonamidate and Phosphinamide Desmuramyldipeptide Analogs. Lett. Pept. Sci. 1998, 5, 109–114. [Google Scholar]
- Gobec, S.; Urleb, U. Synthesis of Phosphono Phthalimido-desmuramyldipeptide Analogs. Phosphorus, Sulfur, Silicon Relat. Elem. 2000, 156, 125–133. [Google Scholar]
- Vo-Quang, Y.; Gravey, A.M.; Simonneau, R.; Vo-Quang, L.; Lacoste, A.M.; Le Goffic, F. Towards New Inhibitors of D-Alanine: D-Alanine Ligase: The Synthesis of 3-Amino Butenylphosphonic and Aminophosphonamidic Acids. Tetrahedron Lett. 1987, 28, 6167–6170. [Google Scholar] [CrossRef]
- Lefrancier, P.; Bricas, E. Synthese de la subunite peptidique du peptidoglycane de la paroi de trois bacteries gram-positif et de peptides de structure analogue. Bull. Soc. Chim. Biol. 1967, 49, 1257–1271. [Google Scholar] [PubMed]
- Hirschmann, R.; Yager, K.M.; Taylor, C.M.; Witherington, J.; Sprengeler, P.A.; Phillips, B.W.; Moore, W.; Smith, A.B. Phosphonate Diester and Phosphonamide Synthesis. Coordinate Analysis by 31P NMR Spectroscopy: Identification of Pyrophosphonate Anhydrides and Highly Reactive Phosphonylammonium Salts. J. Am. Chem. Soc. 1997, 119, 8177–8190. [Google Scholar] [CrossRef]
- Korošec, E.; Poljšak, D.; Urleb, U. Synthesis of N-Acylamino-ethoxyacetic Acid Derivatives. Arch. Pharm. (Weinheim) 1992, 325, 251–252. [Google Scholar] [CrossRef]
- Campbell, D.; Lentini, D.P. Methods For the Synthesis of Phosphonate Esters. WO Patent 94/06808, 1994. [Google Scholar]
- Lefrancier, P.; Choay, J.; Derrien, M.; Lederman, I. Synthesis of N-Acetyl-muramyl-L-alanine-D-isoglutamine, an Adjuvant of Immune-response, and of Some N-Acetyl-muramyl-peptide Analogs. Int. J. Pept. Protein Res. 1977, 9, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nefkens, G.H.L.; Tesser, G.I.; Nivard, R.J.F. A Simple Preparation of Phthaloyl Amino Acids Via a Mild Phthaloilation. Rec. Trav. Chim. Pays-Bas 1960, 79, 688–698. [Google Scholar] [CrossRef]
- Gobec, S.; Urleb, U.; Auger, G.; Blanot, D. Synthesis and Biochemical Evaluation of Some Novel N-Acyl Phosphono- and Phosphinoalanine Derivatives As Potential Inhibitors of the D-Glutamic Acid-Adding Enzyme. Pharmazie 2001, 56, 295–297. [Google Scholar] [PubMed]
- Jacobsen, N.E.; Bartlett, P.A. A Phosphonamidate Dipeptide Analogue as an Inhibitor of Carboxypeptidase A. J. Am. Chem. Soc. 1981, 103, 654–657. [Google Scholar] [CrossRef]
- McLeod, D.A.; Brinkworth, R.I.; Ashley, J.A.; Janda, K.D.; Wirsching, P. Phosphonamidates and Phosphonamidate Esters as HIV-1 Protease Inhibitors. Bioorg. Med. Chem. Lett. 1991, 1, 653–658. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Haile, W.H.; McGuire, J.J.; Coward, J.K. Mechanism-Based Inhibition of Human Folylpolyglutamate Synthetase: Design, Synthesis, and Biochemical Characterisation of a Phosphapeptide Mimic of a Tetrahedral Intermediate. Arch. Biochem. Biophys. 1998, 355, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Keck, G.E.; Andrus, M.B.; Romer, D.R. A Useful New Enantiomerically Pure Synthon from Malic Acid: Chelation-Controlled Activation as a Route to Regiselectivity. J. Org. Chem. 1991, 56, 417–420. [Google Scholar] [CrossRef]
- Campagne, J.-M.; Coste, J.; Jouin, P. (1H-Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium Hexafluorophosphate-mediated and (1H-Benzotriazol-1-yloxy)tripyrrolidinophosphonium Hexafluorophosphate-mediated Activation of Monophosphonate Esters – Synthesis of Mixed Phosphonate Diesters, the Reactivity of the Benzotriazolyl Phosphonic Estes vs the Reactivity of the Benzotriazolyl Carboxylic Esters. J. Org. Chem. 1995, 60, 5214–5223. [Google Scholar] [CrossRef]
- Sample availability: Samples of compounds 5, 7, 9 and 14 are available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
Share and Cite
Gobec, S.; Urleb, U. Synthesis of New Lipophilic Phosphonate and Phosphonamidate Analogues of N-Acetylmuramyl-L-alanyl-D-isoglutamine Related to LK 423. Molecules 2002, 7, 394-404. https://doi.org/10.3390/70400394
Gobec S, Urleb U. Synthesis of New Lipophilic Phosphonate and Phosphonamidate Analogues of N-Acetylmuramyl-L-alanyl-D-isoglutamine Related to LK 423. Molecules. 2002; 7(4):394-404. https://doi.org/10.3390/70400394
Chicago/Turabian StyleGobec, Stanislav, and Uroš Urleb. 2002. "Synthesis of New Lipophilic Phosphonate and Phosphonamidate Analogues of N-Acetylmuramyl-L-alanyl-D-isoglutamine Related to LK 423" Molecules 7, no. 4: 394-404. https://doi.org/10.3390/70400394
APA StyleGobec, S., & Urleb, U. (2002). Synthesis of New Lipophilic Phosphonate and Phosphonamidate Analogues of N-Acetylmuramyl-L-alanyl-D-isoglutamine Related to LK 423. Molecules, 7(4), 394-404. https://doi.org/10.3390/70400394



