Tyrian Purple: 6,6’-Dibromoindigo and Related Compounds
Abstract
:Introduction
The vein [hypobranchial gland] already mentioned is then extracted and about a sextarius [ca. 7 lb] of salt added to each hundred pounds of material. It should be soaked for three days, for the fresher the extract, the more powerful the dye, then boiled in a leaden vessel. Next, five hundred pounds of dye-stuff, diluted with an amphora [about 8 gallons] of water, are subjected to an even and moderate heat by placing the vessels in a flue communicating with a distant furnace.Meanwhile, the flesh which necessarily adheres to the veins is skimmed off and a test is made about the tenth day by steeping a well-washed fleece in the liquefied contents of one of the vessels. The liquid is then heated till the colour answers to expectations. A frankly red colour is inferior to one with a tinge of black. The wool drinks in the dye for five hours and after carding is dipped again and again until all the colour is absorbed.
The Ancient process
The direct process
he "found this species on the shores of the Bristol Channel, which on cracking and picking off the shell, exhibited a white vein lying transversely in a little furrow or cleft next the head of the fish; which must be digged out with the stiff point of a horse hair pencil being made short and tapering; which must be so formed by reason of the viscous claminess of that white liquor in the vein so that by its stiffness it may drive in the matter into the fine linnen or white silk ....... if placed in the Sun will change into the following colours, i.e., if in the winter about noon, if in the summer an hour or two after sunrise and so much before setting (for in the heat of the day the colours will come on so fast, that the succession of each colour will scarce be distinguishable) next to the first light green will appear a deep green; and in a few minutes this will change into a dull sea green; after which, in a few minutes more, it will alter into a watchet blue; from that in a little time more it will be purplish red; after which, lying an hour or two (supposing the Sun still shining) it will be of a very deep purple red; beyond which the Sun can do no more."
On the coasts belonging to the province of Guayaquil the finest purple is found. The animals from which it is derived are contained in shells, about the size of walnuts, and live on rocks washed by the sea. They contain a juice or humour, which is taken out, and yields the true purple. ... Cotton, thread, and other delicate materials are dyed with it. It gives a lively and durable colour, which does not lose its lustre by frequent washings, but is rather improved thereby, and does not fade through long-continued use and exposure. Near the port of Nicoya in the province of Guatemala [now Costa Rica] the same kind of shellfish is found and is used for dyeing cotton ... Various processes are employed for extracting the juice or humour . Some kill the animal. They take it out of its shell, and, having laid it on the back of the hand, press and squeeze it with a knife from the head to the tail, and then separate the expressed juice, the rest of the animal matter being thrown away. They treat in this way a number of animals until they have sufficient quantity of juice. They then draw through the thread which they wish to dye, and no more is required ... Others express the juice without killing the animal. They do not take it entirely out of the shell, but only press it so as to cause a certain quantity to be ejected, with which the threads are dyed. The shells are then laid again on the stones from which they were taken. They recover, and after some time give a fresh quantity of juice, but not so much as the first time. If the operation is repeated three or four times, the quantity is very small and the animal dies of exhaustion.
The process of dyeing the thread illustrates the patient assiduity of the Indians. It is taken to the seaside, when a sufficient number of shells are collected, which being dried from the sea water, the work is commenced. Each shell is taken up singly, and a slight pressure upon the valve which closes its mouth forces out a few drops of the colouring fluid, which is then almost destitute of colour. In this each thread is dipped singly, and after absorbing enough of the precious liquid, is carefully drawn out between the thumb and finger, and laid aside to dry. Whole days and nights are spent in this tedious process, until the work is completed. At first the thread is of a dull blue colour, but upon exposure to the atmosphere acquires the desired tint. The fish is not destroyed by the operation, but is returned to the sea, where it lays in a new stock of colouring matter for a future occasion.
The identity of the purple
| Authors | Date | Species / artefact | Technique(s) |
|---|---|---|---|
| Friedlander [36] | 1909 | Murex brandaris | elemental analysis |
| Friedlander [37] | 1922 | Purpura lapillus Purpura aperta | elemental analysis |
| Driessen L A [38] | 1944 | textile | photodebromination of leuco-dibromoindigo |
| van Alpen [39] | 1944 | textile | photodebromination of leuco-dibromoindigo |
| Bruin [40] | 1966 | Murex trunculus | ESR |
| Baker & Sutherland [41] | 1968 | Dicathais orbita | elemental analysis; MS |
| Sasaki K [42] | 1975 | Rapana thomasiana | IR, X-RAY |
| Gibaja Oviedo & Salazar de Cavero [43] | 1977 | Chanque (Peru) | UV/VIS |
| Taylor G W [44] | 1983 | textiles | VIS |
| McGovern & Michel [45] | 1985 | Sarepta pot shard | PIXE, ESCA, FTIR, photodebromination of leuco-dbi |
| Daniels [46,47] | 1985 | textile from Enkomi | VIS |
| McGovern, Lazar, Michel [48,49] | 1990 | Peruvian textiles Paracas (900-200BC) Nazca (200BC – 600AD) Purpura patula pansa | MS |
| Wouters [50] | 1991 | HPLC | |
| Koren 1995[51] | 1994 | Murex trunculus | HPLC |
| Kosugi Y & Matsumoto K 1994 [52] | 1994 | Rapana venosa | MS |
| Shimoyama S & Noda Y [53] | 1994 | Rapana thomasiana | 3D fluorescence spectra |
| Clark & Cooksey [54] | 1997 | Nucella lapillus | HPLC |
| Cooksey & Withnall [55] | 1998 | Nucella lapillus | HPLC |
| Shimoyama S [56] | 1999 | coloured textile remnant | 3D fluorescence spectra |
| Cooksey, Withnall, Patel, Naegel [57] | 2000 | Purpura pansa | HPLC |
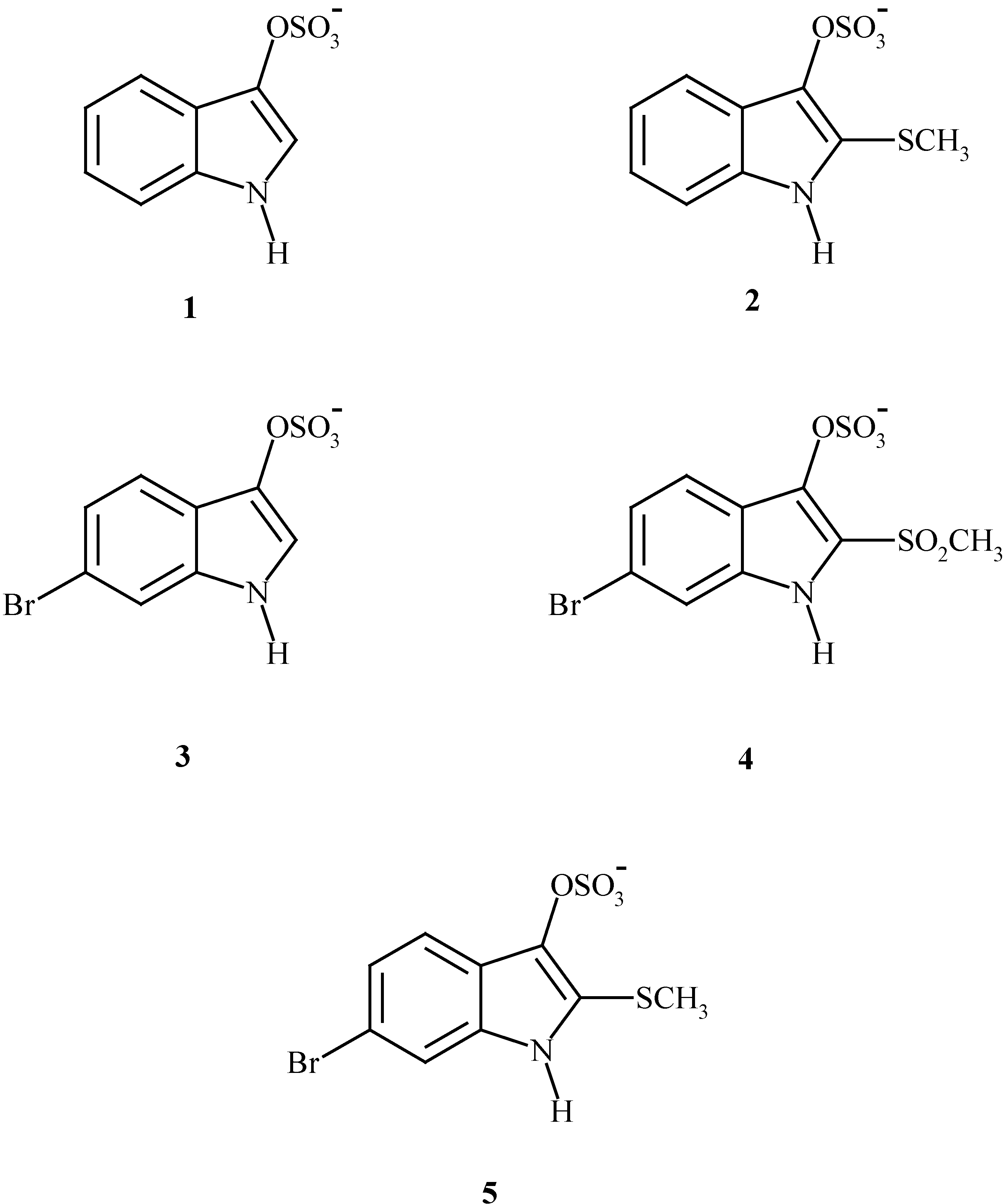
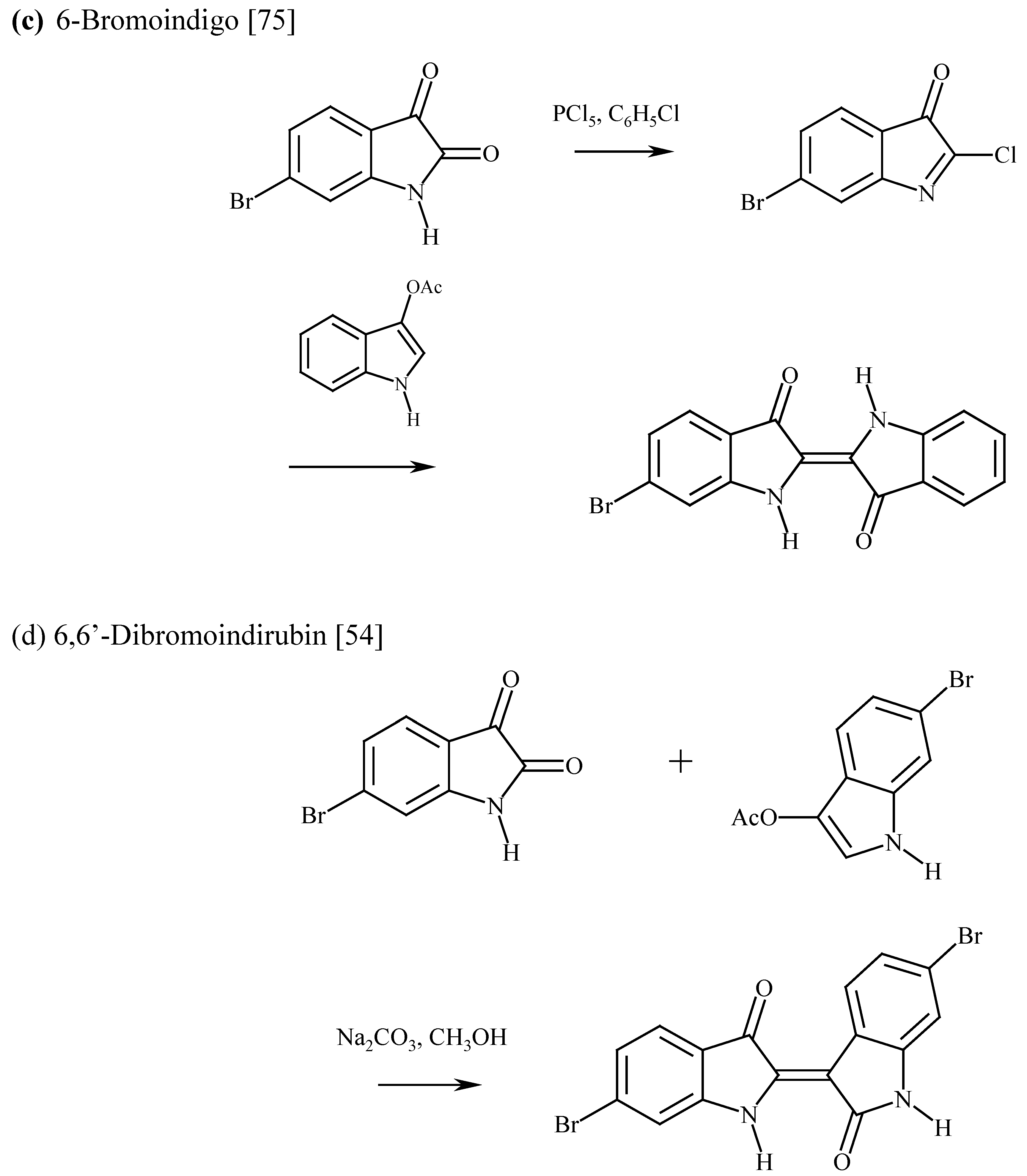
The synthesis of 6,6’-dibromoindigo
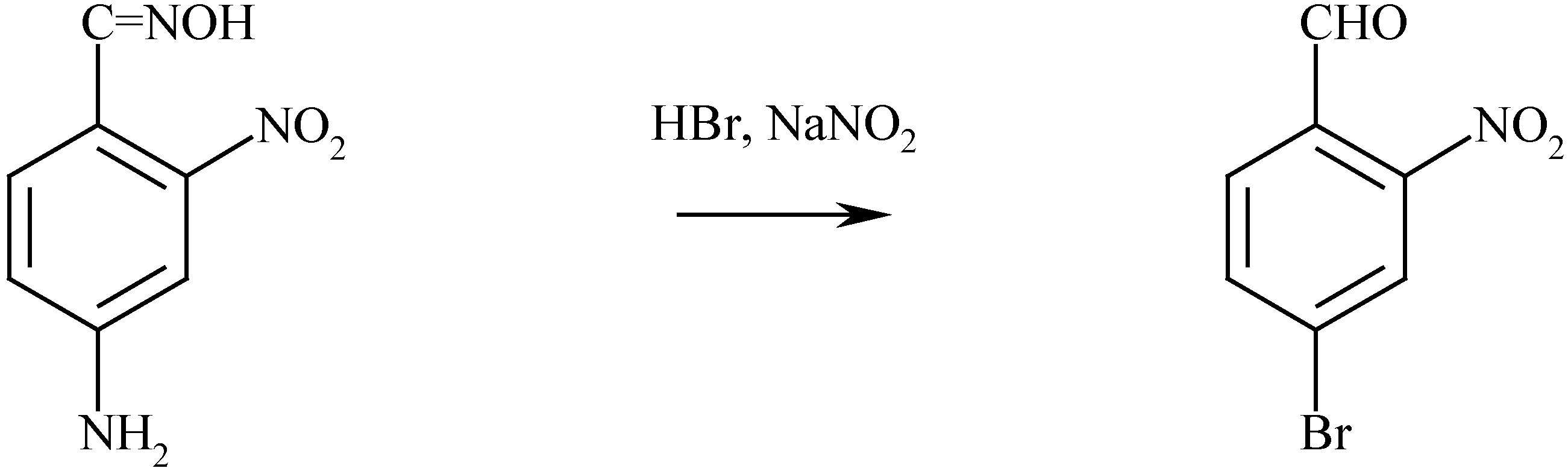
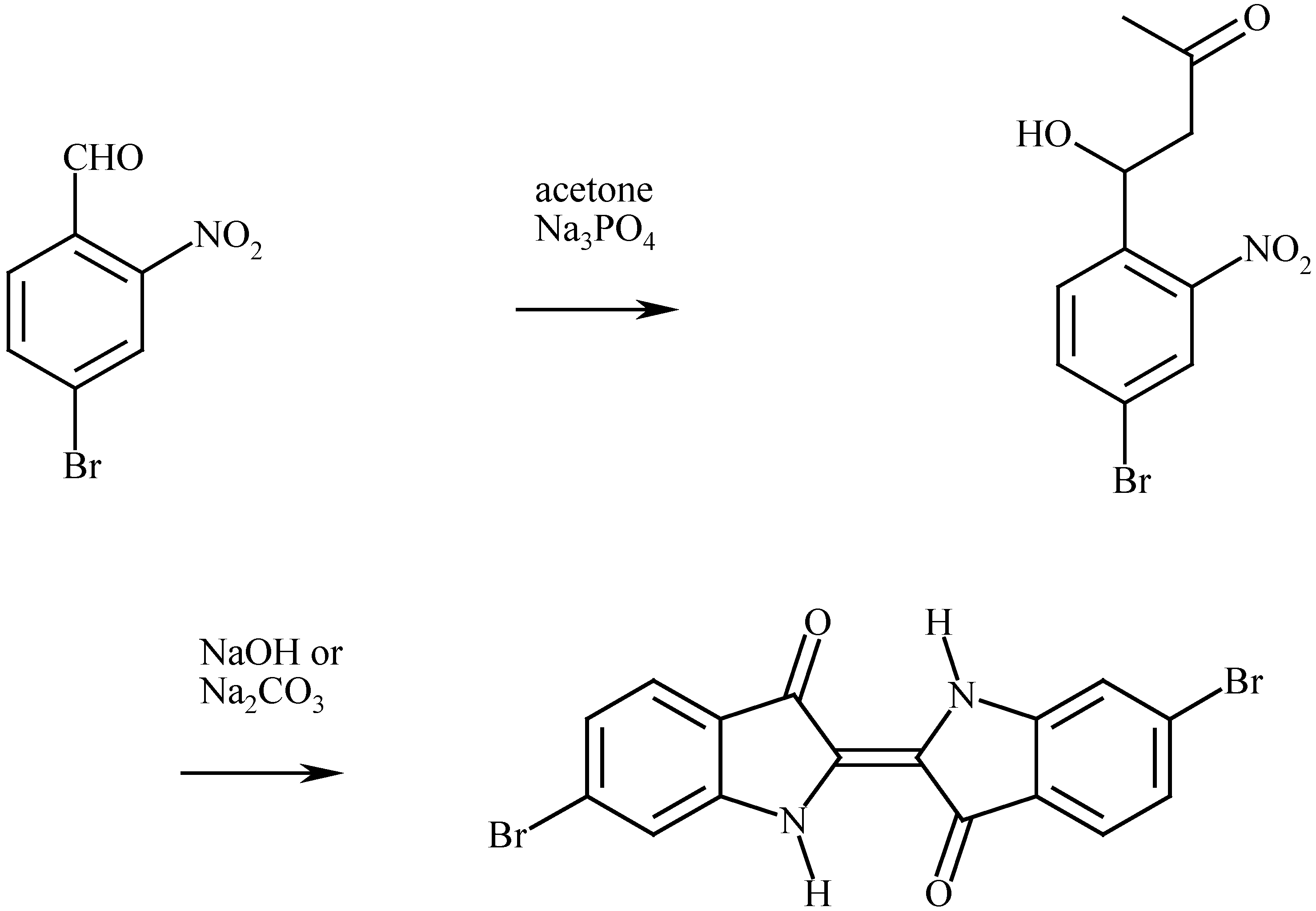
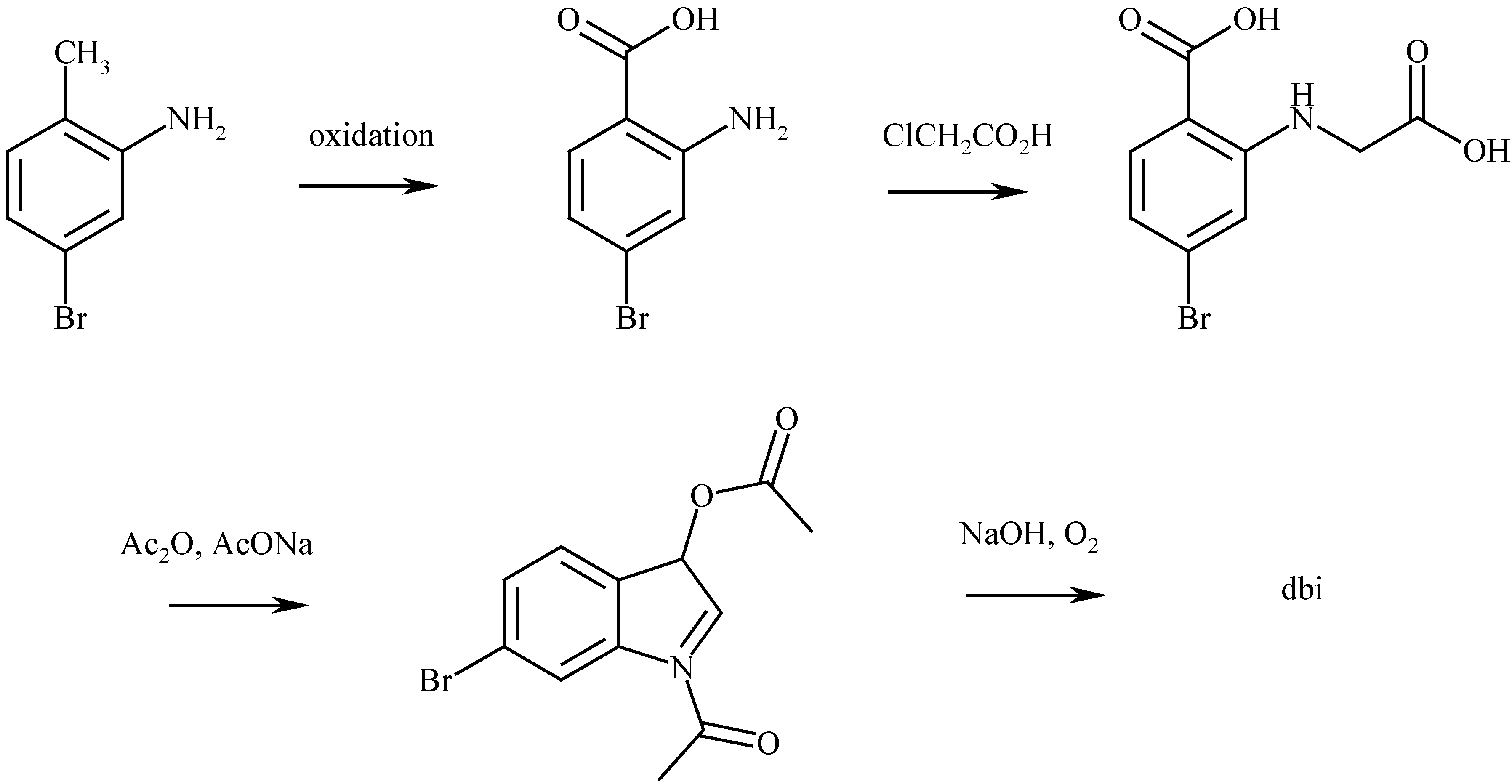
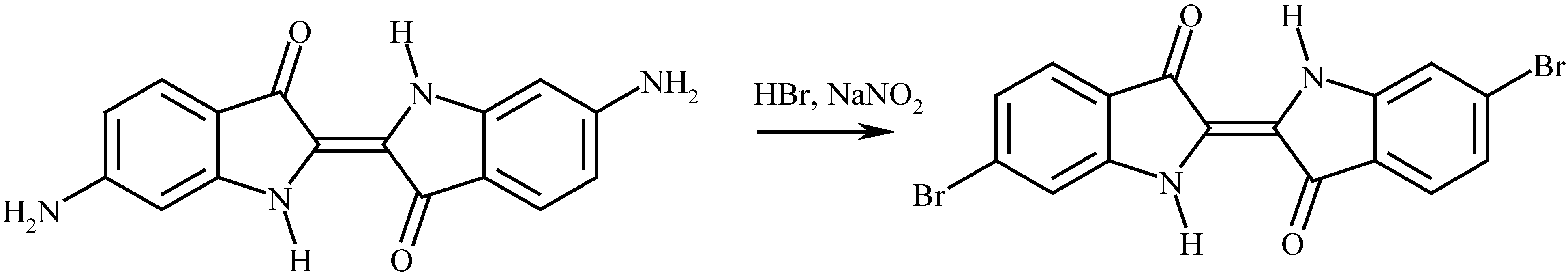

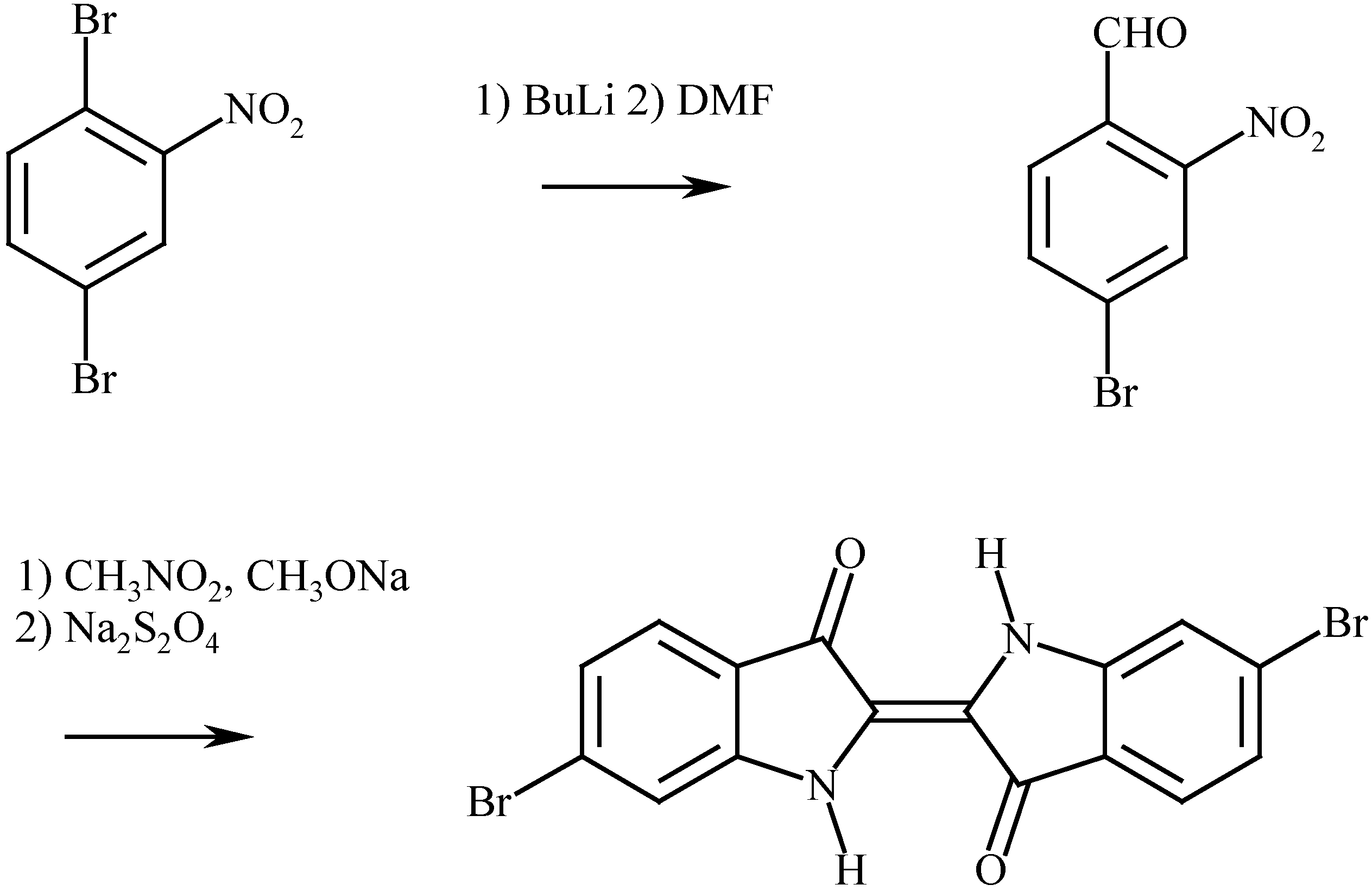

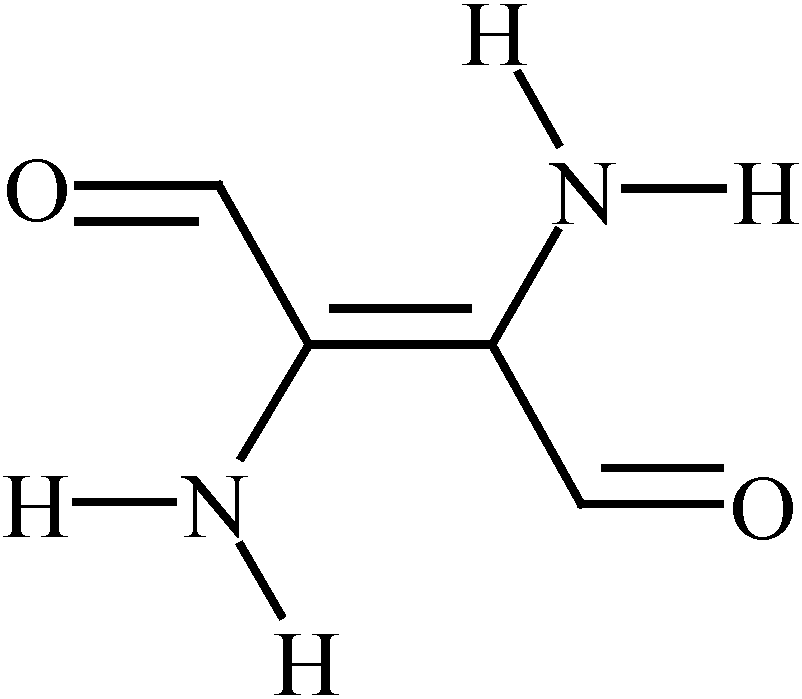
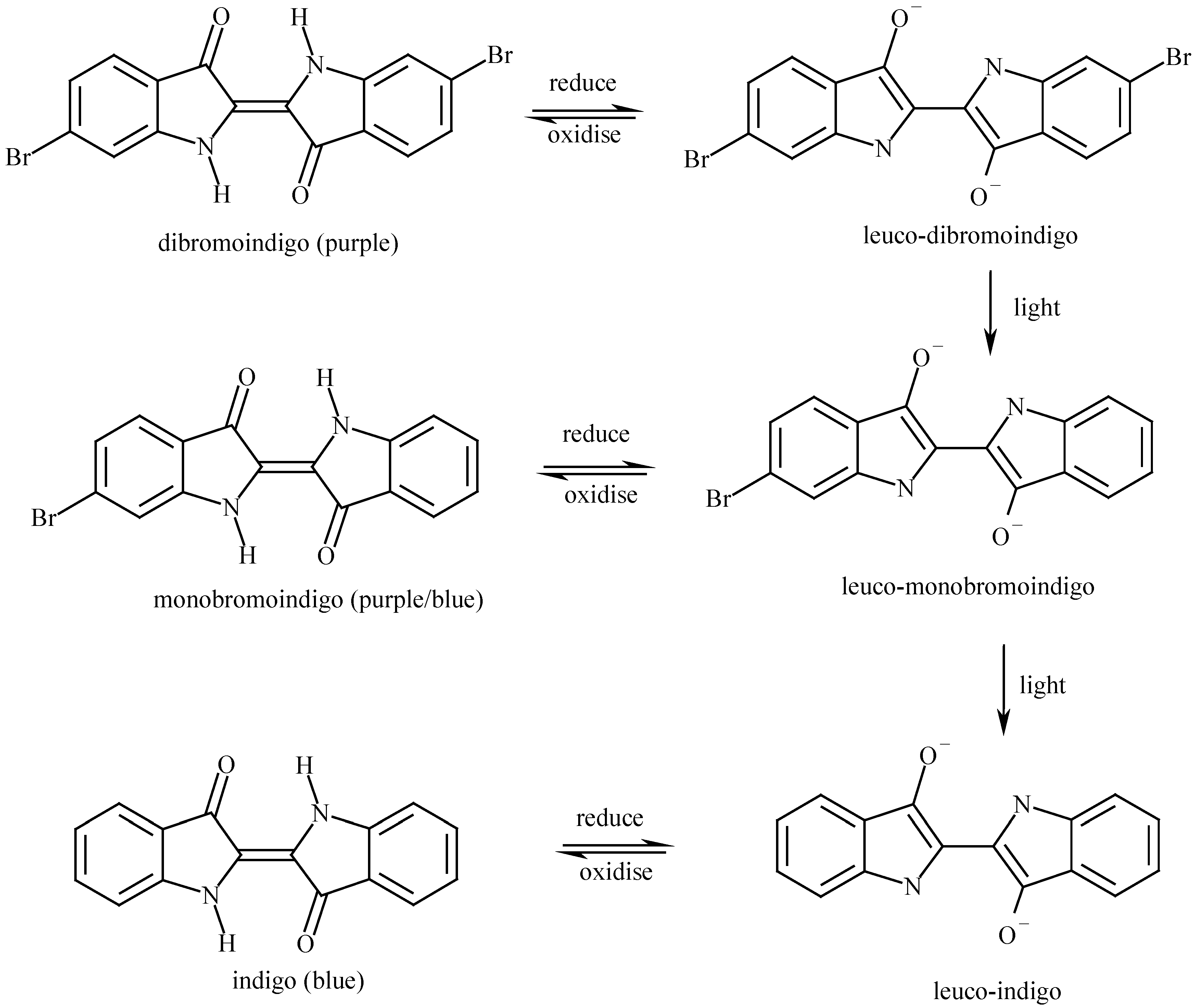
The properties of 6,6’-dibromoindigo
| Bromine substituent(s) | Solvent | λmax/nm | reference |
|---|---|---|---|
| 6,6’- | tce | 585 | [73] |
| 6,6’- | xyl | 590.5 | [74] |
| 6- | tce | 601 | [75] |
| 7,7’- | tce | 605 | [73] |
| none | tce | 605 | [76] |
| 7- | tce | 605 | [75] |
| 5,5’- | xyl | 605.5 | [74] |
| 7,7’- | tce | 606 | [73] |
| 4,4’- | tce | 610 | [73] |
| 4- | tce | 610 | [75] |
| 4,5,6,7,4’,5’,6’,7- | xyl | 611.5 | [74] |
| 5,7,5’- | xyl | 612.5 | [74] |
| 5,7,5’,7’- | xyl | 613 | [74] |
| 4,4’- | tce | 613 | [73] |
| 5- | tce | 613 | [75] |
| 4,5,7,4’,5’- | chl | 615 | [77] |
| 4,5,7,4’,5’,7’- | xyl | 616 | [74] |
| 5,5’- | tce | 621 | [73] |
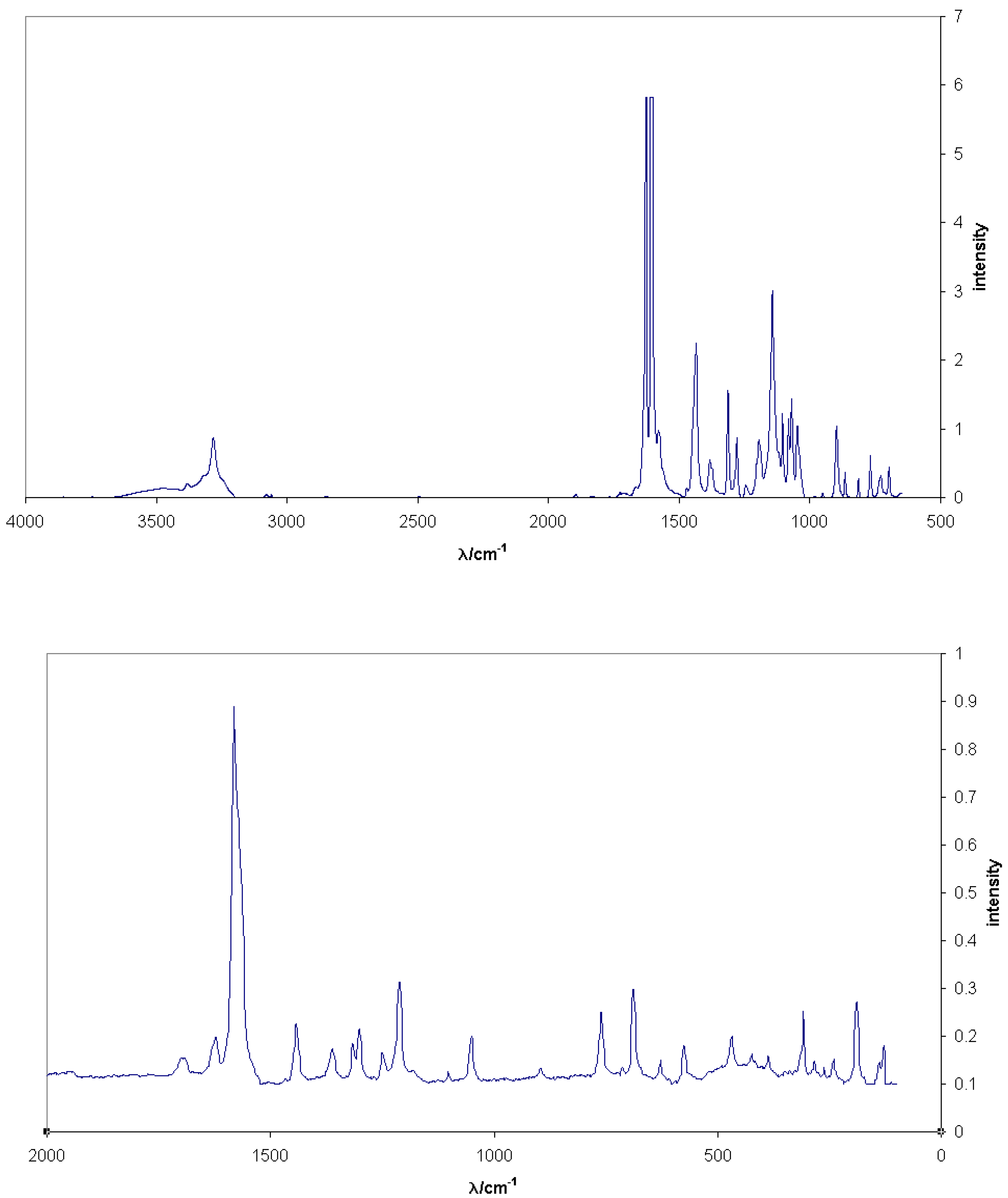

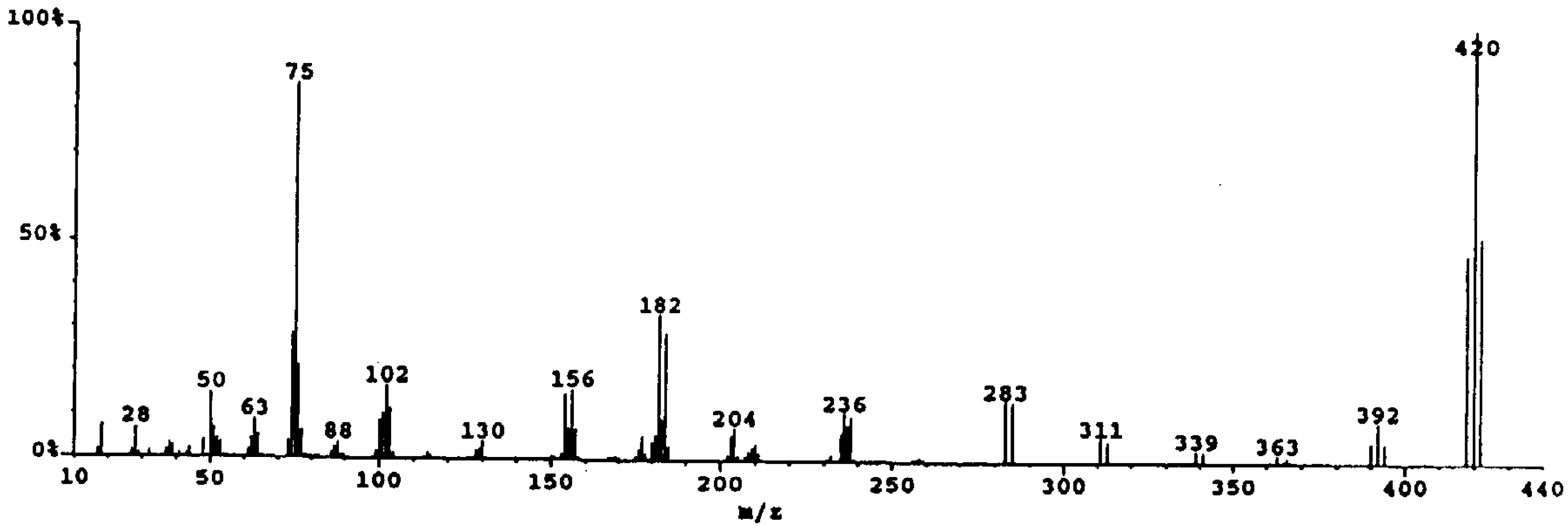
The analysis of Tyrian purple
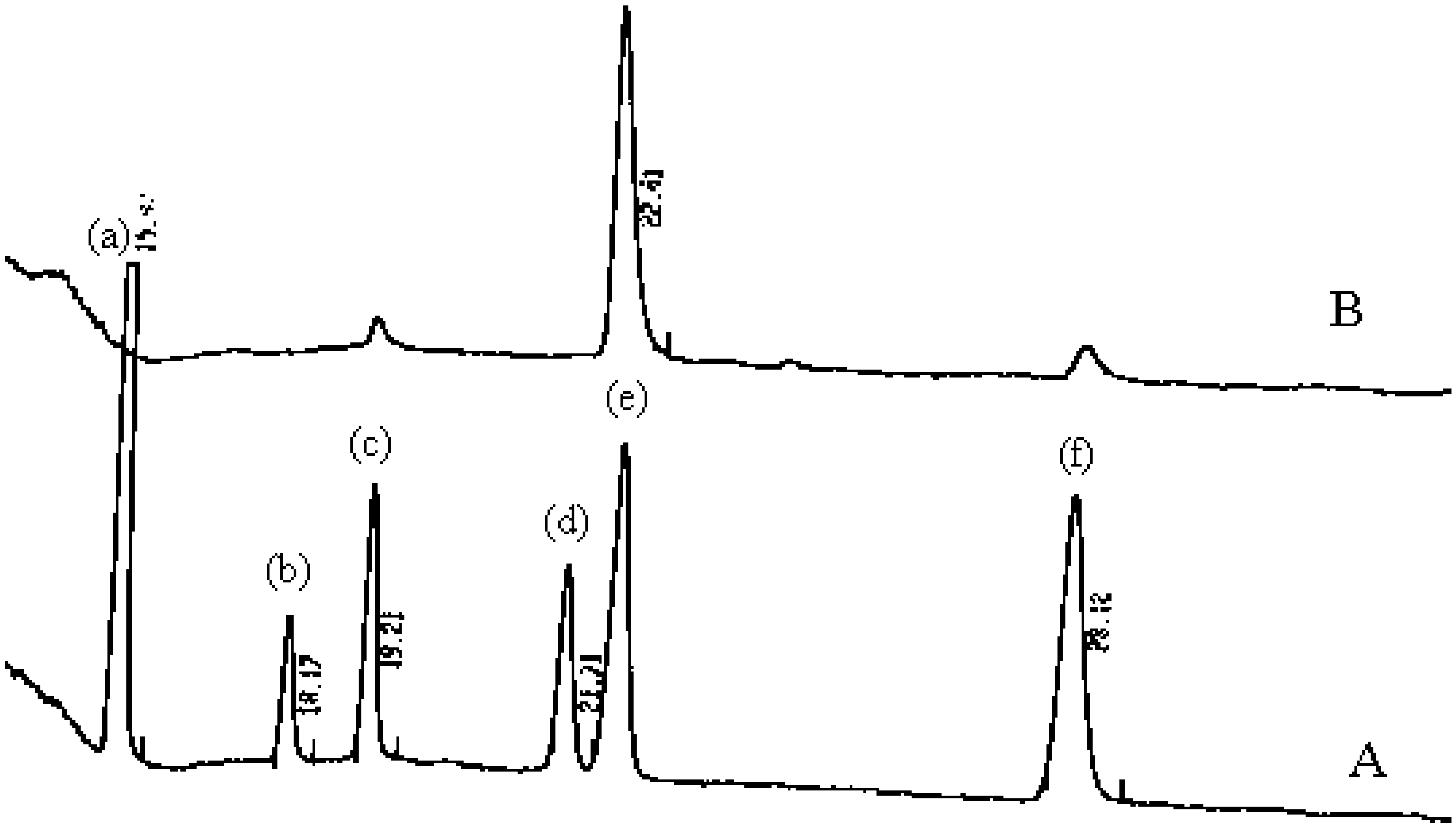
| Mollusc species | Indigo | Indirubin | 6-bromo indigo | 6,6’-dibromo indigo | 6,6’-dibromo indirubin | Reference |
|---|---|---|---|---|---|---|
| Murex brandaris | 0 | 0 | 3 | 83 | 14 | [97] |
| Thais haemastoma | 0 | 0 | 3 | 91 | 6 | [97] |
| Nucella lapillus | 0 | 0 | 3 | 88 | 9 | [98] |
| Nucella lapillus | 8 | 0 | 1 | 77 | 14 | [57] |
| Purpura pansa | 0 | 0 | 9 | 90 | 1 | [57] |
| Purpura pansa | 0 | 0 | 16 | 77 | 7 | [99] |
| Murex trunculus | 55 | 7 | 35 | 3 | 0 | [97] |
| Murex trunculus | 4 | 0 | 18 | 76 | 2 | [51] |
| Rf | |||
|---|---|---|---|
| Indigo | 6-Bromoindigo | 6,6’-Dibromoindigo | |
| 3:2 ethyl acetate – cyclohexane | 0.53 | 0.58 | 0.62 |
| 1:9 methanol – chloroform | 0.79 | 0.80 | 0.81 |
| 1:1 benzene - chloroform | 0.14 | 0.20 | 0.30 |
| Solvent mixture | Rf | |||
|---|---|---|---|---|
| indigo | dibromoindigo | |||
| mono | bis | mono | bis | |
| 3:2 toluene – ethyl acetate | 0.63 | 0.74 | 0.75 | 0.85 |
| 3:2 heptane – ethyl acetate | 0.47 | 0.54 | 0.56 | 0.63 |
| 3:1 hexane – ethyl acetate | 0.23 | 0.34 | 0.34 | 0.43 |
| 1:1 dichloromethane – ethyl acetate | 0.15 | 0.27 | 0.22 | 0.40 |
| 1:1 chloroform – ethyl acetate | 0.06 | 0.15 | 0.16 | 0.35 |
| 3:2 heptane - dichloromethane | 0.07 | 0.15 | 0.14 | 0.26 |
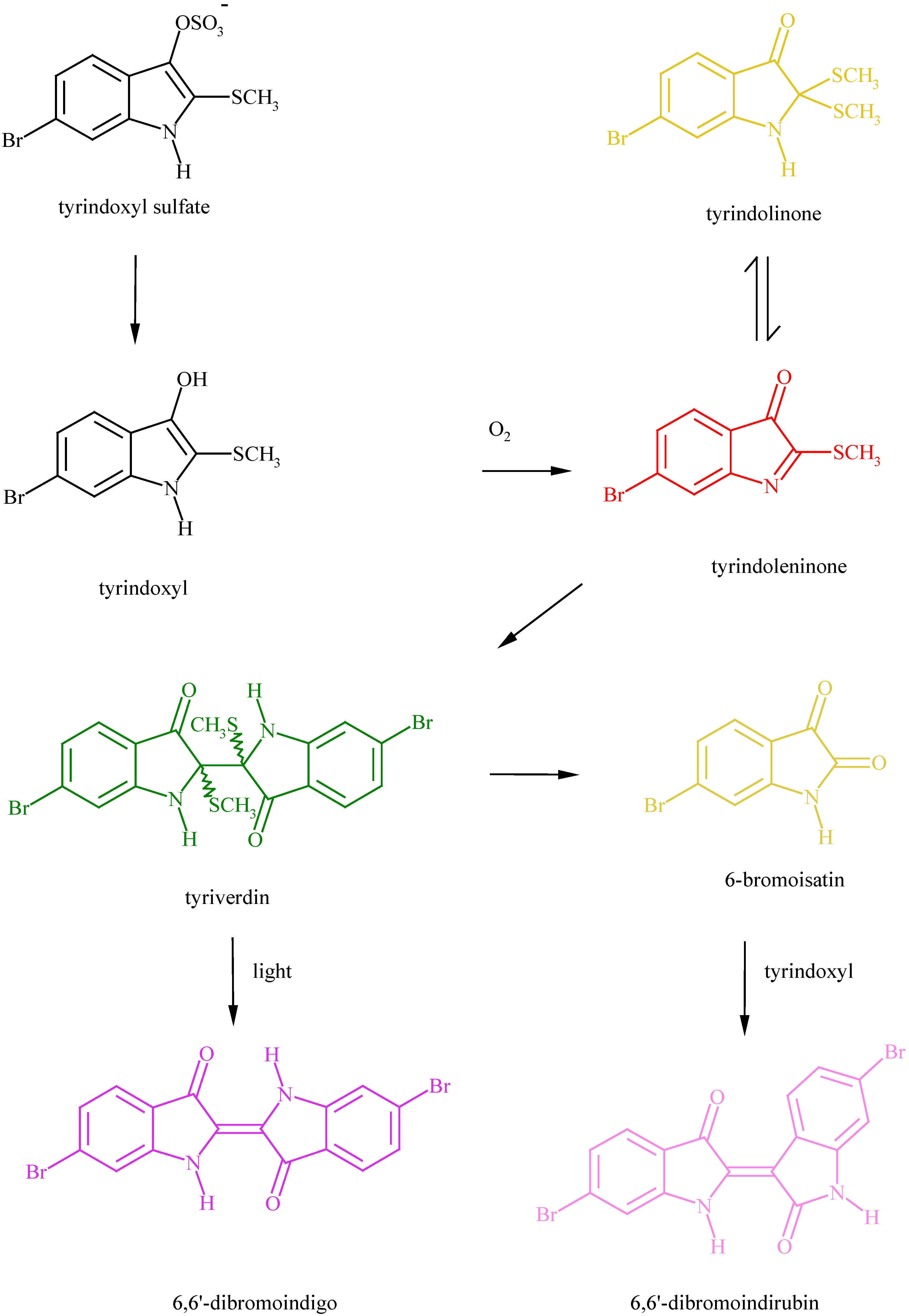
The precursors of the purple.
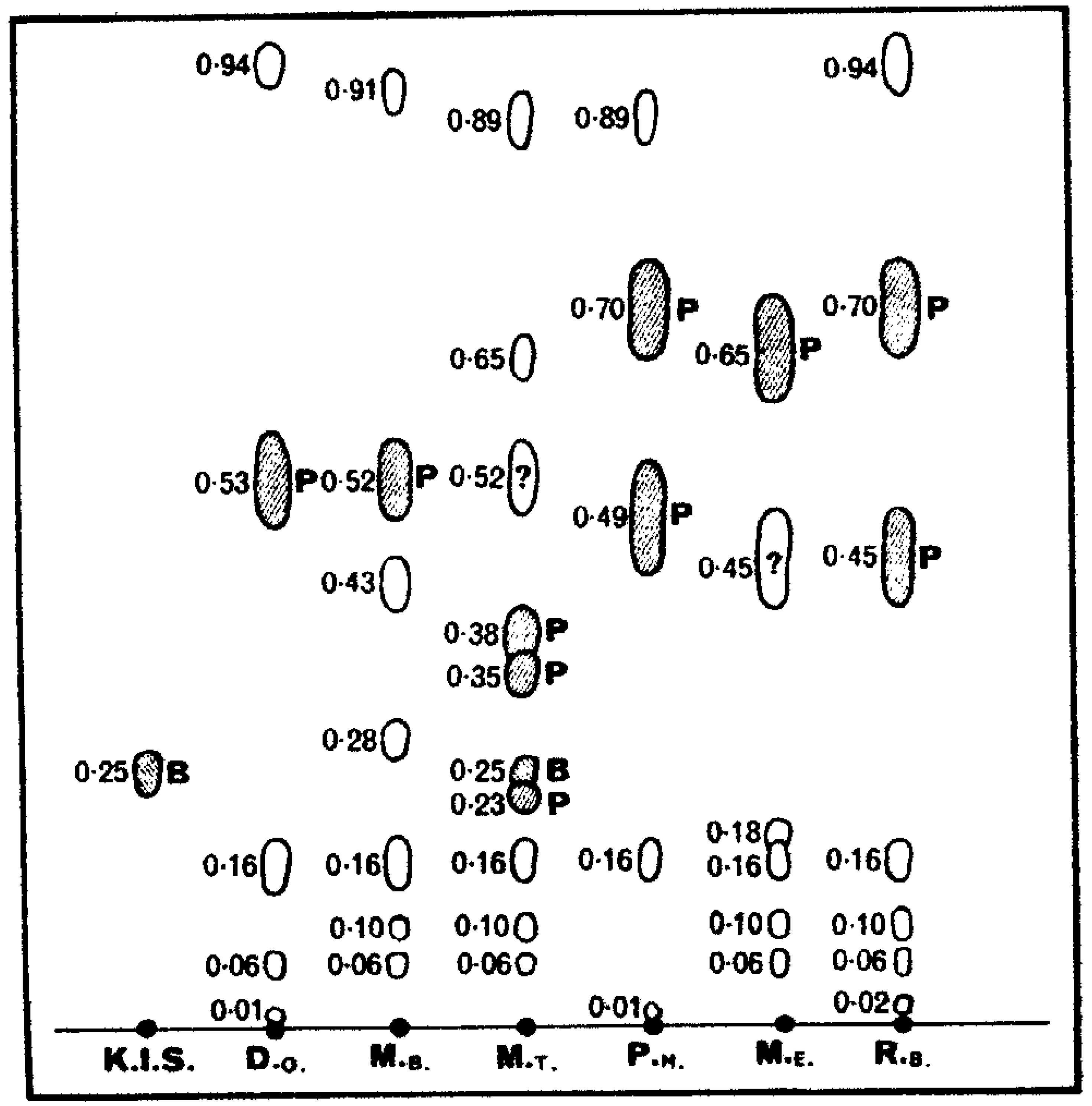
| Nucella lapillusa | Thais clavigerab | Dicathais orbitac | Dicathais orbita (eggs)d | |
| Tyrindoleninone | 0.88 | 0.85 | 0.8 | 0.5 |
| Tyrindolinone | 0.58 | 0.55 | 0.5 | 0.4 |
| Tyriverdin | 0.32 | 0.3 |

The minor components in the purple
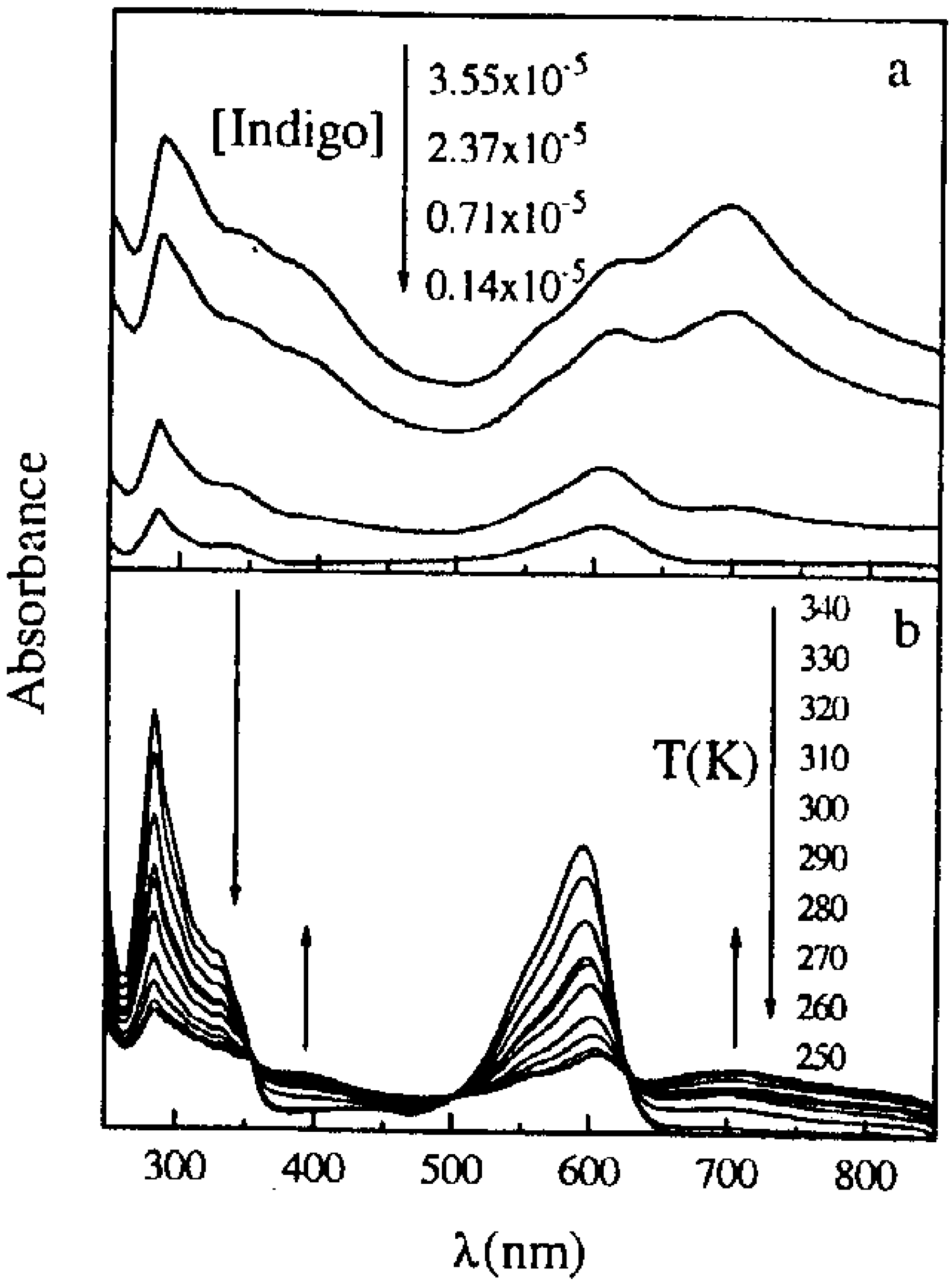
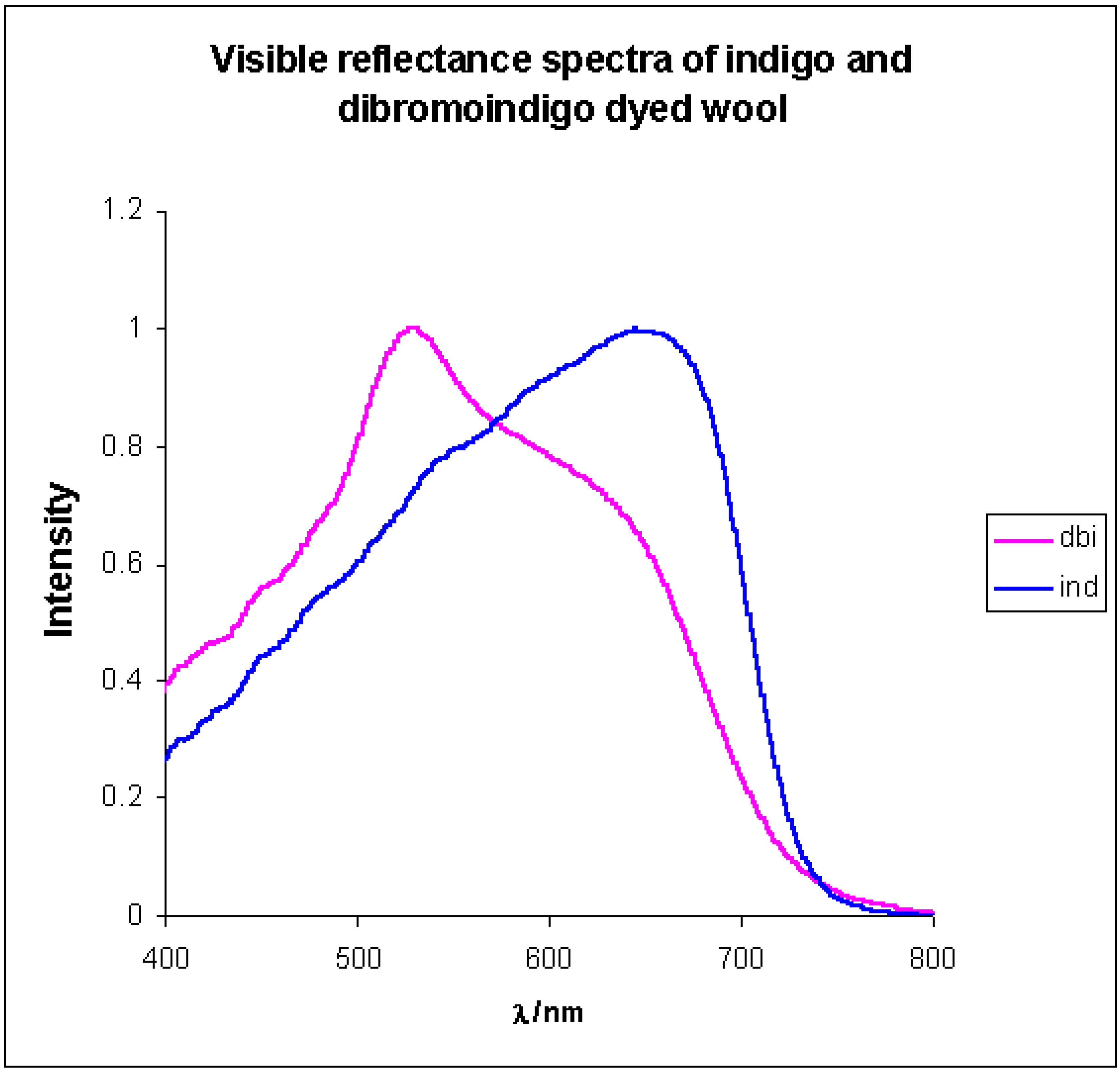
Chemical properties of 6,6’-Dibromoindigo
Further information
A quel usage pourrait-on employer la pourpre? Aujourd’hui que les manufactures de produits chimiques versent à torrent dans l’industrie des matières qui, avec la plus grande facilité et la plus grandes perfection, peuvent servir aux teintures les plus delicates et les plus riches, comment pourrait-on espérer de voir ce peu de matières animale donnant du violet, quoique fort beau et fort tenace, être employé par l’industrie? Il n’est guère probable que la pourpre revienne en honneur.

References and Notes
- McGovern, P.E.; Michel, R.H. Royal Purple dye: tracing the chemical origins of the industry. Anal. Chem. 1985, 57, 1514A–1522A. [Google Scholar]
- Jackson, J.W. The Geographical Distribution of the Shell-Purple Industry; Longmans, Green and Co: London, 1917; pp. 1–29. [Google Scholar]
- Pliny the Elder. Historia Naturalis; book IX, sections 60-65, chs. XXXVI-XLI as translated by Bailey; 1929. [Google Scholar]
- Cole, W. Purple Fish. Phil. Trans. Roy. Soc. 1685, 15, 1278–1286. [Google Scholar]
- Reinhold, M. The history of purple as a status symbol in antiquity. Collect. Latomus. 1970, 116, 1–73. [Google Scholar]
- Dedekind, A. Ein Beitrag zur Purpurkunde.
- Becker, C. Did the people in Ayios Mamas produce purple-dye during the Middle Bronze Age? Considerations on the prehistoric production of purple-dye in the Mediterranean. In Animals and Man in the Past; ARC-Publicatie: Groningen, the Netherlands, 2001; pp. 121–134. [Google Scholar]
- Born, W. Purpura Shell-Fish. Ciba Rev. 1937, 1, 106–110. [Google Scholar]
- Born, W. Purple in Classical Antiquity. Ciba Rev. 1937, 1, 111–118. [Google Scholar]
- Michel, R.H.; McGovern, P.E. The chemical processing of Royal Purple dye: ancient descriptions as elucidated by modern science. Archeomaterials. 1987, 1, 135–143. [Google Scholar]
- Michel, R.H.; McGovern, P.E. The chemical processing of Royal Purple dye: ancient descriptions as elucidated by modern science, part II. Archeomaterial. 1990, 4, 97–104. [Google Scholar]
- Doumet, J. Étude sur la couleur pourpre ancienne et tentative de reproduction du procédé de teinture de la ville de Tyr décrit par Pline l’Ancien; Imprimerie Catholique: Beirut, 1980. [Google Scholar]
- Cheek, G.T.; Barthel, R.V. Electrochemical studies of indigoid systems. Proc. - Electrochem. Soc. 93-11(Proceedings of the Fifth International Symposium on Redox Mechanisms and Interfacial Properties of Molecules of Biological Importance, 1993) 1993, 165–74. [Google Scholar]
- Elsner, O.; Spanier, E. Dyeing with Murex extracts, an unusual dyeing method of wool to the Biblical sky blue. Proc. Int. Wool Text. Res. Conf., 7th. 1985, 5, 118–130. [Google Scholar]
- Padden, A.N.; John, P.; Collins, M.D.; Hutson, R.; Hall, A.R. Indigo-reducing Clostridium isatidis isolated from a variety of sources, including a tenth century Viking woad vat. J. Archaeol. Sci. 2000, 27, 953–956. [Google Scholar]
- Padden, A.N.; Dillon, V.M.; Edmonds, J.; Collins, M.D.; Alvarez, N.; John, P. An indigo-reducing moderate thermophile from a woad vat, Clostridium isatidis sp. nov. Int. J. Syst. Bacteriol. 1999, 49, 1025–1031. [Google Scholar]
- Padden, A.N.; Dillon, V.M.; John, P.; Edmonds, J.; Collins, M.D.; Alvarez, N. Clostridium used in mediaeval dyeing. Nature 1998, 396, 225. [Google Scholar]
- Purple snail meeting. Rousillon, January 2001.
- Edmonds, J. Tyrian or Imperial purple dye, Historic Dye Series No 7, ISBN 0 9534133 65 2000, 41pp.
- Verhecken, A. Experiments with the dyes from European purple-producing shellfish. Dyes Hist. Archaeol. 1994, 12, 32–35. [Google Scholar]
- Verhecken, A. Experiences with mollusc purple. La Conchiglia 1990, 22, 250–252. [Google Scholar]
- Letellier, A. Recherches sur la pourpre produite par le Purpura lapillus. Archiv. Zool. exper. et gen (2) 1890, 8, 361. [Google Scholar]
- Schunck, E. Notes on the Purple of the Ancients. 1. The Chromogen of Purpura Capillus. 2. Properties of the Colouring Matter formed by Insolation from the Chromogen of Purpura Capillus. J. Chem. Soc. 1879, 35, 589–596. [Google Scholar]
- Réaumur, R.A. F. de. Mém. Acad. Roy. Sci. Amsterdam 1711, 168.
- Duhamel, H.L. Mém. Acad. Roy. Sci. Paris 1736.
- Lacaze-Duthiers, H. Mémoire sur le Pourpre. Mém. Soc. Imp. Sci. Agricult. Arts Lille 1859, 6, 303–380. [Google Scholar]
- Juan, Don Jorge; de Ulloa, Don Antonio. Relación Historica del viage a la America Meridional, Madrid. 1748; book 4; pp. 242–244. [Google Scholar]
- Squier, E. G. Nicaragua: its people, scenery, monuments, and the proposed interoceanic canal, with numerous original maps and illustrations, New York. 1852, vol 1, 286, quoted by Schunck E 1880 [35].. [Google Scholar]
- Nuttall, Z. A Curious survival in Mexico of the use of the Purpura Shell-fish for dyeing. In Putnam Anniversary Volume; The Torch Press: Cedar Rapids, Iowa, 1909; pp. 366–384. [Google Scholar]
- Thompson, J. Shellfish Purple: The Use of Purpura patula pansa on the Pacific Coast of Mexico. Dyes Hist. Archaeol. 1995, 13, 3–6. [Google Scholar]
- Rios-Jara, E.; León, H.G; Lizárraga-Chávez, L.; Michel-Morfín, J.E. Producción y tiempo de recuperación del tinte de Plicopurpura patula pansa (Neogastropoda: Muricidae) en Jalisco, México. Rev Biol. Trop. 1994, 42, 537–545. [Google Scholar]
- Michel-Morfin, J.E.; Chavez, E.A. Effect of repetitive dye extraction over yield and survival rate of the purple snail Plicopurpura pansa (Gould, 1853). J. Shellfish Res. 2000, 19, 913–917. [Google Scholar]
- Michel-Morfin, J.E; Chavez, E.A.; Landa, V. Population parameters and dye yield of the purple snail Plicopurpura pansa (Gould, 1853) of west central Mexico. J. Shellfish Res. 2000, 19, 919–925. [Google Scholar]
- Bizio, B. Scoperta del principio purpureo nei due Murex brandaris e trunculus Lina., e studio delle sue proprietà. Ann. Sci. Lombardo-Veneto 1833, 346–364. [Google Scholar]
- Schunck, E. Notes on the Purple of the Ancients. 3. Purple dyeing in modern times. J. Chem. Soc. 1880, 613–617. [Google Scholar]
- Friedlander, P. Uber den Farbstoff des antiken Purpurs aus murex brandaris. Ber. Dtsch. Chem. Ges. 1909, 42, 765–770. [Google Scholar]
- Friedlander, P. Uber die Farbstoffe aus Purpura aperta and Purpura lapillus. Ber. Dtsch. Chem. Ges. 1922, 55, 1656–1658. [Google Scholar]
- Driessen, L.A. Über eine charakteristische Reaktion des antiken Purpurs auf der Faser. Melliand Textilber. 1944, 25, 66. [Google Scholar]
- Van Alphen, J. Remarks on the Action of Light on Indigo Dyes in a Reducing Medium. Rec. Trav. Chim. Pays Bas 1944, 63, 95–96. [Google Scholar]
- Bruin, F. Royal Purple and the dye industries of the Mycenaeans and Phoenicians. Sociétés et Compagnies de Commerce en Orient et dans l'Océan Indien (Acts du huitième colloque international d'histoire maritime, Beyrouth — 5-10 Septembre 1966) 1966, 73–90. [Google Scholar]
- Baker, J.T.; Sutherland, M.D. Pigments of marine animals VIII. Precursors of 6,6'-Dibromo-indigotin (Tyrian Purple) from the mollusc Dicathais Orbita Gmelin. Tetrahedron Lett. 1968, 43–46. [Google Scholar]
- Sasaki, K. Chemical structure of ancient purple dye. Tyrian purple. Senryo To Yakuhin 1975, 20, 36–45. [Google Scholar]
- Gibaja Oviedo, S.; Salazar de Cavero, L. Production of Tyrian purple from the Concholepas mollusk "Chanque". Bol. Soc. Quim. Peru 1977, 43, 139–40. [Google Scholar]
- Taylor, G.W. Detection of shellfish purples on textiles. Dyes Hist. Archaeol. 1983, 2. [Google Scholar]
- McGovern, P.E.; Michel, R.H. Royal Purple dye: tracing the chemical origins of the industry. Anal. Chem. 1985, 57, 1514A–1522A. [Google Scholar]
- Daniels, V. Dye analysis of two fragments from Enkomi. Dyes Hist. Archaeol. 1985, 4, 15–18. [Google Scholar]
- Daniels, V. Further work on the dye analysis of textile fragments from Enkomi. Dyes Hist. Archaeol. 1987, 6, 3–7. [Google Scholar]
- McGovern, P.E.; Lazar, J.; Michel, R.H. The analysis of indigoid dyes by mass spectrometry. J. Soc. Dyers Colour. 1990, 106, 22–25. [Google Scholar]
- McGovern, P.E.; Lazar, J.; Michel, R.H. Caveats on the analysis of indigoid dyes by mass spectrometry. J. Soc. Dyers Colour. 1991, 107, 280–281. [Google Scholar]
- Wouters, J. A new method for the analysis of blue and purple dyes in textiles. Dyes Hist. Archaeol. 1992, 10, 17–21. [Google Scholar]
- Koren, Z.C. High-performance Liquid Chromatographic Analysis of an Ancient Tyrian Purple Dyeing Vat from Israel. Isr. J. Chem. 1995, 35, 117–124. [Google Scholar]
- Kosugi, Y.; Matsumoto, K.A. convenient and rapid detection method of Kaimurasaki coloring matter by MS. Bunseki Kagaku. 1994, 43, 1133–1136. [Google Scholar]
- Shimoyama, S.; Noda, Y. Non-destructive three-dimensional fluorescence technique. Dyes Hist. Archaeol. 1994, 12, 50–61. [Google Scholar]
- Clark, R.J.H.; Cooksey, C.J. Bromoindirubins: the synthesis and properties of minor components of Tyrian purple and the composition of the colorant from Nucella lapillus. J. Soc. Dyers Colour. 1997, 113, 316–321. [Google Scholar]
- Cooksey, C.J.; Withnall, R. Chemical studies on Nucella lapillus. Dyes Hist. Archaeol. Lyon 1997, (in press). [Google Scholar]
- Shimoyama, S. Studies on the non-destructive determination of dyestuffs used in ancient colored cloths and ukiyo-e prints by the three-dimensional display of fluorescence spectra. Bunseki Kagaku 1999, 48, 951–952. [Google Scholar]
- Withnall, R.; Patel, D.; Cooksey, C.J.; Naegel, L. Chemical Studies of the Purple Dye of Purpura pansa. Dyes Hist. Archaeol. Edinburgh 2000, (in press). [Google Scholar]
- Sachs, F.; Kempf, R. Uber p-Halogen-o-nitrobenzaldehyde. Ber. Dtsch. Chem. Ges. 1903, 36, 3299–3303. [Google Scholar]
- Baeyer, A.; Drewson, V. Darstellung von Indigblau aus Orthonitrobenzaldehyd. Ber. Dtsch. Chem. Ges. 1882, 2856–2864. [Google Scholar]
- Sachs, F.; Sichel, E. Ueber p-substituirte o-Nitrobenzaldehyde. Ber. Dtsch. Chem. Ges. 1904, 37, 1861–1874. [Google Scholar]
- Grandmougin, E.; Seyder, P. Uber Indigo. V:Uber halogenierte Indigo und Derivate. Ber. Dtsch. Chem. Ges. 1914, 47, 2365–2373. [Google Scholar]
- Harley-Mason, J. A new synthesis of indigo. J. Chem. Soc. 1950, 2907. [Google Scholar]
- Barber, H.J.; Stickings, C.E. Phenanthrene-3:6-diamidine. J. Chem. Soc. 1945, 167–169. [Google Scholar]
- Pinkney, J.M.; Chambers, J.A. Synthesising Tyrian purple. Educ. Chem. 1979, 16, 144–145. [Google Scholar]
- Torimoto, N.; Morimoto, S.; Shingaki, T. Synthesis of Tyrian Purple as a teaching material. Kagaku to Kyoiku. 1991, 39, 198–201. [Google Scholar]
- Voss, G.; Gerlach, H. Regioselektiver Brom/Lithium-Austausch bei 2,5-Dibrom-1-nitrobenzol. Eine einfache Synthese von 4-Brom-2-nitrobenzaldehyde und 6,6'-Dibromindigo. Chem. Ber. 1989, 122, 1199–1201. [Google Scholar]
- Cooksey, C.J. unpublished observation.
- Kröhnke, F. Neuere Methoden der preparativen organischen Chemie. Synthesen mit Hilfe von Pyridiniumsaltzen (IV). Angew. Chem. 1963, 75, 317–329. [Google Scholar]
- Cooksey, C.J. Making Tyrian purple. Dyes Hist. Archaeol. 1995, 13, 7–13. [Google Scholar]
- Lüttke, W.; Klessinger, M. Theoretische und spektroskopische Untersuchungen an Indigofarbstoffen, I. Infrarot- und Lichtabsorptionsspektren einfacher Indigofarbstoffe. Chem. Ber. 1964, 97, 150–163. [Google Scholar]
- Lüttke, W.; Klessinger, M. Theoretische und spektroskopische Untersuchungen an Indigofarbstoffen, II. Das Chromophore System der Indigofarbstoffe. Tetrahedron 1963, 19, (Suppl. 2). 315–335. [Google Scholar]
- Serrano-Andrés, L.; Roos, B.O. A theoretical study of the indigoid dyes and their chromophore. Chem.-Eur. J. 1997, 3, 717–725. [Google Scholar]
- Friedländer, P.; Bruckner, S.; Deutsch, G. Über Brom- und Methoxyderivate des Indigos. Ann. Chem. 1912, 388, 23–49. [Google Scholar]
- Formanek, J. Über den Einfluß verschiedener Substituenten auf Farbe und Absorptions Spektrum des Indigo, Thioindigo und Indirubin. Z. Angew. Chem. 1928, 41, 1133–1141. [Google Scholar]
- Clark, R.J.H.; Cooksey, C.J. Monobromoindigos: a new general synthesis, the characterization of all four isomers and an investigation into the purple colour of 6,6'-dibromoindigo. New J. Chem. 1999, 23, 323–328. [Google Scholar]
- Sadler, P.W. Absorption Spectra of Indigoid Dyes. J. Org. Chem. 1956, 21, 316–318. [Google Scholar]
- Brode, W.R.; Pearson, E.G.; Wyman, G M. The Relation between the Absorption Spectra and the Chemical Constitution of Dyes. XXVII. cis-trans Isomerism and Hydrogen Bonding in Indigo Dyes. J. Am. Chem. Soc. 1954, 76, 1034–1036. [Google Scholar]
- Miliani, C.; Romani, A.; Favaro, G.A. Spectrophotometric and fluorimetric study of some anthraquinoid and indigoid colorants used in artistic paintings. Spectrochim. Acta Ser A 1998, 54, 581–588. [Google Scholar]
- Shen, B.; Olbrich-Stock, M.; Posdorfer, J.; Schindler, R.N. An Optical and Spectro-electrochemical Investigation of Indigo Carmine. Z. Phys. Chem. 1991, 173, 251–255. [Google Scholar]
- José-Yacamán, M.; Rendón, L.; Arenas, J.; Puche, M.C.S. Maya Blue Paint: An Ancient Nanostructured Material. Science 1996, 273, 223–225. [Google Scholar]
- Kleber, R.; Masschelein-Kleiner, L.; Thissen, J. Étude et Identification du ‘Bleu Maya’. Stud. Conserv. 1967, 12, 41–56. [Google Scholar]
- Artioli, G.; Galli, E.; Burattini, E.; Cappuccio, G.; Simeoni, S. Palygorskite from Bolca, Italy - A Characterization by High-Resolution Synchrotron-Radiation Powder Diffraction and Computer Modeling. Neues Jahrb. Mineral.,Monatsh. 1994, 217–219. [Google Scholar]
- Cooksey, C.J. unpublished observation.
- Baker, J.T. Tyrian purple. Ancient dye, a modern problem. Endeavour 1974, 33, 11–17. [Google Scholar]
- Tatsch, E.; Schrader, B. Near-Infrared Fourier Transform Raman Spectroscopy of Indigoids. J. Raman Spectr. 1995, 26, 467–473. [Google Scholar]
- Jackson, A.H.; Jenkins, R.T.; Grinstein, M.; Ferramola de Sancovich, A-M.; Sancovich, H.A. The isolation and identification of indigoid pigments from urine. Clin. Chim. Acta 1988, 172, 245–252. [Google Scholar]
- Voss, G. The analysis of indigoid dyes as leuco forms by NMR spectroscopy. J. Soc. Dyers Colour. 2000, 116, 80–90. [Google Scholar]
- Withnall, R.; Clark, R.J.H.; Cooksey, C.J.; Daniels, M.A.M. Non-destructive, in situ identification of indigo/woad and shellfish purple by Raman microscopy and visible reflectance spectroscopy. Dyes His. Archaeol. 1993, 11, 19–24. [Google Scholar]
- Clark, R.J.H.; Cooksey, C.J.; Daniels, M.A.M.; Withnall, R. Indigo – red white and blue. Educ. Chem. 1996, 16–19. [Google Scholar]
- Gibbs, P.J.; Jordan, G.J.; Sedden, K.R.; Cooksey, C.J.; Brovenko, N.M.; Tiomkin, E.N.; Petrosyan, Y.A. The in situ identification of indigo on ancient papers. Eur. Mass Spectrom. 1995, 1, 417–421. [Google Scholar]
- Golding, B.T.; Pierpoint, C. Indigo blue. Educ. Chem. 1986, 71–73. [Google Scholar]
- Posner, T. Beiträge zur Kenntnis der Indigo-Gruppe, VI: Über die Einwirkung von Säurechloriden auf Indigo und Indigo-Derivate, sowie über die Raumformel des Indigos. Ber. Dtsch. Chem. Ges. 1926, 59, 1799–1833. [Google Scholar]
- von Eller, H. Stéréochemie de l’indigo dans l’etat cristallin. C. R. Hebd. Seances Acad. Sci. 1954, 239, 975–976. [Google Scholar]
- Süsse, P.; Krampe, C. 6,6'-Dibromo-indigo, a main component of Tyrian purple. Its crystal structure and light absorption. Naturwissenschaften 1979, 66, 110. [Google Scholar]
- Larsen, S.; Watjen, F. The crystal and molecular structures of tyrian purple (6,6'-dibromoindigotin) and 2,2'-dimethoxyindigotin. Acta Chem. Scand. Ser. A 1980, A34, 171–176. [Google Scholar]
- Süsse, P.; Steins, M.; Kupcik, V. Z. Kristallogr. 1988, 184, 269.
- Wouters, J.; Verhecken, A. Composition of Murex dyes. J. Soc. Dyers Colour. 1992, 108, 404. [Google Scholar]
- Cooksey, C.J.; Withnall, R. Chemical studies on Nucella lapillus. Dyes Hist. Archaeol. Lyon 1997, (in press). [Google Scholar]
- Wouters, J. personal communication.
- Bide, M.J.; Choi, H. The thin layer chromatography of vat dyes. J. Soc. Dyers Colour. 1992, 108, 133–138. [Google Scholar]
- Cooksey, C.J. TLC of the Indigoid Colorants in Shellfish Purple. Dyes Hist. Archaeol. 1996, 14, 70–77. [Google Scholar]
- Bouchilloux, S.; Roche, J. Sur la pourpre des Murex trunculus et ses précurseurs. C. R. Soc. Biol. Lyon 1954, 148, 1583–1587. [Google Scholar]
- Bouchilloux, S.; Roche, J. Sur les prochromogenes et les pigments purpuriques de Murex trunculus Linné. C. R. Soc. Biol., Lyon. 1954, 148, 1732–1734. [Google Scholar]
- Malaszkiewicz, J. Chromogene und Farbstoff-Komponenten der Purpurschnicke Murex trunculus. Ph.D.Thesis, University of Saarbrücken, Saarbrücken, 1967. [Google Scholar]
- Fouquet, H. Bau und Reaktionen natürlicher Chromogene indigoider Farbstoffe bei Purpurschnecken. Ph.D. Thesis, University of Saarbrücken, Saarbrücken, 1970. [Google Scholar]
- Fouquet, H.; Bielig, H.J. Biological Precursors and Genesis of Tyrian Purple. Angew Chem (Int Edit) 1971, 10, 816–817. [Google Scholar] Fouquet, H.; Bielig, H.J. Biologische Vorstufen und Genese von antikem Purpur. Angew Chem 1971, 83, 856–857. [Google Scholar]
- Spanier, E. Rediscovering Royal Purple and Biblical Blue. Oceanus 1990, 33, 75. [Google Scholar]
- Michel, R.H.; Lazar, J.; McGovern, P.E. The chemical composition of the indigoid dyes derived from the hypobranchial glandular secretions of Murex molluscs. J. Soc. Dyers Colour. 1992, 108, 145–150. [Google Scholar]
- Melzer, R.R.; Brandhuber, P.; Zimmermann, T.; Smola, U. Der Purpur: Farben aus dem Meer. Biol. Unserer Zeit. 2001, 31, 30–39. [Google Scholar]
- Fujise, Y.; Miwa, K.; Ito, S. Structure of tyriverdin, the intermediate precursor of Tyrian purple. Chem. Lett. 1980, 6, 631–632. [Google Scholar]
- Baker, J.T.; Duke, C.C. Isolation of Choline and Choline ester salts of Tyrindoxyl sulfate. Tetrahedron Lett. 1976, 1233–1234. [Google Scholar]
- Baker, J.T.; Duke, C.C. The Chemistry of the indoleninones. II. Isolation from the hypobranchial glands of marine molluscs of 6-bromo-2,2-dimethylthioindolin-3-one and 6-bromo-2-methylthioindoleninone as alternative precursors to Tyrian Purple. Aust. J. Chem. 1973, 26, 2153–2157. [Google Scholar]
- Hiyoshi, Y.; Fujise, Y. Tyrian purple. As a learning tool for natural product chemistry. Kagaku to Kyoiku 1992, 40, 390–3. [Google Scholar]
- Benkendorff, K.; Bremner, J.B.; Davis, A.R. Tyrian purple precursors in the egg masses of the Australian muricid, Dicathais orbita: A possible defensive role. J. Chem. Ecol. 2000, 26, 1037–1050. [Google Scholar]
- Fujise, Y. Chemistry of the Production of Tyrian Purple and Related Natural Products. Kagakushi 1999, 26, 34–44. [Google Scholar]
- Benkendorff, K.; Bremner, J.B.; Davis, A.R. Indole derivatives from the egg masses of Muricid molluscs. Molecules 2001, 6, 70–78. [Google Scholar]
- Christophersen, C.; Wätjen, F.; Buchardt, O.; Anthoni, U. A revised structure of tyriverdin. The precursor of Tyrian purple. Tetrahedron 1978, 34, 2779–81. [Google Scholar]
- Sadler, P.W. J. Org. Chem. 1956, 21, 169–70.
- Holt, S.J.; Sadler, P.W. Studies in enzyme cytochemistry. II. Synthesis of indigogenic substrates for esterases. Proc. Roy. Soc. London, B. 1958, 148, 481–494. [Google Scholar]
- Baker, J.T.; Duke, C.C. The Chemistry of the indoleninones. III. Reactions of 2-(Methylthio)indoleninones with Diazomethane. Aust. J. Chem. 1976, 29, 1023–1030. [Google Scholar]
- Imming, P.; Zentgraf, M.; Imhof, I. Welche Farbe hatte der antike Purpur? Textilveredlung 2000, 35, 22–24. [Google Scholar]
- Ettinger, L.; Friedlander, P. Uber 6,6’-dibrom-indirubin. Ber. Dtsch. Chem. Ges. 1912, 45, 2081–2083. [Google Scholar]
- Grosjean, D.; Sensharma, D.K.; Cass, G.R. Fading of colorants by atmospheric pollutants: mass spectrometry studies. Sci. Total Environ. 1994, 152, 125–34. [Google Scholar]
- Cooksey, C.J. The Synthesis of minor Components of Shellfish Purple: Bromoisatin, Bromoindigotin and Bromoindirubins, from Dibromoindigotin. Bei. Waidtag. 1998, 7, 71–74. [Google Scholar]
- Voss, G.; Schramm, W. Selectively C-deuterated indigotins. Helv. Chim. Acta 2000, 83, 2884–2892. [Google Scholar]
- Schweppe, H. Handbuch der Naturfarbstoffe. Vorkommen, Verwendung, Nachweis; Ecomed: Landsberg/Lech, 1993; pp. 304–314. [Google Scholar]
- Spanier, E.; Karmon, N.; Linder, E. Bibliography concerning various aspects of the purple dye. Levantina. 1982, pp. 437–447. updated at http://www.tekhelet.com/pdf/ta02.pdf.
- Cooksey, C.J. Bibliography: Tyrian (Shellfish) Purple. Dyes Hist. Archaeol. 1994, 12, pp. 57–66. updated at http://www.chriscooksey.demon.co.uk/tyrian/cjcbiblio.html.
- Cardon, D.; du Chatenet, G. Guide des Teintures Naturelles; Delachaux et Niestlé: Lausanne, 1990; pp. 338–354. [Google Scholar]
- Clark, R.J.H.; Cooksey, C.J.; Daniels, M.A.M.; Withnall, R. Indigo, woad, and Tyrian Purple: important vat dyes from antiquity to the present. Endeavour 1993, 17, 191–199. [Google Scholar]
- Spanier, E (Ed.) The Royal Blue and The Biblical Purple; Argaman and Tekhelet; Keter Publishing House Jerusalem Ltd.: Jerusalem, 1987.
- ‘How could the purple be used? Today when chemical manufacturers produce a torrent of materials for industry, which, with great ease and perfection, provide fine and strong colours, how can we expect to see this small animal which provides the purple, though beautiful and long-lasting, being used by industry? It is hardly likely that the purple will return to favour.’
- http://www.kremer-pigmente.de/intl.catalog/epigmen08.htm.
- Sample Availability: Samples of 6,6'-dibromoindigo, 6-bromoindigo and indirubin are available from MDPI.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cooksey, C.J. Tyrian Purple: 6,6’-Dibromoindigo and Related Compounds. Molecules 2001, 6, 736-769. https://doi.org/10.3390/60900736
Cooksey CJ. Tyrian Purple: 6,6’-Dibromoindigo and Related Compounds. Molecules. 2001; 6(9):736-769. https://doi.org/10.3390/60900736
Chicago/Turabian StyleCooksey, Christopher J. 2001. "Tyrian Purple: 6,6’-Dibromoindigo and Related Compounds" Molecules 6, no. 9: 736-769. https://doi.org/10.3390/60900736




