Zn Mediated Regioselective Barbier Reaction of Propargylic Bromides in THF/aq. NH4Cl Solution
Abstract
:Introduction
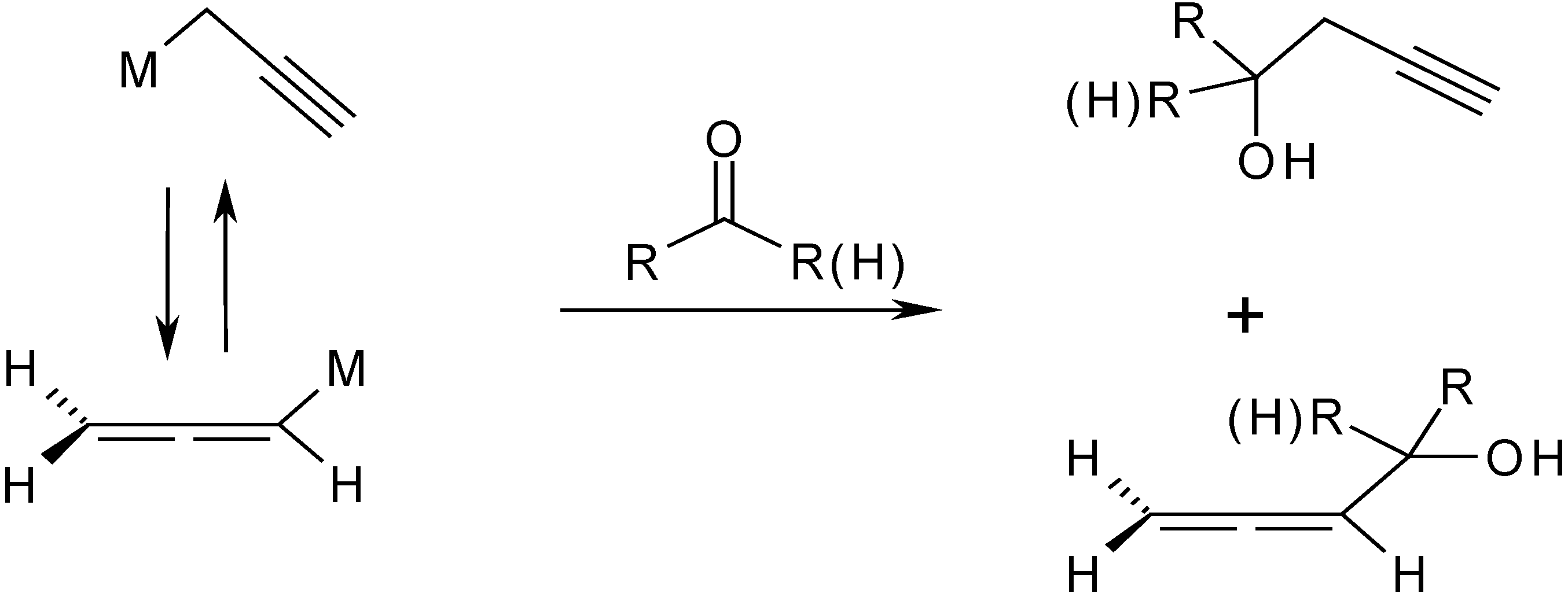
Results and Discussion
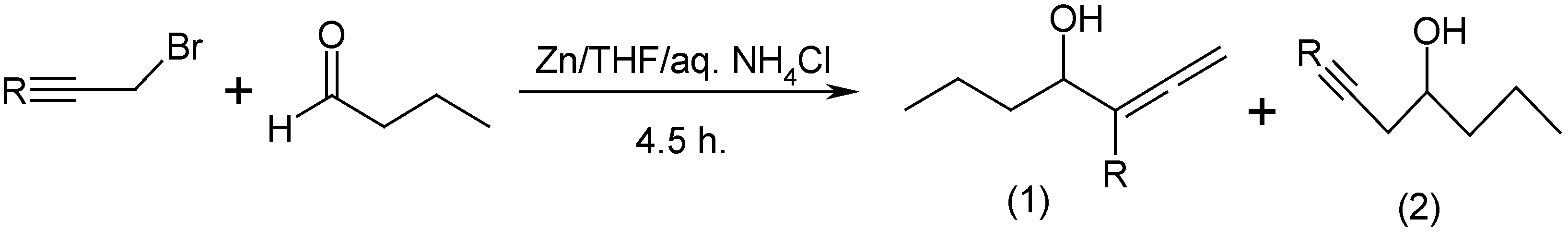
| Propargylic bromide (R) | Yield, % | Allenic (1) : Propargylic (2) alcohol |
| C6H13 | 47 | 100 : 0 |
| H | 25 | 0 : 100 |
Conclusions
Experimental
General
Typical synthetic procedure
Spectral Data
Acknowledgements
References and Notes
- Trost, B. M. Comprehensive Organic Synthesis; Fleming, I., Ed.; Pergamon Press: Oxford, 1991; Vol. 1, Part 1; pp. 255–266. [Google Scholar]
- Li, C.J. Aqueous Barbier-Grignard type reaction: scope, mechanism, and synthetic applications. Tetrahedron 1996, 52, 5643–5668. [Google Scholar]
- Cho, Y. S.; Lee, J. E.; Pae, A. N.; Choi, K. I.; Koh, H. Y. Indium and zinc mediated Barbier type reactions: allylation and propargylation reactions of 6-oxopenicillanate and 7-oxocephalosporanate. Tetrahedron 1999, 40, 1725–1728. [Google Scholar]
- Kim, S. H.; Han, E. H. Zinc mediated Barbier type propargylation of cyclic imides. Tetrahedron Lett. 2000, 41, 6479–6482. [Google Scholar]
- Iseki, K.; Kuroki, Y.; Kobayashi, Y. Asymmetric allenylation of aliphatic aldehydes catalyzed by a chiral formamide. Tetrahedron: Asymmetry 1998, 9, 2889–2894. [Google Scholar]
- Kurono, N.; Sugita, K.; Tokuda, M. Regioselective propargylation of aldehydes and ketones by electrochemical reaction using zinc and aluminum anodes. Tetrahedron 2000, 56, 847–854. [Google Scholar]
- Isaac, M. B.; Chan, T.-H. Indium-mediated coupling of aldehydes with prop-2-ynyl bromides in aqueous media. J. Chem. Soc., Chem. Commun. 1995, 1003–1004. [Google Scholar]
- Cabezas, J. A.; Alvarez, L. X. Propargylation of carbonyl compounds: an efficient method for the synthesis of homopropargyl alcohols. Tetrahedron Lett. 1998, 39, 3935–3938. [Google Scholar]
- Bieber, L. W.; Silva, M. F.; Costa, R. C.; Silva, L. O. S. Zinc Barbier reaction of propargyl halides in water. Tetrahedron Lett. 1998, 39, 3655–3658. [Google Scholar]
- Brandsma, L. Preparative Acetylenic Chemistry; Elsevier: Amsterdam, 1971; p. 158. [Google Scholar]
- Favre, E.; Gaudemar, M. J. Organomet. Chem. 1974, 76, 297–304.
- Sample Availability: Samples of compound 1 are available from MDPI.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jõgi, A.; Mäeorg, U. Zn Mediated Regioselective Barbier Reaction of Propargylic Bromides in THF/aq. NH4Cl Solution. Molecules 2001, 6, 964-968. https://doi.org/10.3390/61200964
Jõgi A, Mäeorg U. Zn Mediated Regioselective Barbier Reaction of Propargylic Bromides in THF/aq. NH4Cl Solution. Molecules. 2001; 6(12):964-968. https://doi.org/10.3390/61200964
Chicago/Turabian StyleJõgi, Artur, and Uno Mäeorg. 2001. "Zn Mediated Regioselective Barbier Reaction of Propargylic Bromides in THF/aq. NH4Cl Solution" Molecules 6, no. 12: 964-968. https://doi.org/10.3390/61200964



