Amino Acid Based Synthesis of Chiral Long Chain Diamines and Tetramines
Abstract
:Introduction
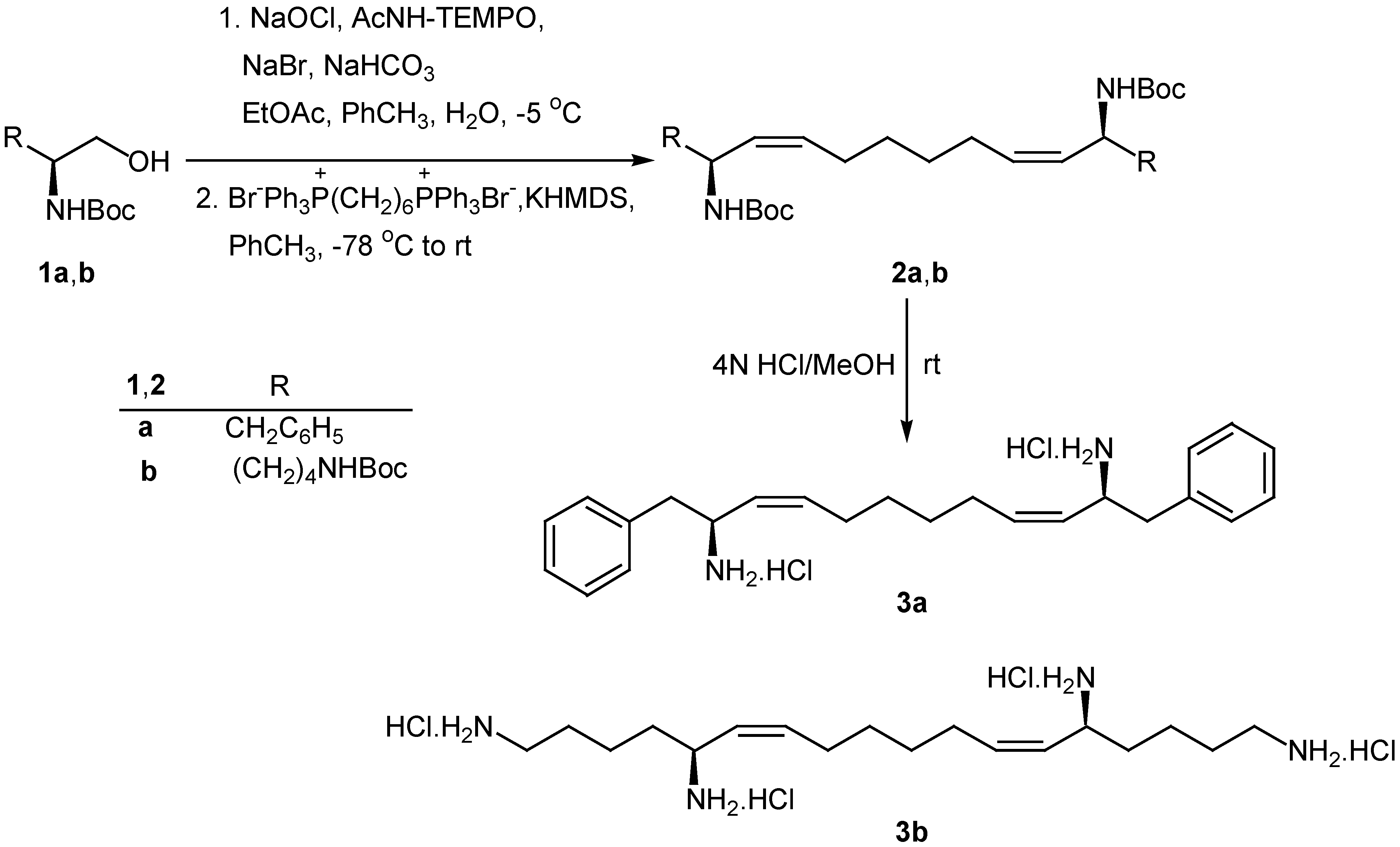
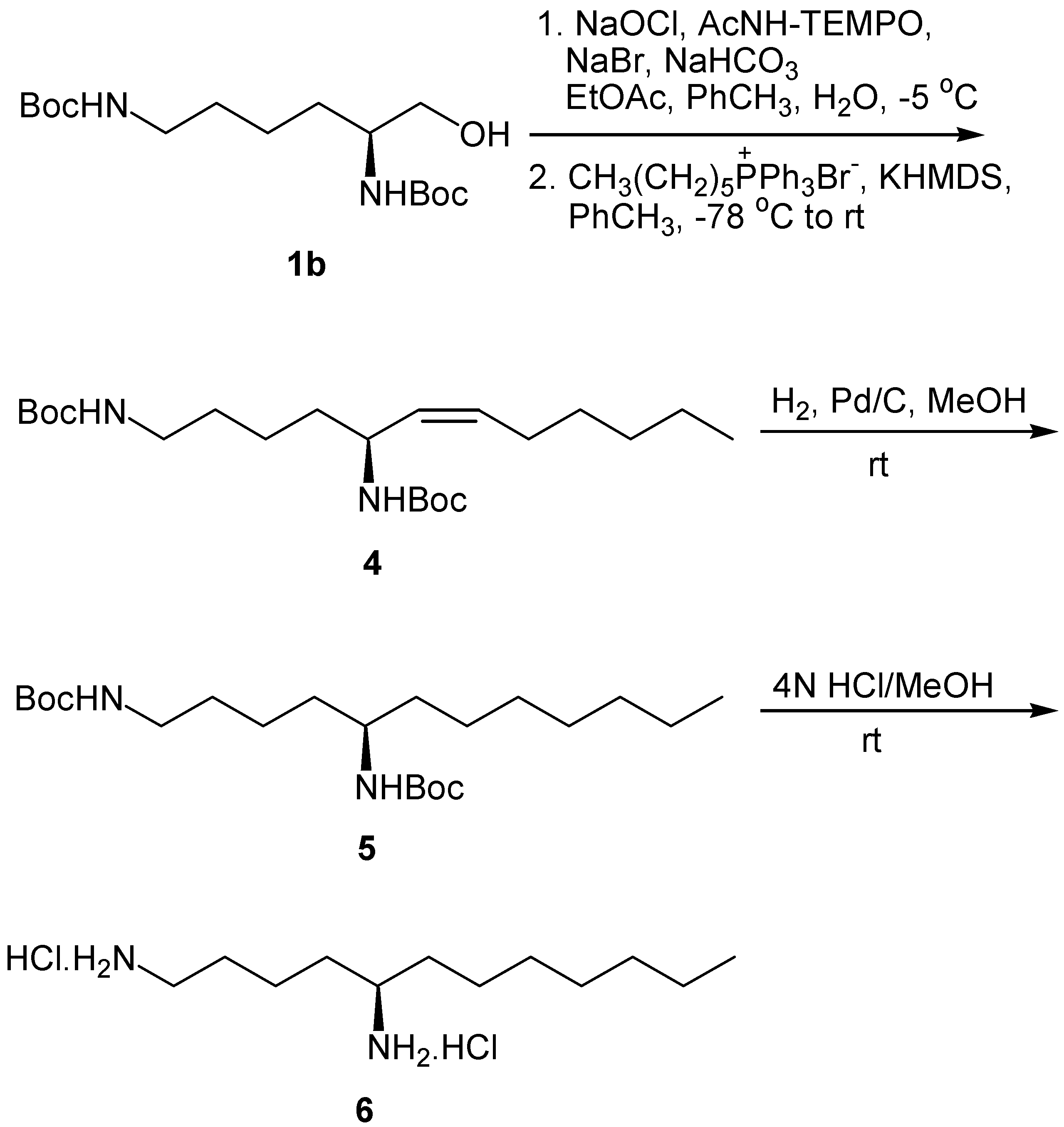
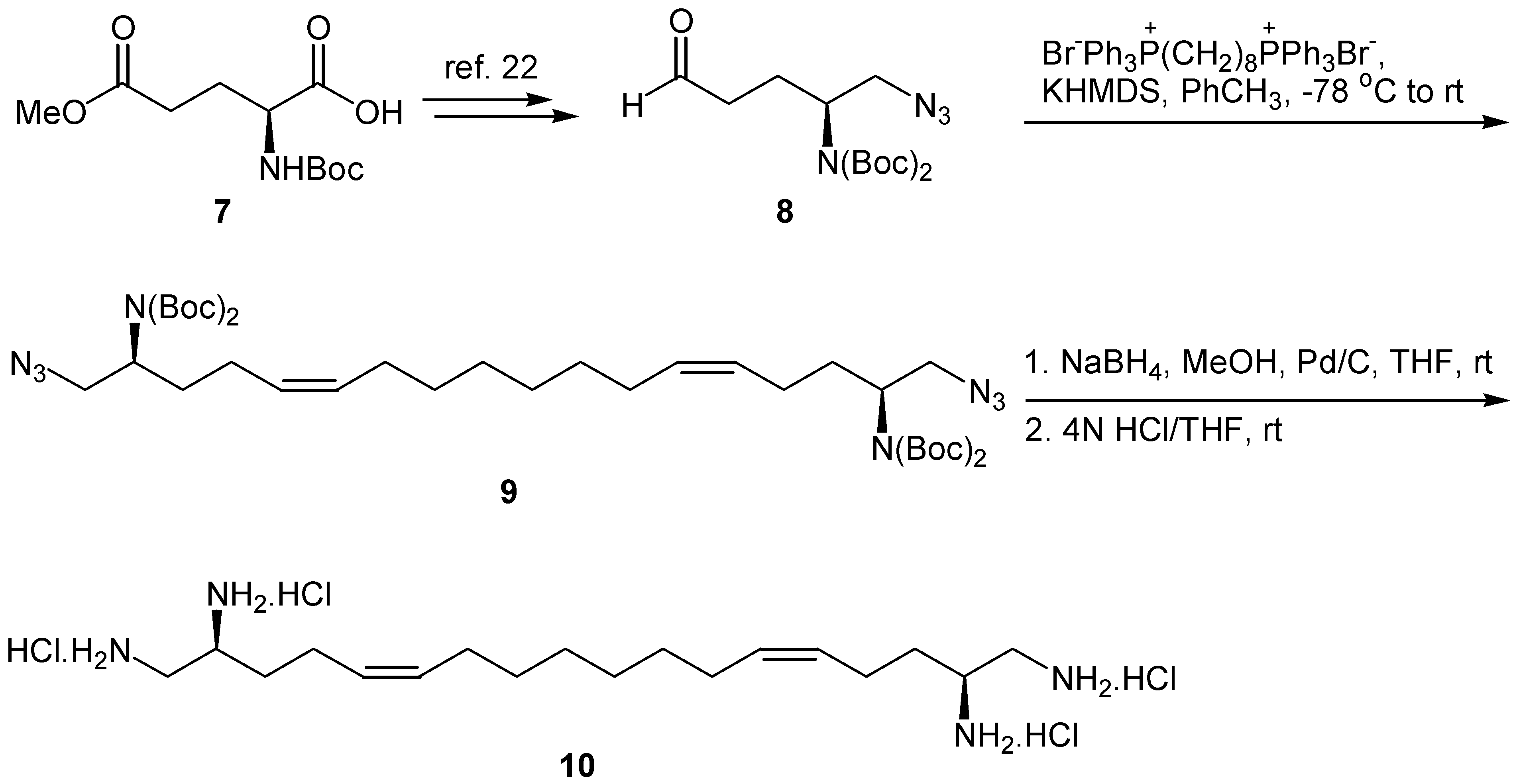
Results and Discussion
Conclusions
Experimental
General
General Procedure for the Preparation of N-Protected Unsaturated Polyamines 2a,b and 4.
 –4.7 (c 0.9, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 0.88–1.25 [m, 4H, innermost (CH2)2], 1.44 [br s, 18H, 2 × (CH3)3], 1.68–2.03 (m, 4H, 2 × CH2CH=CH), 2.69 (dd, 2H, J = 10.2, 12.8 Hz, 2 × CHHC6H5), 2.93 (dd, 2H, J = 4.4, 12.8 Hz, 2 × CHHC6H5), 4.34 (br, 2H, 2 × NH), 4.54 (m, 2H, 2 × CHN), 5.19 (dd, 2H, J = 10.0, 10.8 Hz, 2 × CH=CHCH2), 5.36 (dt, 2H, J = 7.7, 10.0 Hz, 2 × CH=CHCH2), 7.12–7.33 (m, 10H, 2 × C6H5); 13C‑NMR (50 MHz, CDCl3) δ 27.6, 28.4, 28.8, 42.1, 49.3, 79.2, 126.3, 128.1, 128.9, 129.7, 132.6, 137.7, 155.0; Anal. Calcd for C34H48N2O4 (548.77): C, 74.42; H, 8.82; N, 5.10. Found: C, 74.71; H, 8.91; N, 4.85.
–4.7 (c 0.9, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 0.88–1.25 [m, 4H, innermost (CH2)2], 1.44 [br s, 18H, 2 × (CH3)3], 1.68–2.03 (m, 4H, 2 × CH2CH=CH), 2.69 (dd, 2H, J = 10.2, 12.8 Hz, 2 × CHHC6H5), 2.93 (dd, 2H, J = 4.4, 12.8 Hz, 2 × CHHC6H5), 4.34 (br, 2H, 2 × NH), 4.54 (m, 2H, 2 × CHN), 5.19 (dd, 2H, J = 10.0, 10.8 Hz, 2 × CH=CHCH2), 5.36 (dt, 2H, J = 7.7, 10.0 Hz, 2 × CH=CHCH2), 7.12–7.33 (m, 10H, 2 × C6H5); 13C‑NMR (50 MHz, CDCl3) δ 27.6, 28.4, 28.8, 42.1, 49.3, 79.2, 126.3, 128.1, 128.9, 129.7, 132.6, 137.7, 155.0; Anal. Calcd for C34H48N2O4 (548.77): C, 74.42; H, 8.82; N, 5.10. Found: C, 74.71; H, 8.91; N, 4.85. +0.5 (c 1.0, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 1.05–1.68 [m, 52H, 4 × C(CH3)3, innermost (CH2)2, 2 × (CH2)3CHN], 1.89–2.17 (m, 4H, 2 × CH2CH=CH), 3.06 (dt, 4H, J = 6.4, 6.6 Hz, 2 × CH2N), 4.27 (m, 2H, 2 × CHN), 4.52 (br, 2H, 2 × CHNH), 4.67 (br, 2H, 2 × CH2NH), 5.13 (dd, 2H, J = 10.2, 10.6 Hz, 2 × CH=CHCH2), 5.40 (dt, 2H, J = 7.4, 10.2 Hz, 2 × CH=CHCH2); 13C-NMR (50 MHz, CDCl3) δ 22.8, 27.6, 28.3, 29.0, 29.7, 35.8, 40.3, 47.6, 79.0, 130.4, 132.2, 155.2, 156.0. Anal. Calcd for C38H70N4O8 (711.00): C, 64.19; H, 9.92; N, 7.88. Found: C, 64.38; H, 9.81; N, 7.98.
+0.5 (c 1.0, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 1.05–1.68 [m, 52H, 4 × C(CH3)3, innermost (CH2)2, 2 × (CH2)3CHN], 1.89–2.17 (m, 4H, 2 × CH2CH=CH), 3.06 (dt, 4H, J = 6.4, 6.6 Hz, 2 × CH2N), 4.27 (m, 2H, 2 × CHN), 4.52 (br, 2H, 2 × CHNH), 4.67 (br, 2H, 2 × CH2NH), 5.13 (dd, 2H, J = 10.2, 10.6 Hz, 2 × CH=CHCH2), 5.40 (dt, 2H, J = 7.4, 10.2 Hz, 2 × CH=CHCH2); 13C-NMR (50 MHz, CDCl3) δ 22.8, 27.6, 28.3, 29.0, 29.7, 35.8, 40.3, 47.6, 79.0, 130.4, 132.2, 155.2, 156.0. Anal. Calcd for C38H70N4O8 (711.00): C, 64.19; H, 9.92; N, 7.88. Found: C, 64.38; H, 9.81; N, 7.98. +4.3 (c 1.1, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 0.87 (t, 3H, J = 6.6 Hz, CH3), 1.18–1.62 [m, 30H, 2 × (CH3)3, (CH2)3CH3, (CH2)3CHN], 2.02–2.18 (m, 2H, CH2CH=CH), 3.08 (dt, 2H, J = 6.4, 6.6 Hz, CH2N), 4.22–4.47 (m, 2H, NH, CH), 4.55 (br, 1H, NH), 5.14 (dd, 1H, J = 10.0, 10.8 Hz, CHCH=CH), 5.44 (dt, J = 7.7, 10.0 Hz, CHCH=CH); 13C-NMR (50 MHz, CDCl3) δ 14.0, 22.3, 22.8, 27.8, 28.3, 28.4, 29.2, 29.7, 31.1, 35.9, 40.3, 47.6, 79.0, 130.2, 132.6, 155.2, 156.0. Anal. Calcd for C22H42N2O4 (398.59): C, 66.29; H, 10.62; N, 7.03. Found: C, 66.57; H, 10.73; N, 6.80.
+4.3 (c 1.1, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 0.87 (t, 3H, J = 6.6 Hz, CH3), 1.18–1.62 [m, 30H, 2 × (CH3)3, (CH2)3CH3, (CH2)3CHN], 2.02–2.18 (m, 2H, CH2CH=CH), 3.08 (dt, 2H, J = 6.4, 6.6 Hz, CH2N), 4.22–4.47 (m, 2H, NH, CH), 4.55 (br, 1H, NH), 5.14 (dd, 1H, J = 10.0, 10.8 Hz, CHCH=CH), 5.44 (dt, J = 7.7, 10.0 Hz, CHCH=CH); 13C-NMR (50 MHz, CDCl3) δ 14.0, 22.3, 22.8, 27.8, 28.3, 28.4, 29.2, 29.7, 31.1, 35.9, 40.3, 47.6, 79.0, 130.2, 132.6, 155.2, 156.0. Anal. Calcd for C22H42N2O4 (398.59): C, 66.29; H, 10.62; N, 7.03. Found: C, 66.57; H, 10.73; N, 6.80.General Procedure for the Preparation of Free Unsaturated Polyamines 3a,b and 6.
 +1.9 (c 0.6, EtOH); 1H-NMR (200 MHz, CD3OD) δ 0.76–1.08 [m, 4H, innermost (CH2)2], 1.50–1.92 (m, 4H, 2 × CH2CH=CH), 2.76 (dd, 2H, J = 10.2, 12.9 Hz, 2 × CHHPh), 3.14 (dd, 2H, J = 4.4, 12.9 Hz, 2 × CHHPh), 4.20 (m, 2H, 2 × CHN), 5.32 (dd, 2H, J = 10.0, 10.8 Hz, 2 × CHCH=CH), 5.56 (dt, 2H, J = 7.7, 10.0 Hz, 2 × CHCH=CH), 7.13–7.47 (m, 10H, 2 × C6H5); 13C‑NMR (50 MHz, CD3OD) δ 28.4, 29.4, 40.8, 51.4, 125.2, 128.4, 130.0, 131.0, 137.1, 138.6; MS (FAB) m/z (%): 349 (M++1, 100), 332 (28), 240 (12). Anal. Calcd for C24Cl2H34N2 (421.46): C, 68.40; H, 8.13; N, 6.65. Found: C, 68.19; H, 8.25; N, 6.43.
+1.9 (c 0.6, EtOH); 1H-NMR (200 MHz, CD3OD) δ 0.76–1.08 [m, 4H, innermost (CH2)2], 1.50–1.92 (m, 4H, 2 × CH2CH=CH), 2.76 (dd, 2H, J = 10.2, 12.9 Hz, 2 × CHHPh), 3.14 (dd, 2H, J = 4.4, 12.9 Hz, 2 × CHHPh), 4.20 (m, 2H, 2 × CHN), 5.32 (dd, 2H, J = 10.0, 10.8 Hz, 2 × CHCH=CH), 5.56 (dt, 2H, J = 7.7, 10.0 Hz, 2 × CHCH=CH), 7.13–7.47 (m, 10H, 2 × C6H5); 13C‑NMR (50 MHz, CD3OD) δ 28.4, 29.4, 40.8, 51.4, 125.2, 128.4, 130.0, 131.0, 137.1, 138.6; MS (FAB) m/z (%): 349 (M++1, 100), 332 (28), 240 (12). Anal. Calcd for C24Cl2H34N2 (421.46): C, 68.40; H, 8.13; N, 6.65. Found: C, 68.19; H, 8.25; N, 6.43. +2.4 (c 2.5, EtOH); 1H-NMR (200 MHz, CD3OD) δ 1.47 [br, 8H, innermost (CH2)2, 2 × CH2CH2CHN], 1.55–1.97 (m, 8H, 2 × CH2CHN, 2 × CH2CH2N), 2.19 (br, 4Η, 2 × CH2CH=CH), 2.94 (t, 4H, J = 7.2 Hz, 2 × CH2N), 4.10 (br, 2H, 2 × CHN), 5.35 (dd, 2H, J = 10.2, 10.6 Hz, 2 × CHCH=CH), 5.82 (dt, 2H, J = 7.4, 10.2 Hz, 2 × CHCH=CH); 13C-NMR (50 MHz, CD3OD) δ 22.4, 27.0, 27.6, 29.0, 32.9, 39.3, 53.7, 124.9, 137.5; MS (FAB) m/z (%): 311 (M++1, 100), 277 (18). Anal. Calcd for C18Cl4H42N4.H2O (474.39): C, 45.57; H, 9.35; N, 11.81. Found: C, 45.31; H, 9.54; N, 11.70.
+2.4 (c 2.5, EtOH); 1H-NMR (200 MHz, CD3OD) δ 1.47 [br, 8H, innermost (CH2)2, 2 × CH2CH2CHN], 1.55–1.97 (m, 8H, 2 × CH2CHN, 2 × CH2CH2N), 2.19 (br, 4Η, 2 × CH2CH=CH), 2.94 (t, 4H, J = 7.2 Hz, 2 × CH2N), 4.10 (br, 2H, 2 × CHN), 5.35 (dd, 2H, J = 10.2, 10.6 Hz, 2 × CHCH=CH), 5.82 (dt, 2H, J = 7.4, 10.2 Hz, 2 × CHCH=CH); 13C-NMR (50 MHz, CD3OD) δ 22.4, 27.0, 27.6, 29.0, 32.9, 39.3, 53.7, 124.9, 137.5; MS (FAB) m/z (%): 311 (M++1, 100), 277 (18). Anal. Calcd for C18Cl4H42N4.H2O (474.39): C, 45.57; H, 9.35; N, 11.81. Found: C, 45.31; H, 9.54; N, 11.70. ‑1.3 (c 0.8, EtOH); 1H-NMR (200 MHz, CD3OD) δ 0.90 (t, 3H, J = 6.4 Hz, CH3), 1.19–1.82 [m, 18H, (CH2)6, (CH2)3CH], 2.96 (t, 2H, J = 7.6 Hz, CH2N), 3.18 (m, 1H, CH); 13C-NMR (50 MHz, CD3OD) δ 14.4, 23.2, 23.7, 26.1, 28.3, 30.2, 30.5, 32.9, 33.2, 33.6, 40.4, 52.9. Anal. Calcd for C12Cl2H30N2 (273.29): C, 52.74; H, 11.07; N; 10.25. Found: C, 52.51; H, 11.33; N, 10.09.
‑1.3 (c 0.8, EtOH); 1H-NMR (200 MHz, CD3OD) δ 0.90 (t, 3H, J = 6.4 Hz, CH3), 1.19–1.82 [m, 18H, (CH2)6, (CH2)3CH], 2.96 (t, 2H, J = 7.6 Hz, CH2N), 3.18 (m, 1H, CH); 13C-NMR (50 MHz, CD3OD) δ 14.4, 23.2, 23.7, 26.1, 28.3, 30.2, 30.5, 32.9, 33.2, 33.6, 40.4, 52.9. Anal. Calcd for C12Cl2H30N2 (273.29): C, 52.74; H, 11.07; N; 10.25. Found: C, 52.51; H, 11.33; N, 10.09.(5R)-1,5-Di[(tert-butoxycarbonyl)amino]-dodecane (5)
 –2.3 (c 1.0, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 0.85 (t, 3H, J = 6.4 Hz, CH3), 1.15–1.62 [m, 18H, 2 × (CH3)3], 3.08 (dt, 2H, J = 6.4, 6.6 Hz, CH2N), 3.37–3.61 (m, 1H, CH2NH), 4.28 (d, 1H, J = 9.2 Hz, CHNH), 4.64 (br, 1H, CH); 13C-NMR (50 MHz, CDCl3) δ 14.0, 22.6, 23.0, 25.8, 28.3, 28.4, 29.2, 29.5, 29.7, 31.7, 35.2, 35.6, 40.3, 50.3, 78.8, 155.8, 156.0; Anal. Calcd for C22H44N2O4 (400.60): C, 65.96; H, 11.07; N; 6.99. Found: C, 65.89; H, 11.01; N, 7.03.
–2.3 (c 1.0, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 0.85 (t, 3H, J = 6.4 Hz, CH3), 1.15–1.62 [m, 18H, 2 × (CH3)3], 3.08 (dt, 2H, J = 6.4, 6.6 Hz, CH2N), 3.37–3.61 (m, 1H, CH2NH), 4.28 (d, 1H, J = 9.2 Hz, CHNH), 4.64 (br, 1H, CH); 13C-NMR (50 MHz, CDCl3) δ 14.0, 22.6, 23.0, 25.8, 28.3, 28.4, 29.2, 29.5, 29.7, 31.7, 35.2, 35.6, 40.3, 50.3, 78.8, 155.8, 156.0; Anal. Calcd for C22H44N2O4 (400.60): C, 65.96; H, 11.07; N; 6.99. Found: C, 65.89; H, 11.01; N, 7.03.(2S,17S,5Z,13Z))-1,18-Diazido-2,17-di[bis(tert-butoxycarbonyl)amino]-octadeca-5,13-diene (9)
 = –3.5 (c 1.2, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 1.28 [br s, 8H, (CH2)4], 1.43–1.65 [m, 38H, 4 × C(CH3)3, 2 × CHHCHN], 1.74–1.90 (m, 2H, 2 × CHHCHN), 1.91–2.13 (m, 8H, 2 × CH2CH=CHCH2), 3.29 (dd, 2H, J = 12.2, 5.6 Hz, 2 × CHHN3), 3.76 (dd, 2H, J = 12.2, 9.4, 2 × CHHN3), 4.35 (m, 2H, 2 × CHN), 5.23–5.46 (m, 4H, 2 × CH=CH); 13C‑NMR (50 MHz, CDCl3) δ 24.0, 27.2, 28.0, 29.3, 29.7, 30.2, 53.6, 56.6, 82.5, 128.0, 131.1, 153.2; MS (FAB) m/z (%): 785 (M++Na, 100), 736 (8). Anal. Calcd for C38H66N8O8 (762.99): C, 59.82; H, 8.72; N; 14.69. Found: C, 59.56; H, 8.99; N, 14.63.
= –3.5 (c 1.2, CHCl3); 1H-NMR (200 MHz, CDCl3) δ 1.28 [br s, 8H, (CH2)4], 1.43–1.65 [m, 38H, 4 × C(CH3)3, 2 × CHHCHN], 1.74–1.90 (m, 2H, 2 × CHHCHN), 1.91–2.13 (m, 8H, 2 × CH2CH=CHCH2), 3.29 (dd, 2H, J = 12.2, 5.6 Hz, 2 × CHHN3), 3.76 (dd, 2H, J = 12.2, 9.4, 2 × CHHN3), 4.35 (m, 2H, 2 × CHN), 5.23–5.46 (m, 4H, 2 × CH=CH); 13C‑NMR (50 MHz, CDCl3) δ 24.0, 27.2, 28.0, 29.3, 29.7, 30.2, 53.6, 56.6, 82.5, 128.0, 131.1, 153.2; MS (FAB) m/z (%): 785 (M++Na, 100), 736 (8). Anal. Calcd for C38H66N8O8 (762.99): C, 59.82; H, 8.72; N; 14.69. Found: C, 59.56; H, 8.99; N, 14.63.(2S,17S,5Z,13Z)-Octadeca-5,13-diene-1,2,17,18-tetramine tetrahydrochloride (10)
Acknowledgements
References
- For reviews see: (a) Kuksa, V.; Buchan, R.; Lin, P. K. T. Synthesis of polyamines, their derivatives, analogues and conjugates. Synthesis 2000, 1189–1207. [Google Scholar] (b) Karigiannis, G.; Papaioannou, D. Structure, biological activity and synthesis of polyamine analogues and conjugates. Eur. J. Org. Chem. 2000, 1841–1863. [Google Scholar]
- Lucet, D.; Le Gall, T.; Mioskowski, C. The chemistry of vicinal diamines. Angew. Chem., Int. Ed. 1998, 37, 2580–2627. [Google Scholar]
- Donner, B. G. Conversion of chiral amino acids to enantiomerically pure α-methylamines. Tetrahedron Lett. 1995, 36, 1223–1226. [Google Scholar]
- Kokotos, G.; Markidis, T.; Constantinou-Kokotou, V. Synthesis of chiral triamines and diamines from amino acids. Synthesis 1996, 1223–1226. [Google Scholar]
- Gurjar, M. K.; Pal, S.; Rao, A. V. R. Synthesis of novel C2-symmetric and pseudo C2-symmetric based diols, epoxides and dideoxy derivatives of HIV protease inhibitors. Tetrahedron 1997, 53, 4769–4778. [Google Scholar]
- Yamauchi, T.; Higashiyama, K.; Kubo, H.; Ohmiya, S. Synthesis of C2-symmetrical chiral diamines: diastereoselective addition to bis(1,3-oxazolidinyl)alkanes with Grignard reagents. Tetrahedron: Asymmetry 2000, 11, 3003–3015. [Google Scholar]
- Mamos, P.; Karigiannis, G.; Athanassopoulos, C.; Bichta, S.; Kalpaxis, D.; Papaioannou, D.; Sindona, G. Simple total syntheses of N-substituted polyamine derivatives using N-tritylamino acids. Tetrahedron Lett. 1995, 36, 5187–5190. [Google Scholar]
- Kokotos, G.; Constantinou-Kokotou, V.; Noula, C.; Hadjipavlou-Litina, D. Synthetic routes to lipidic diamines and amino alcohols: a class of potential antiinflammatory agents. Lipids 1999, 34, 307–311. [Google Scholar]
- Kokotos, G.; Theodorou, V.; Constantinou-Kokotou, V.; Gibbons, W. A.; Roussakis, C. Synthesis and in vitro cytotoxicity of lipophilic platinum(II) complexes. Bioorg. Med. Chem. Lett. 1998, 8, 1525–1530. [Google Scholar]
- Constantinou-Kokotou, V.; Kokotos, G.; Roussakis, C. Synthesis and in vitro cytotoxicity of lipidic alcohols and amines. Anticancer Res. 1998, 18, 3439–3442. [Google Scholar]
- Markidis, T.; Padrón, J. M.; Martín, V. S.; Peters, G. J.; Kokotos, G. Synthesis and in vitro cytotoxicity of long chain 2-amino alcohols and 1,2-diamines. Anticancer Res. 2001, 21, 2835–2840. [Google Scholar]
- Karikas, G.; Constantinou-Kokotou, V.; Kokotos, G. An HPLC method for the measurement of polyamines and lipidic amines binding to DNA. J. Liq. Chrom. & Rel. Technol. 1997, 20, 1789–1796. [Google Scholar]
- Constantinou-Kokotou, V.; Karikas, G.; Kokotos, G. Study of aminoglycoside-nucleic acid interactions by an HPLC method. Bioorg. Med. Chem. Lett. 2001, 11, 1015–1018. [Google Scholar]
- For reviews see: (a) Jurczak, J.; Golebiowski, A. Optically active N-protected α-amino aldehydes in organic synthesis. Chem. Rev. 1989, 89, 149–164. [Google Scholar] (b) Reetz, M. T. Synthesis and diastereoselective reactions of N,N-dibenzylamino aldehydes and related compounds. Chem. Rev. 1999, 99, 1121–1162. [Google Scholar]
- Jurczak, J.; Gryko, D.; Kobrzycka, E.; Gruza, H.; Prokopowicz, P. Effective and mild method for preparation of optically active α-amino aldehydes via TEMPO oxidation. Tetrahedron 1998, 54, 6051–6064. [Google Scholar]
- Kokotos, G. A convenient one-pot conversion of N-protected amino acids and peptides into alcohols. Synthesis 1990, 299–301. [Google Scholar]
- Kokotos, G.; Noula, C. Selective one-pot conversion of carboxylic acids into alcohols. J. Org. Chem. 1996, 61, 6994–6996. [Google Scholar]
- Ma, Z.; Bobbitt, J. M. Organic oxoammonium salts. 3. A new convenient method for the oxidation of alcohols to aldehydes and ketones. J. Org. Chem. 1991, 56, 6110–6114. [Google Scholar]
- Leanna, M. R.; Sowin, T. J.; Morton, H. E. Synthesis of α-amino and α-alkoxy aldehydes via oxoammonium oxidation. Tetrahedron Lett. 1992, 33, 5029–5032. [Google Scholar]
- Schlosser, M.; Schaub, B.; de Oliveira-Neto, J.; Jeganathan, S. Practical guidance for obtaining optimum cis-selectivities in Wittig reactions with triphenylphosphonio-alkanides. Chimia 1986, 40, 244–245. [Google Scholar]
- Maryanoff, B. E.; Reitz, A. B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 1989, 89, 863–927. [Google Scholar]
- Markidis, T.; Kokotos, G. A novel approach to the synthesis of chiral terminal 1,2-diamines. J. Org. Chem. 2001, 66, 1919–1923. [Google Scholar]
- Dawson, M. I.; Vasser, M. Synthesis of prostaglandin synthetase substrate analogues. 1. (Z)-14-Hydroxy-12,13-methano-8-nonadecenoic acid. J. Org. Chem. 1977, 42, 2783–2785. [Google Scholar]
- For synthesis of racemic 1,5-bis-p-tolouenesulfonylamino-dodecane see: Wiesner, K.; Valenda, Z.; Orr, D. E.; Liede, V.; Kohan, G. Stucture of pithecolobine. III. The synthesis of the 1,5- and 1,3-deoxypithecolobines. Can. J. Chem. 1968, 46, 3617–3624. [Google Scholar]
- Sample Availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Loukas, V.; Markidis, T.; Kokotos, G. Amino Acid Based Synthesis of Chiral Long Chain Diamines and Tetramines. Molecules 2002, 7, 767-776. https://doi.org/10.3390/71000767
Loukas V, Markidis T, Kokotos G. Amino Acid Based Synthesis of Chiral Long Chain Diamines and Tetramines. Molecules. 2002; 7(10):767-776. https://doi.org/10.3390/71000767
Chicago/Turabian StyleLoukas, Vassilios, Theodoros Markidis, and George Kokotos. 2002. "Amino Acid Based Synthesis of Chiral Long Chain Diamines and Tetramines" Molecules 7, no. 10: 767-776. https://doi.org/10.3390/71000767



