General
Mps are uncorrected. Elemental analyses were carried out in the Microanalytical Laboratories of the Faculty of Science, Cairo University, and the results are given in
Table III. IR spectra (KBr disks) were measured on a FT IR/5300 spectrometer.
1H-NMR spectra (δ/ppm) were recorded for DMSO-d
6 solutions on Varian Gemini (200 MHz) or Varian Mercury (300 MHz) spectrometers. Mass spectra were obtained on a Shimadzu GC-MS-QP 1000 EX spectrometer.
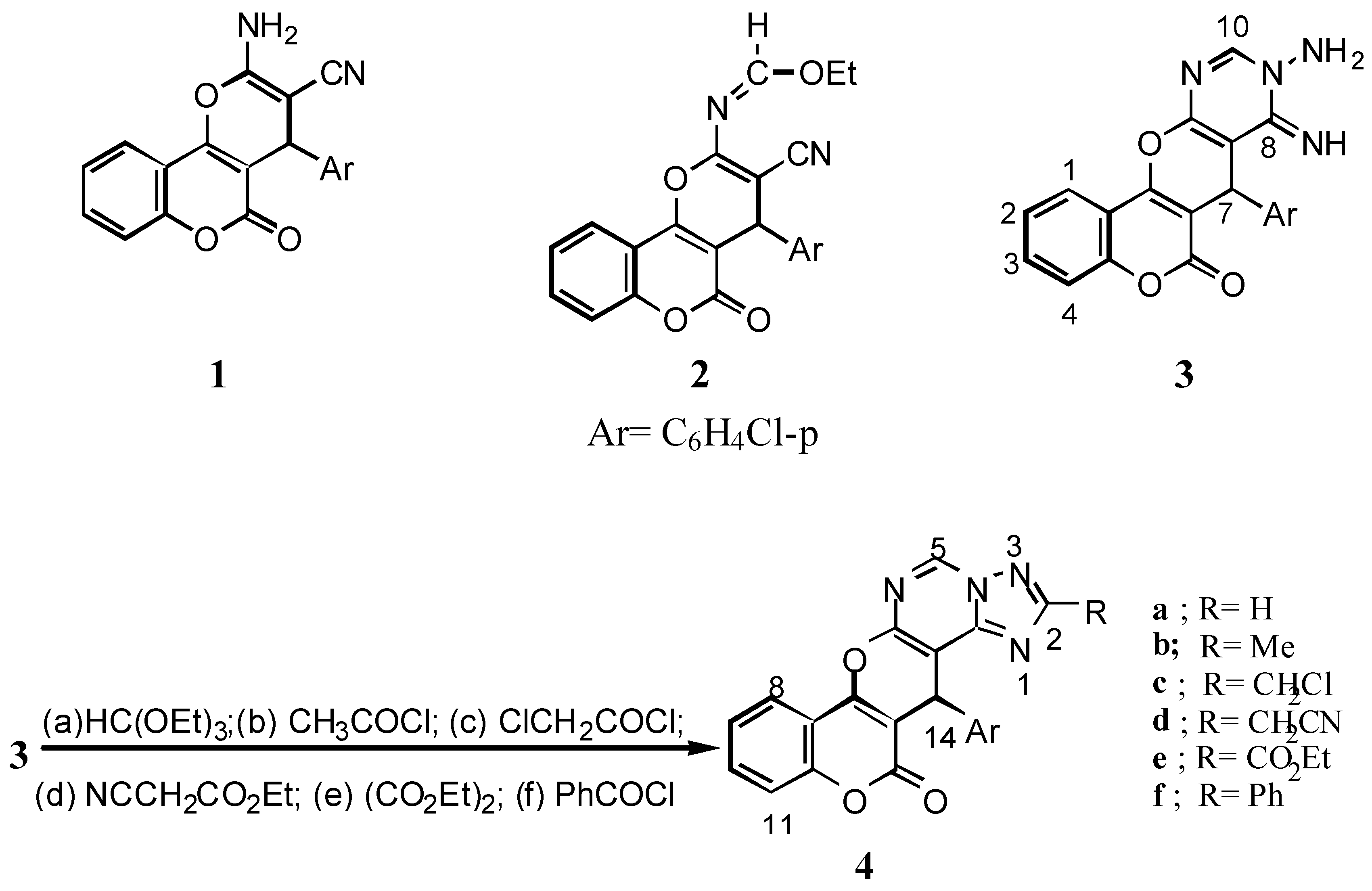
2-Amino-4-(4’-chlorophenyl)-3-cyano-4H,5H-pyrano[3,2-c][1]benzopyran-5-one (
1): A solution of 4-hydroxycoumarin (0.01 mol) in ethanol (30 mL) and α-cyano-
p-chlorocinnamonitrile (0.01 mol) was heated for 30 min. to give
1 (85% yield), m.p. 261°C (lit. [
12] 258-260°C).
4-(4’-Chlorophenyl)-3-cyano-2-ethoxymethyleneamino-4H,5H-pyrano[3,2-c][1]benzopyran-5-one (
2): A mixture of
1 (3.49g, 0.01 mol), triethyl orthoformate (0.01 mol) and acetic anhydride (20 mL) was refluxed for 5 h. The solvent was removed under vacuum. The residue obtained was recrystallized from benzene to give
2 (73% yield), m.p. 236°C (lit. [
12] 221-223°C).
9-Amino-7-(4’-chlorophenyl)-8,9-dihydro-8-imino-6H,7H-[1]benzopyrano[3’,4’:5,6]-pyrano[2,3-d]-pyrimidine-6-one (3): A solution of 2 (4.06 g, 0.01 mol) and hydrazine hydrate (99%, 5mL) in ethanol (50 mL) was stirred for 45 min at room temperature. The colourless solid obtained was filtered off and crystallized from benzene to give 3 (76% yield); IR: 3568, 3547 (NH2), 3337 (NH), 1718 (δ-lactone C=O), 1653 (C=N). 1H-NMR: 9.79(br, 1H, NH), 8.65 (s, 1H, H-10), 7.30-8.05 (m, 10H, arom., NH2), 5.44 (s, 1H, H-7).
14-(4’-Chlorophenyl)-13H,14H-[1]benzopyrano[3’,4’:5,6]pyrano[3,2-e][1,2,4]triazolo [1,5-c]-pyrimidine-13-one (4a): A solution of 3 (3.91g, 0.01 mol) and triethyl orthoformate (0.01 mol) in dry benzene (30 mL) was refluxed for 6h to give 4a (78% yield); IR: 2940 (CH stretching), 1720 (δ-lactone C=O).
14-(4’-Chlorophenyl)-2-methyl-13H,14H[1]benzpyrano[3’,4’:5,6]pyrano[3,2-e][1,2,4]-triazolo[1,5-c]-pyrimidine-13-one (4b): To a solution of 3 (3.91 g, 0.01 mol) in dry benzene (30 mL) acetyl chloride was added (0.01 mol) dropwise with stirring. The reaction mixture was boiled at reflux for 3 h and after cooling poured into ice-water (50 mL) to give 4b (78% yield); IR 2940 (CH stretching), 1720 (δ-lactone C=O); MS: m/z 416 (M+, 3.84%), 418 (M+2, 1.3%), (ratio 3:1), 402 (35.6), 404 (12.87), (ratio 3:1), 291 (100), 264 (8.98), 200 (1.49), 165 (3.47), 121 (2.89), 75(4.72).
2-Chloromethyl-14-(4’-chlorophenyl)-13,14H-[1]benzopyrano[3’,4’:5,6]pyrano-[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine-13-one (4c): Compound 4c was prepared from 3 (3.91 g, 0.01 mol) and chloroacetyl chloride (0.01 mol) according to the procedure described for 4b; (71% yield); IR: 3086 (CH stretching), 1724 (δ-lactone C=O); MS: m/z 450 (M+, 25.1%), 452 (M+2, 15.9%), 454 (M+4, 3.32), 339 (100), 341 (35.35) (ratio 3:1), 264 (13.85), 236 (17.26), 185 (13.81), 129 (26.66), 55 (78).
14-(4’-Chloromethyl)-2-cyanomethyl-13H,14H-[1]benzopyrano[3’,4’:5,6]pyrano[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine-13-one (4d): A mixture of 3 (3.91g, 0.01 mol), ethyl cyanoacetate (0.01 mol) and absolute ethanol (20 mL) was refluxed for 6 h. On cooling, the solid obtained was crystallized from benzene to give 4d (67% yield); IR: 3068, 2928, 2816 (CH stretching), 2262 (CN), 1724 (δ-lactone C=O); MS: m/z 441 (M+, 16.9%), 443 (M+2, 6.04%), (ratio 3:1), 330 (63.38), 264 (7.24), 237 (4.47), 173 (2.9), 117(4), 89 (6.13).
14-(4’-Chlorophenyl)-2-ethoxycarbonyl-13H,14H-[1]benzopyrano[3’,4’:5,6]-pyrano[3,2-e]-[1,2,4]-triazolo[1,5-c]pyrimidine-13-one (4e): Prepared from 3 (3.91g, 0.01 mol) and diethyl oxalate (0.01 mol) according to the procedure described for 4d; (78% yield); ΙR: 3052, 2985 (CH stretching), 1741 (ester C=O), 1724 (δ-lactone C=O); 1H-NMR: 9.88 (s, 1H, H-5), 7.26-8.32 (m, 8H, arom.), 5.51 (s, 1H, H-14), 4.41 (q, 2H, CH2, J= 6.7 Hz), 1.34 (t, 3H, CH3, J= 6.7 Hz).
14-(4’-Chlorophenyl)-2-phenyl-13H,14H-[1]benzopyrano[3’,4’:5,6]pyrano[3,2-e]-[1,2,4]triazolo-[1,5-c]pyrimidine-13-one (4f): Prepared from 3 (3.91g, 0.01 mol) and benzoyl chloride (0.02 mol) according to the procedure described for 4a; (67% yield); MS: m/z 478 (M+, 33.66%), 480 (M+2, 12.71%), (ratio 3:1), 367 (100), 291 (1.2), 264 (4.02), 165 (2.37), 121(2.8), 77 (7.48).
Methyl N-[7-(4’-chlorophenyl)-8-imino-6-oxo-6H,7H-[1]benzopyrano[3’,4’:5,6]pyrano-[2,3-d]-pyrimidyl-9]carbamate (5a): Prepared from 3 (3.91g, 0.01 mol) and methyl chloroformate (0.01 mol) according to the procedure described for 4a (reaction time: 30 min.), to give 5a (71% yield): IR: 3500- 2421 centered at 3067 (NH, CH stretching), 1737 (C=O), 1720 (δ-lactone C=O); 1H-NMR: 9.43 (br, 1H, C=NH), 8.92 (s, 1H, H-10), 7.37-8.01 (m, 8H, arom.), 6.88 (br, 1H, NHCO), 5.51(s, 1H, H-7), 3.58 (s, 3H, CH3); MS: m/z 450 (M+, 0%), 377 (46.0), 266 (100%), 239 (41.8), 121 (54.8), 92 (24.1).
14-(4’-Chlorophenyl)-2,3-dihydro-2H,13H,14H-[1]benzopyrano[3’,4’:5,6]pyrano[3,2-e][1,2,4]-triazolo[1,5-c]pyrimidine-2,13-dione (5b): Prepared in 60% yield from 3 (3.91g, 0.01 mol) and methyl chloroformate (0.01 mol) according to the procedure described for 4a; IR: 3422 (NH), 3069, 2853 (CH stretching), 1713 (δ-lactone C=O ), 1653 (C=O); 1H-NMR: 12.58 (br, 1H, NH), 8.65 (s, 1H, H-5), 7.30-8.06 (m, 8H, arom.), 5.45 (s, 1H, H-14); MS: m/z 418 (M+, 4.9%), 401 (3.7), 291 (100%), 239 (3.4), 186 (1.0), 121 (11.0), 92 (6.5), 75 (12.7).
14-(4’-Chlorophenyl)-2,3-dihydro-13-oxo-2H-13H,14H-[1]benzopyrano[3’,4’:5,6]-pyrano[3,2-e]-[1,2,4]triazolo[1,5-c]pyrimidine-2-thione (5c): A mixture of 3 (3.91g, 0.01 mol), ethanol (30 mL), KOH (0.3 g) and carbon disulfide (3 mL) was refluxed for 15 h. After removal of the ethanol, water was added and the alkaline solution was acidified with acetic acid to give the thione 5c (55% yield); IR: 3398 (NH), 1714 (δ-lactone C=O), 1041 (C=S); MS: m/z 434 (M+, 1%), 324 (3.3), 266 (100), 212 (3.51), 174 (3.53), 144 (1.07), 104 (1.69), 76 (5.48).
Ethyl 14-(4’-chlorophenyl)-2,13-dioxo-2,3-dihydro-2H-13H,14H-[1]benzopyrano-[3’,4’:5,6]-pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine-3-carboxylate (5d): Prepared in 45% yield from 3 (3.91g, 0.01 mol) and ethyl chloroformate (0.01 mol) according to the procedure described for 4a; IR: 3068, 2991 (CH stretching), 1747 (ester C=O), 1736 (δ-lactone C=O), 1714 (C=O); 1H-NMR: 9.03 (s, 1H, H-5), 7.30-8.39 (m, 8H, arom.), 4.84 (s, 1H, H-14), 4.04 (q, 2H, CH2, J= 7 Hz), 1.18 (t, 3H, CH3, J= 7 Hz).
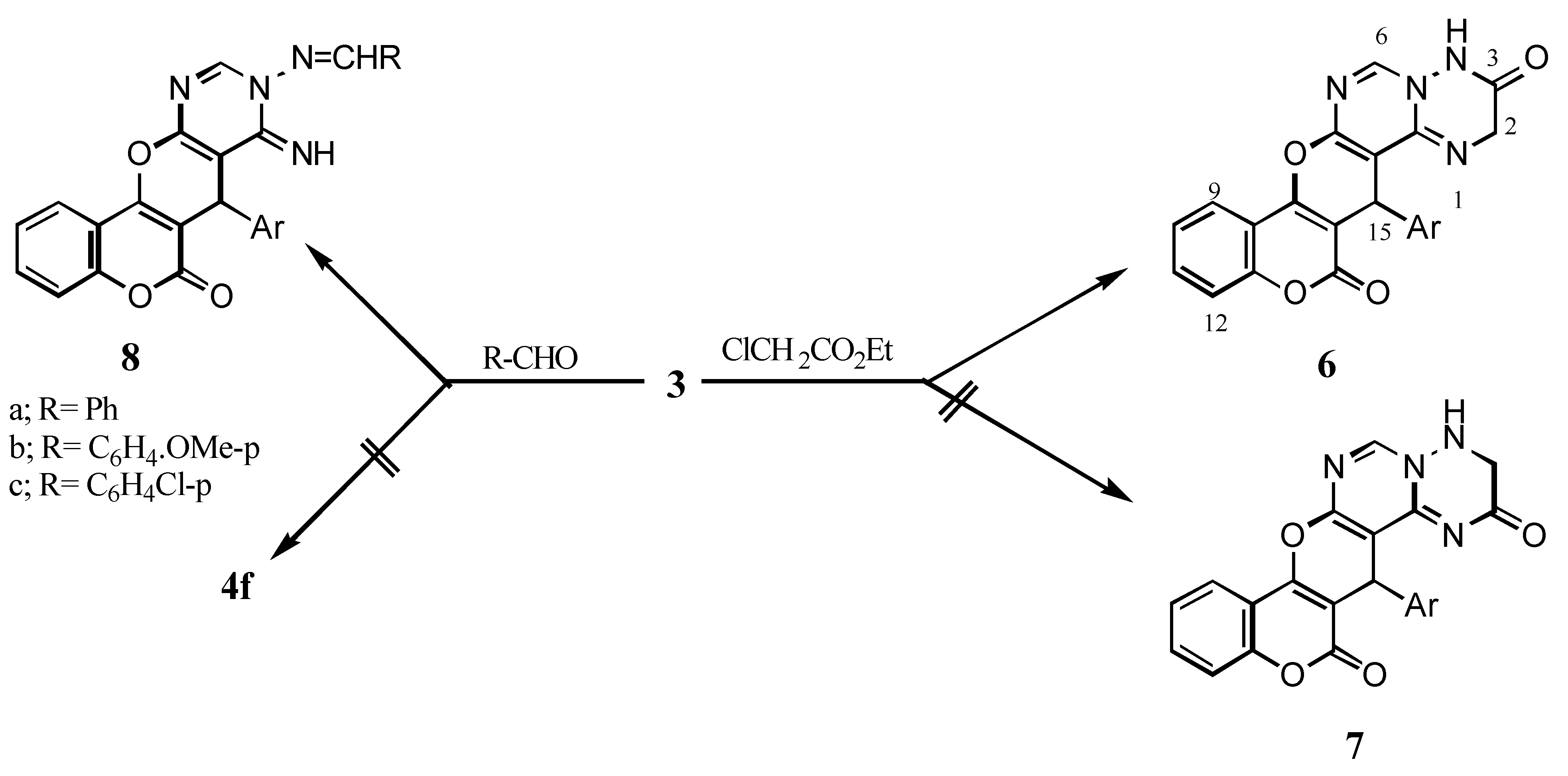
15-(4’-Chlorophenyl)-3,4-dihydro-2H,-14H,15H-[1]benzopyrano[3’,4’:5,6]pyrano[2,3-d]pyrimido-[1,6-b][1,2,4]triazine-3,14-dione (6): A mixture of 3 (3.91g, 0.01 mol), ethyl chloroacetate (0.01 mol), methanol (30 mL) and sodium metal (0.023 g, 0.01 mol) was refluxed for 6 h. The reaction mixture was cooled, then poured into cold water to give 6 (64% yield); IR: 3468 (NH), 1726 (δ-lactone C=O), 1650 (C=O); 1H-NMR: 9.78 (s, 1H, H-6), 7.36-8.17 (m, 9H, arom.+ NH), 5.47 (s, 1H, H-15), 4.95 (s, 2H, CH2).
9-Arylmethylideneamino-7-(4’-chlorophenyl)-8,9-dihydro-8-imino-6H,7H-[1]benzopyrano-[3’,4’:5,6]pyrano[2,3-d]pyrimidine-6-ones (8a-c): A mixture of 3 (3.91g, 0.01 mol), benzaldehyde, p-anisaldehyde or p-chlorobenzaldehyde (0.01 mol), piperidine (0.5 mL) and dioxane (30 mL) was refluxed for 16 h to give 8a-c (77-85% yield after workup); 8a: IR: 3231 (NH), 3067, 3026 (CH stretching) 1713 (δ-lactone C=O); 1H-NMR: 11.30 (br, 1H, C=NH), 8.48 (s, 1H, H-10), 8.24 (s, 1H, N=CH), 7.27-7.98 (m, 13H, arom.), 6.06 (s, 1H, H-7); 8b: IR: °3279 (NH), 2909, 2833 (CH stretching), 1707 (δ-lactone C=O); 1H-NMR: 11.14 (br, 1H, C=NH), 8.45 (s, 1H, H-10), 8.16 (s, 1H, N=CH), 7.01-7.98 (m, 12H, arom.), 6.27 (s, 1H, H-7), 3.81 (s, 3H, OCH3); 8c: IR: 3284 (NH), 1721 (δ-lactone C=O); 1H-NMR: 11.33 (br, 1H, C=NH), 8.48 (s,1H, H-10), 8.21(s, 1H, N=CH), 7.27-7.96 (m, 12H, arom.), 5.95 (s, 1H, H-7); MS: m/z 514 (M+, 7.38%), 516 (M+2, 4.22), 5.18 (M+4, 0.73), 403 (6.79), 292 (1.53), 266 (37.09), 185 (11.63), 121 (31.24), 55 (100).
Table III.
Characterization data for newly synthesized compounds
Table III.
Characterization data for newly synthesized compounds
| Compd. No. | M.P.(T/°C)a | Molecular formula (Molecular weight) | Elemental analyses Found (Required) % |
|---|
| C | H |
|---|
| 3 | 250c | C20H13ClN4O3 (392.80) | 61.1 (61.15) | 3.3 (3.31) |
| 4a | 282 | C21H11ClN4O3 (402.80) | 62.5 (62.61) | 2.7 (2.73) |
| 4b | 279 | C22H13ClN4O3 (416.82) | 63.3 (63.39) | 3.0 (3.12) |
| 4c | 310 | C22H12Cl2N4O3 (451.27) | 58.5 (58.54) | 2.6 (2.66) |
| 4d | 307 | C23H12ClN5O3 (441.83) | 62.4 (62.52) | 2.6 (2.71) |
| 4e | 292b | C24H15ClN4O5 (474.86) | 60.6 (60.70) | 3.1 (3.16) |
| 4f | 298b | C27H15ClN4O3 (478.90) | 67.6 (67.71) | 3.1 (3.13) |
| 5a | 248b | C22H15ClN4O5 (418.84) | 58.4 (58.67) | 3.1 (3.33) |
| 5b | 285 | C21H11ClN4O4 (418.80) | 60.1 (60.29) | 2.4 (2.63) |
| 5c | 275 | C21H11ClN4O3S (434.86) | 57.9 (57.99) | 2.5 (2.53) |
| 5d | 286b | C24H15ClN4O6 (490.86) | 58.6 (58.72) | 3.0 (3.05) |
| 6 | 289 | C22H13ClN4O4 (432.82) | 60.9 (61.04) | 2.9 (3.00) |
| 8a | 316 | C27H17ClN4O3 (480.91) | 67.3 (67.43)) | 3.4 (3.53) |
| 8b | 290 | C28H19ClN4O4 (510.94) | 65.7 (65.82) | 3.6 (3.72) |
| 8c | 345 | C27H16Cl2N4O3 (515.36) | 62.8 (62.92) | 3.0 (3.10) |