ElectronTransfer Induced Ring Opening of α-Epoxyketones: Spirodioxolane Formation
Abstract
:Introduction
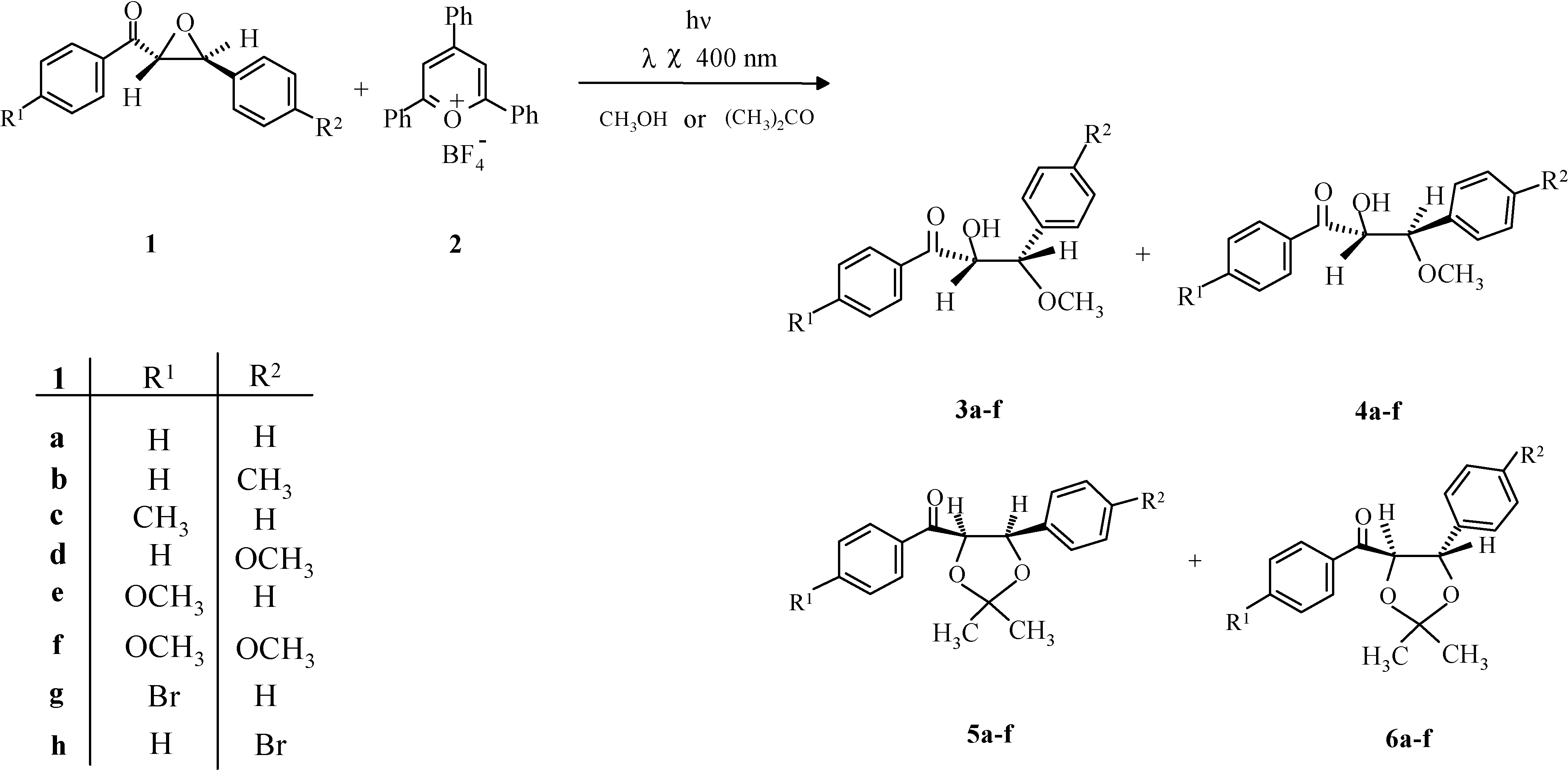
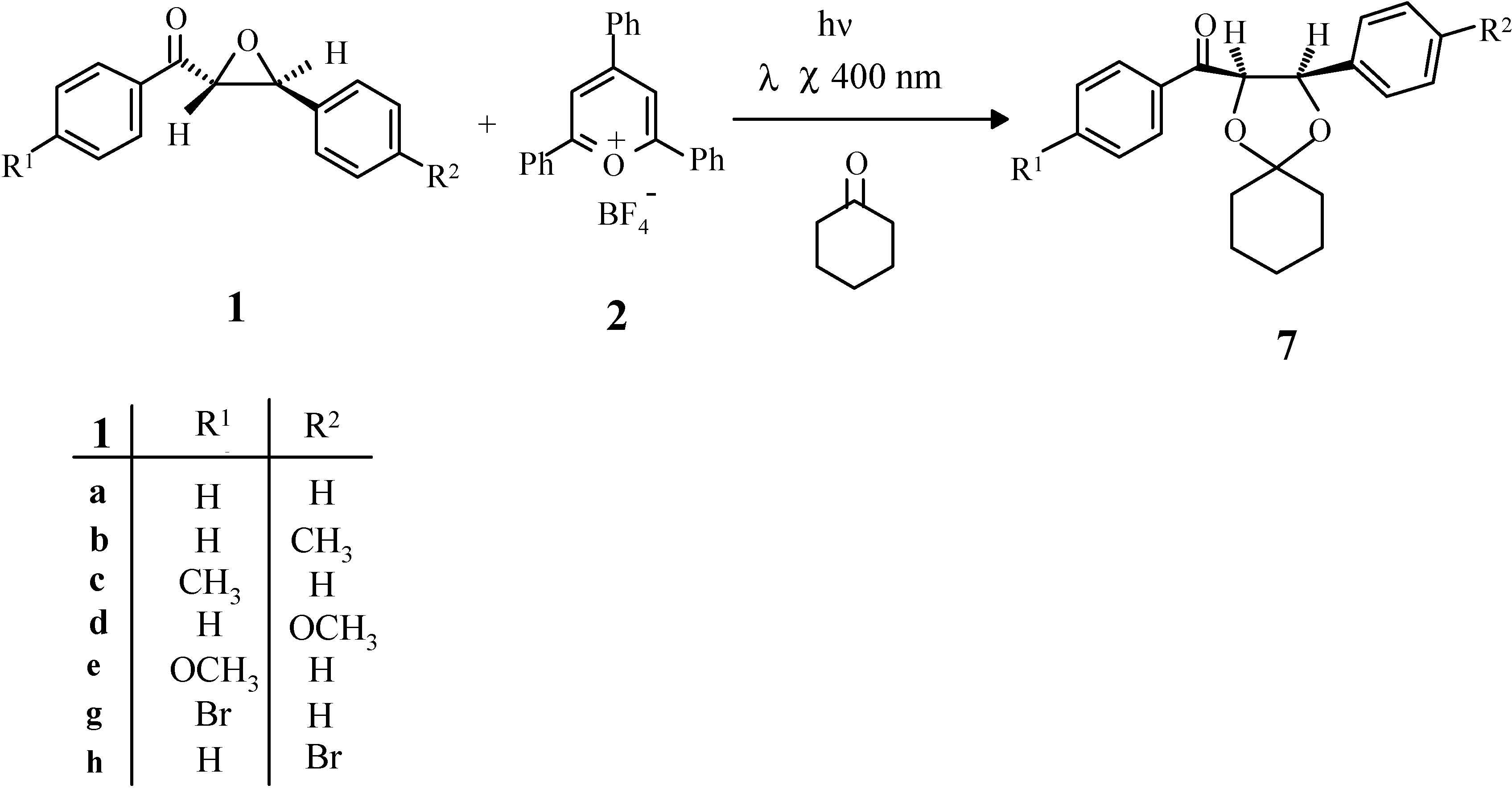
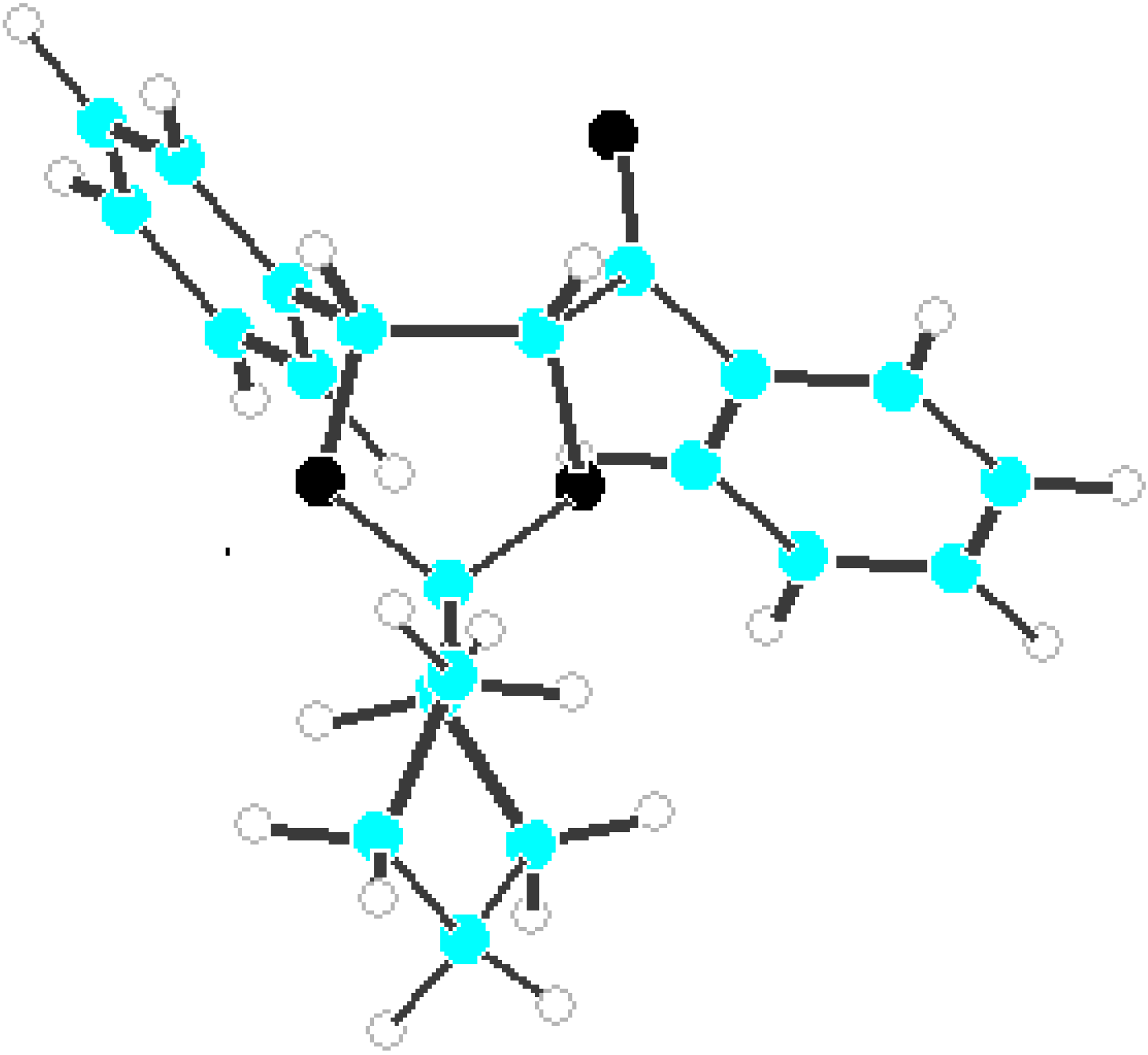
Results and discussion
| Compd. | CO (cm-1) | Prod. | CO (cm-1) |
| 1a | 1670 | 7a | 1695 |
| 1b | 1660 | 7b | 1680 |
| 1c | 1665 | 7c | 1685 |
| 1d | 1660 | 7d | 1690 |
| 1e | 1665 | 7e | 1680 |
| 1g | 1670 | 7g | 1690 |
| Compd. | δA 4-H | δB 3-H | JAB |
| 7a | 5.52 | 5.80 | 8.0 |
| 7b | 5.46 | 5.78 | 7.6 |
| 7c | 5.49 | 5.74 | 7.6 |
| 7d | 5.59 | 5.77 | 7.6 |
| 7e | 5.50 | 5.74 | 7.4 |
| 7g | 5.60 | 5.70 | 7.4 |
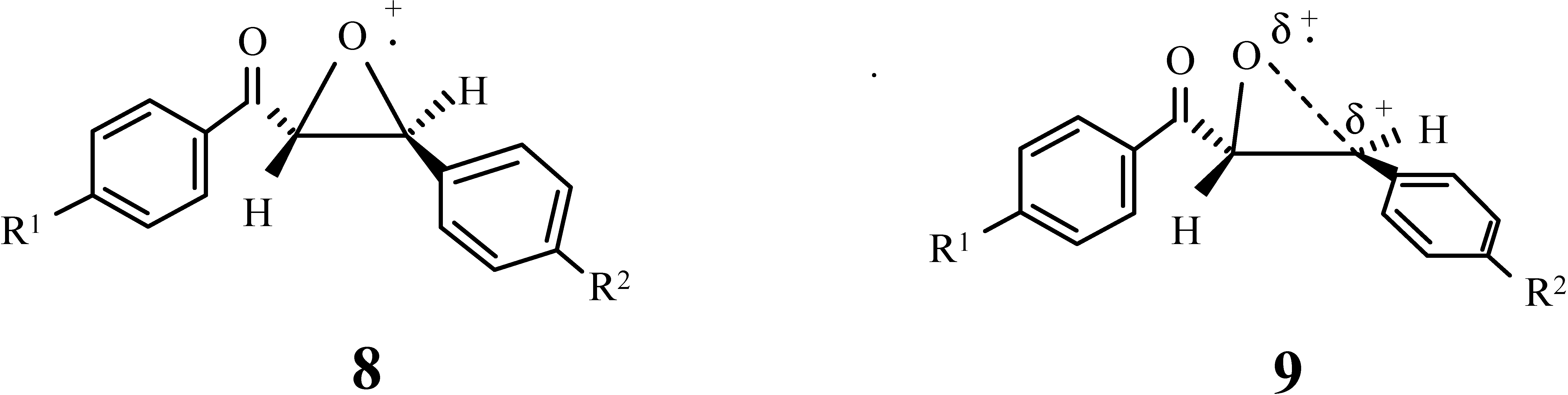
- Irradiation of 1a-e and 1g in the absence of the photocatalyst 2 at λ ≥ 400 nm (blank experiment) does not result the formation of any product.
- Upon irradiation of 1h and 2 in the presence of cyclohexanone unchanged starting materials were obtained even after 15 hours irradiation. This indicates that the formation of 8 or 9 will be destabilized by the electron withdrawing character of the bromine on the phenyl ring directly attached to the epoxide ring. As we show in Table 3, the ring opening will be facilitated, when the phenyl group bears the electron donor group such as methyl or methoxy group (1b, 1d).
| Compd. | Irrad. Timeb (hr) | Product | Yield %c |
| 1a | 5 | 7a | 27 |
| 1b | 2.5 | 7b | 31 |
| 1c | 5 | 7c | 26 |
| 1d | 2 | 7d | 33 |
| 1e | 5 | 7e | 30 |
| 1g | 15 | 7g | 24 |
Acknowledgments
Experimental
General
General experimental procedure for the conversions of 1a-e, 1g-h by photoexcited 2 in the presence of cyclohexanone.
References
- Schaap, A.P.; Siddiqui, S.; Prasad, G.; Maqsudur Rahman, A.F.M.; Oliver, J.P. Stereoselective Formation of Cis Ozonides by Electron-Transfer Photooxygenation of Naphthyl-Substituted Epoxides. Stereochemical Assignments of Ozonides by X-ray Crystallography and Chromatographic Resolution. J. Am. Chem. Soc. 1984, 106, 6087–6088. [Google Scholar] [CrossRef]
- Futamura, S.; Kusunose, S.; Ohta, H.; Kamiya, J. Photoinduced Electron Transfer Reaction. Part 3. 9,10-Dicyanoanthracene-sensitized Photo-oxidation of Electron-rich Stilbene Oxides. J. Chem. Soc. Perkin Trans 1 1984, 15–19. [Google Scholar]
- Miyashi, T.; Kamata, M.; Mukai, T. Simultaneous Capture of Two Distinct Radical Ion Intermediates Generated from the EDA Complexes of Three-Membered Compounds with TCNE by Photoexcitation and in the Dark. J. Am. Chem. Soc. 1987, 109, 2780–2788. [Google Scholar] [CrossRef]
- Masaki, Y.; Miura, T.; Ochiai, M. Alcoholysis of Epoxides Catalyzed by Tetracyanoethylene and Dicyanoketene Acetals. Bull. Chem. Soc. Jpn. 1996, 69, 195–205. [Google Scholar] [CrossRef]
- Hasegawa, E.; Ishiyama, K.; Kashiwazaki, H.; Horaguchi, T.; Shimizu, T. Selective Cβ-O Bond Cleavage of Chalcone Epoxides Induced by Pyrilium Salt Sensitized Photoreactions and Dark Reactions with Cerium(IV) Salts. Tetrahedron Lett. 1990, 31, 4045–4048. [Google Scholar] [CrossRef]
- Hasegawa, E.; Ishiyama, K.; Horaguchi, T.; Shimizu, T. Selective Cα-O Bond Cleavage of α,β-Epoxy Ketones to Aldols induced by Free Radical processes. J. Chem. Soc. Chem. Commun. 1990, 550–552. [Google Scholar] [CrossRef]
- Hasegawa, E.; Ishiyama, K.; Kato, T.; Horaguchi, T.; Shimizu, T.; Tanaka, S.; Yamashita, Y. Photochemically and Thermally Induced Free-Radical Reactions of α,β-Epoxy Ketones with Tributyltin Hydride: Selective Cα-O Bond Cleavage of Oxiranylmethyl Radicals Derived from α,β-Epoxy Ketones. J. Org. Chem. 1992, 57, 5352–5359. [Google Scholar] [CrossRef]
- Memarian, H.R.; Hesami, A.; Nikpour, F.; Döpp, D. Effect of Substituent on Photoinduced Ring Opening of α-Epoxyketones by 2,4,6-Triphenylpyrylim Tetrafluoroborate (TPT). Indian J. Chem. Sec. B. 2001, 40B, 662–666. [Google Scholar]
- Kagan, J.; Juang, P.Y.; Firth, B.E.; Przybytek, J.T.; Singh, S.P. Catalysis of the Ionic-like Photoaddition of Methanol to Epoxides by Fe(III). Tetrahedron Lett. 1977, 4289–4290. [Google Scholar] [CrossRef]
- Iranpoor, N.; Mohammadpour-Baltork, I. Mild, Efficient and Selective Opening of Epoxides with Alcohols Catalyzed by Ceric (IV) Ammonium Nitrate. Synth. Commun. 1990, 20, 2789–2797. [Google Scholar] [CrossRef]
- Iranpoor, N.; Mohammadpour-Baltork, I.; Shiriny Zardaloo, F. Ceric Ammonium Nitrate, an Efficient Catalyst for Mild and Selective Opening of Epoxides in the Presence of Water, Thiols and Acetic acid. Tetrahedron 1991, 47, 9861–9866. [Google Scholar] [CrossRef]
- Iranpoor, N.; Mohammadpour-Baltork, I. 2,3-Dichloro-5,6-dicyano-p-benzoquinone, an Efficcient, Mild, Neutral and Highly Regioselective Catalyst for Alcoholysis of Epoxides. Tetrahedron Lett. 1990, 31, 735–738. [Google Scholar] [CrossRef]
- Iranpoor, N.; Salehi, P. Highly Efficient, Regio-and Stereoselective Alcoholysis of Epoxides Catalyzed with Iron(III) Chloride. Synthesis 1994, 1152–1154. [Google Scholar]
- Memarian, H.R.; Amini, M.K.; Nikjah, S. (unpublished results).
- Memarian, H.R.; Nikpour, F. Photocatalytic Ring Opening of α-Epoxyketones: 1,3-Dioxolane Formation. Chem. Mon. Accepted.
- Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W. Tabellen zur Strukturaufklärung organischer Verbindungen mit spektroskopischen Methoden; Springer Verlag: Berlin, 1981; p. H70. [Google Scholar]
- Hesse, M.; Meier, H.; Zeeh, B. Spektroskopische Methoden in der Organischen Chemie; George Thieme Verlag: Stuttgart, 1984; pp. 140–144. [Google Scholar]
- William, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry; McGraw-Hill Book Company: London, 1989; p. 135. [Google Scholar]
- Murrov, S.L. Handbook of Photochemistry; Marcel Dekker: New York, 1973; p. 79. [Google Scholar]
- Sample Availability: Samples are available from MDPI.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Memarian, H.R.; Nikpour, F. ElectronTransfer Induced Ring Opening of α-Epoxyketones: Spirodioxolane Formation. Molecules 2002, 7, 63-71. https://doi.org/10.3390/70100063
Memarian HR, Nikpour F. ElectronTransfer Induced Ring Opening of α-Epoxyketones: Spirodioxolane Formation. Molecules. 2002; 7(1):63-71. https://doi.org/10.3390/70100063
Chicago/Turabian StyleMemarian, Hamid R., and Farzad Nikpour. 2002. "ElectronTransfer Induced Ring Opening of α-Epoxyketones: Spirodioxolane Formation" Molecules 7, no. 1: 63-71. https://doi.org/10.3390/70100063




