Flash Vacuum Pyrolysis of 2,5-Diphenyloxazole
Abstract
:Introduction
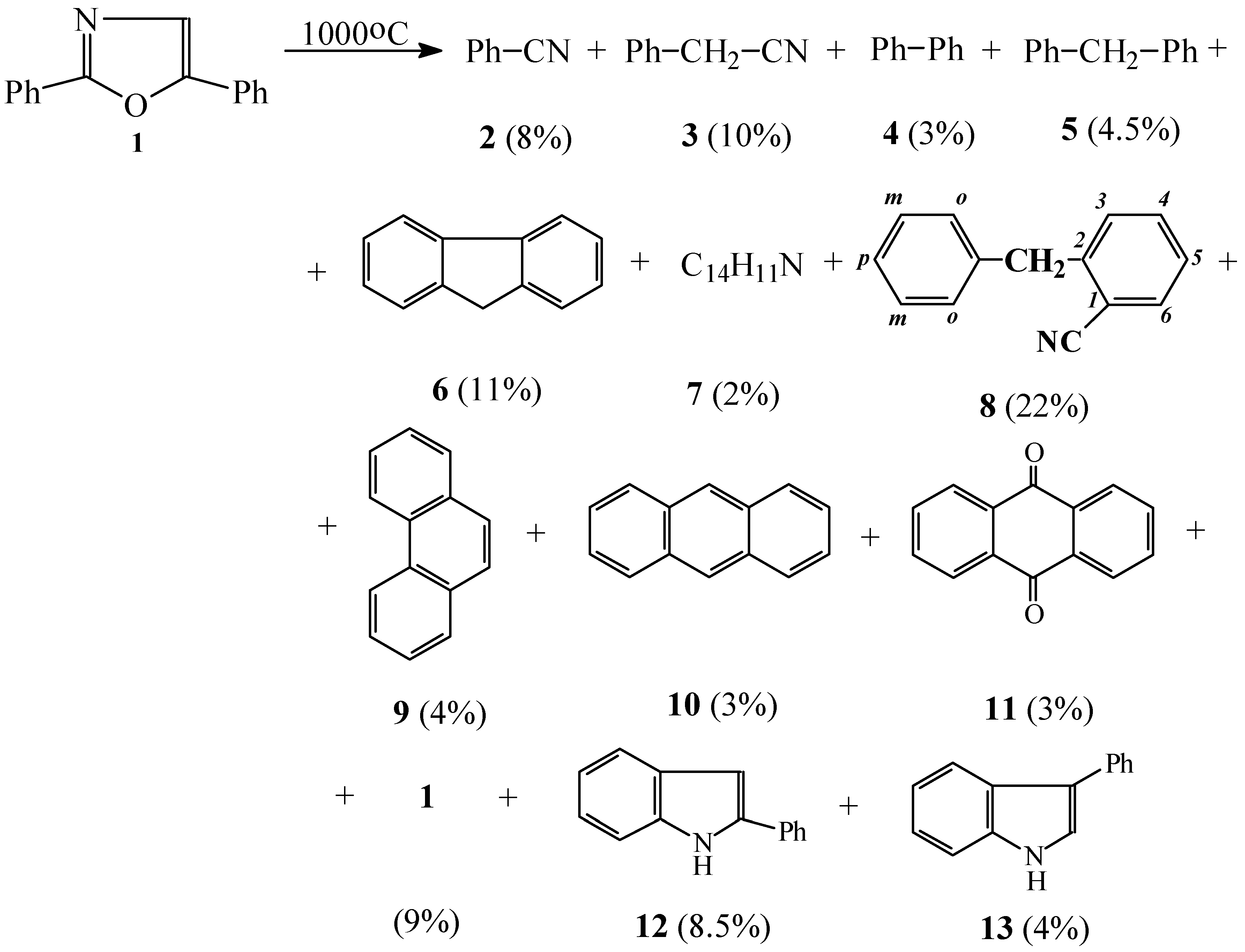
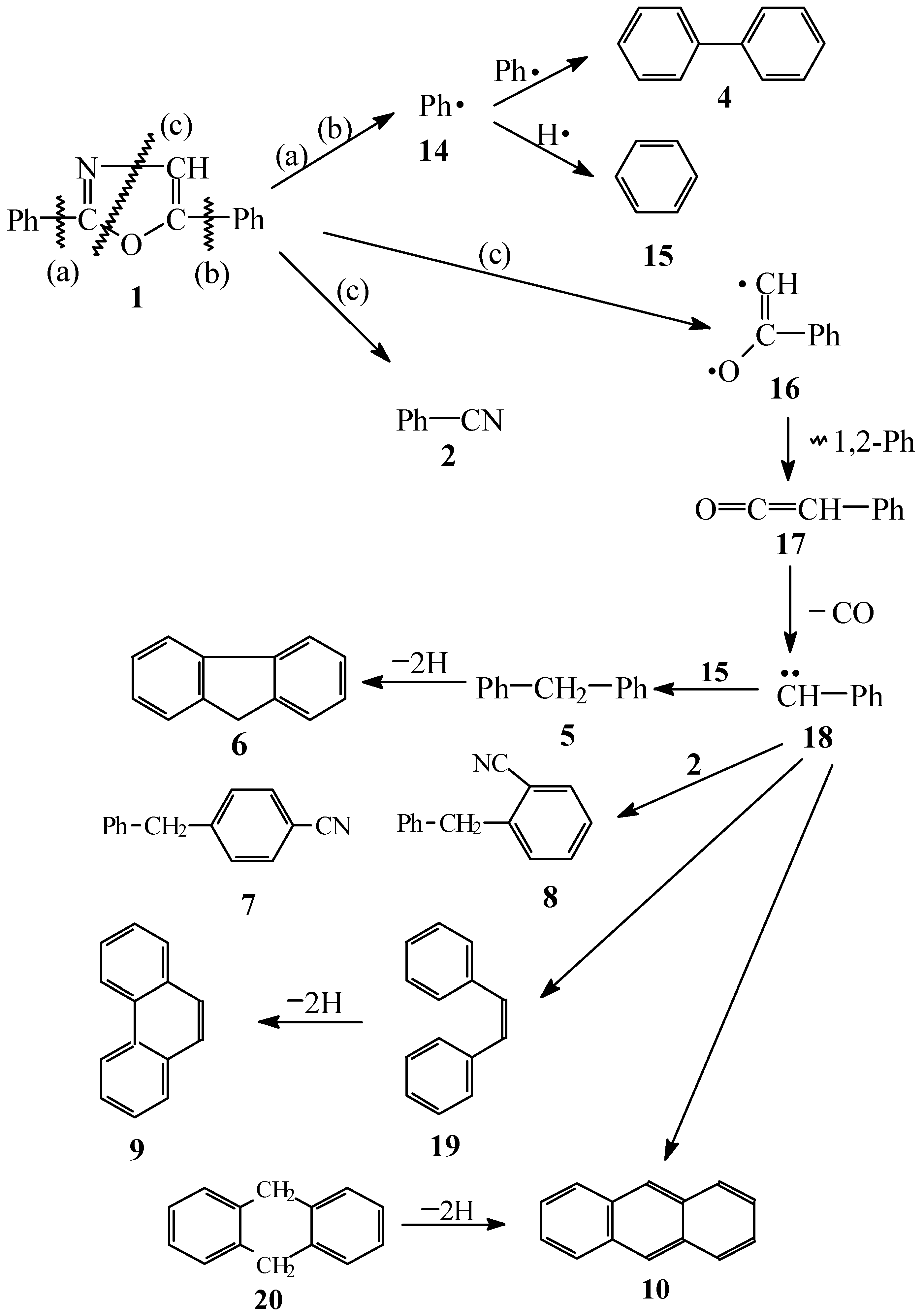
Results and Discussion
Conclusions
Experimental
General
2,5-Diphenyloxazole (1)
FVP of 2,5-Diphenyloxazole
Diphenylmethane (5)
Benzylbenzonitrile (7)
o-Benzylbenzonitrile (8)
2-Phenylindole (12)
3-Phenylindole (13)
Acknowledgements
References and Notes
- Brown, R.F.C. Pyrolytic Methods in Organic Chemistry; Academic Press: New York, 1980; Volume 247–281, pp. 328–335. [Google Scholar]
- Braslavsky, S.; Heicklen, J. The Gas-Phase Thermal and Photochemical Decomposition of Heterocyclic Compounds Containing Nitrogen, Oxygen or Sulfur. Chem. Rev. 1977, 77, 473–511. [Google Scholar] [CrossRef]
- McNab, H. Synthetic Applications of Flash Vacuum Pyrolysis. Contemp. Org. Synth. 1996, 3, 373–396. [Google Scholar] [CrossRef]
- Aldous, G.L.; Bowie, J.H.; Thompson, M.J. Thermal Rearrangements of 3,5-Diphenylisoxazole. J. Chem. Soc. Perkin Trans. I 1976, 16–19. [Google Scholar] [CrossRef]
- Flammang, R.; Laurent, S.; Flammang-Barbieux, M.; Wentrup, C. Formation and Identification of Ionized and Neutral Cumulenes, RN:C:C:C:NH, by Tandem Mass Spectrometry. Org. Mass Spectrom. 1993, 28, 1161–1166. [Google Scholar] [CrossRef]
- Kappe, C.O.; Flamming, R.; Wentrup, C. Synthesis and Flash Vacuum Pyrolysis of Isoxazolo[4,5-d] pyrimidines. Heterocycles 1994, 37, 1615–1622. [Google Scholar]
- Khalafy, J.; Prager, R.H. Flash Vacuum Pyrolysis of some N-Benzyl-benzotriazoles and N-Benzylbenzisoxazolones. Aust. J. Chem. 1998, 51, 925–929. [Google Scholar] [CrossRef]
- Prager, R.H.; Singh, Y. The Chemistry of 5-Oxodihydroisoxazoles. VII. Conversion of Heterocyclyloxazol-5(4H)-ones to Imidazoles by Flash Vacuum Pyrolysis. Tetrahedron 1993, 49, 8147–8158. [Google Scholar] [CrossRef]
- Clark, A.D.; Janowski, W.K.; Prager, R.H. Unusual Rearrangements of 2-aroylimidoyl-2-phenyl-ethylidene to 2,5-disubstituted Oxazoles. Tetrahedron 1999, 55, 3637–3648. [Google Scholar] [CrossRef]
- Flammang, R.; Plisnier, M.; Bouchoux, G.; Happilliard, Y.; Humbert, S.; Wentrup, C. Unimolecular Chemistry of Oxazole and Isoxazole Radical Cations in the Gas Phase. Org. Mass Spectrom. 1992, 27, 317–325. [Google Scholar] [CrossRef]
- Lesniak, S.; Mloston, G.; Heimgartner, H. Flash Vacuum Pyrolysis of 1,3-Thiazole-5(4H)-thiones. Pol. J. Chem. 1998, 72, 1915–1920. [Google Scholar]
- Balaban, A.T.; Banciu, D.M.; Ciorba, V. Annulenes-, Benzo-, Hetero-, Homo-Derivatives and their Valence Isomers; CRC Press: Boca Raton, Fla, 1987; Vol. II, pp. 196–203. [Google Scholar]
- Banciu, D.M.; Popescu, A.; Simion, A.; Draghici, C.; Mangra, C.; Mihaiescu, D.; Pocol, M. FlowVacuum Pyrolysis of Tetrazoles with Annelated Dibenzocycloalkane Skeletons. J. Anal. Appl. Pyrolysis 1999, 48, 129–146. [Google Scholar] [CrossRef]
- Brown, R.F.C. Pyrolytic Methods in Organic Chemistry; Academic Press: New York, 1980; pp. 110, 119, 120–121. [Google Scholar]
- Schissel, P.; Kent, M.E.; McAdoo, D.J.; Hedaya, E. Flash-Vacuum Pyrolysis. VII. Fulvenallene. The Ring Contraction and Expansion of Phenylcarbene. J. Amer. Chem. Soc. 1970, 92, 2147–2149. [Google Scholar] [CrossRef]
- Banciu, D.M.; Stanescu, M.D.; Florea, C.; Petride, A.; Draghici, C.; Cioranescu, E. Benzoannelated Valence Isomers of Homoannulenes. I. Thermal Behaviour of some Dibenzo (C10H9) hydrocarbons. Bull. Soc. Chim. Fr. 1991, 128, 919–925. [Google Scholar]
- Cassirer, H. Zur Kenntniss der o-Cyano- und o-Nitrobenzylchloride. Chem. Ber. 1892, 25, 3081–3030. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Banciu, M.D.; Istrati, D.; Mihaiescu, D.; Draghici, C. Flash Vacuum Pyrolysis of 2,5-Diphenyloxazole. Molecules 2000, 5, 1004-1010. https://doi.org/10.3390/50801004
Banciu MD, Istrati D, Mihaiescu D, Draghici C. Flash Vacuum Pyrolysis of 2,5-Diphenyloxazole. Molecules. 2000; 5(8):1004-1010. https://doi.org/10.3390/50801004
Chicago/Turabian StyleBanciu, Mircea D., Daniela Istrati, Dan Mihaiescu, and Constantin Draghici. 2000. "Flash Vacuum Pyrolysis of 2,5-Diphenyloxazole" Molecules 5, no. 8: 1004-1010. https://doi.org/10.3390/50801004



