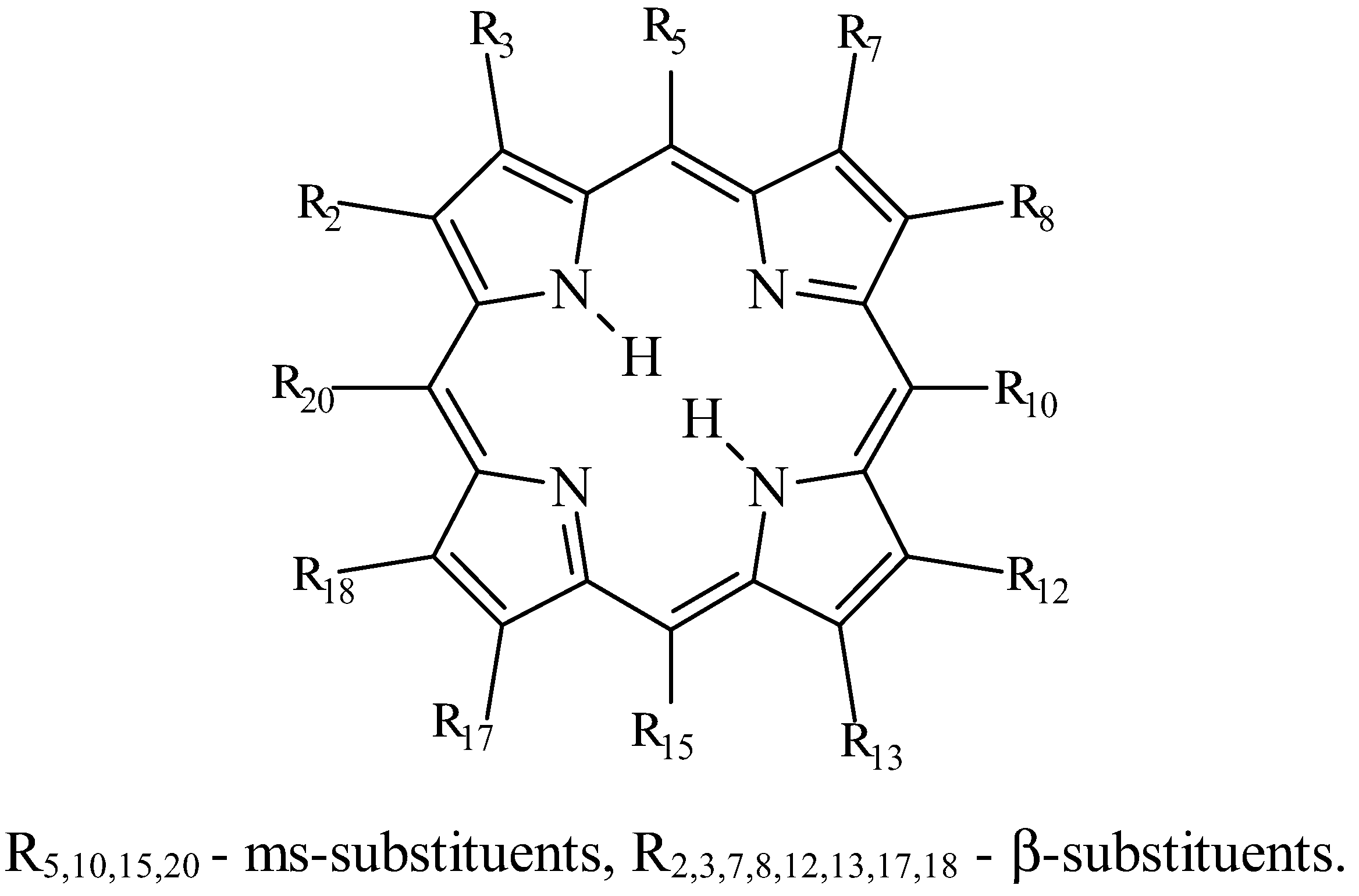
Introduction
Solubility is one of the most important physical constants of the substance similar to melting tem-perature, boiling point, etc.
In spite of the fairly great importance of solubility methods in physicochemical investigations, the solubility values are important primarily for practical reasons, e.g. for the selection of optimum condi-tions for synthesis, rectification, and for the study and practical applications of compounds.
The only method for determination of the solubility value of complex and macrocyclic compounds - porphyrins (1) and their complexes - is experimentation.
Usually the porphyrins display low solubility values in the majority of organic solvents and are also quite insoluble in water due to the relatively high energy of their molecular lattices and their moderate energies of solvation with respect to the low-polarity chemical bonding of the macrocycle.
As the experimental investigations showed, porphyrin solubility values in organic solvents essentially depend on their structure, i.e. on the functional substitution of hydrogen atom in the pyrroles (2,3,7,8,12,13,17,18), ms-positions (5,10,15,20) or NH-groups of the coordination center. A macrocy-cle's solubility can decrease or increase ten, hundred or even a thousand times depending on the nature, number and position of the functional substituents. For alkylporphyrins the last factor is the most essen-tial. In this paper the data obtained mainly in two solvents - benzene and ethanol - are discussed.
Results and Discussion
The length of the alkyl substituents has no considerable effect on the solubility values. The ms-alkyl substituent that causes a maximum increase of the solubility values depends on the porphyrin structure and the nature of the solvent. Among 5,15-dialkylsubstituted 3,7,13,17-tetramethyl-2,8,12,18-tetra- butylporphyrins, the 5,15-methylsubstituted porphyrin displays maximum solubility values.
Methyl groups introducing into the 5,15-positions of β-octamethylporphyrin practically does not change the porphyrin solubility. The majority of octalkylporphyrins with alkyl substitutents (from H to C
4H
9) in β-positions display average solubility values in benzene: (2-4)⋅10
-3 mol/l. (
Table 1). The num- ber of the alkyl groups, their length within the above-mentioned limits and their positions in the case of β-octalkylporphyrin isomers causes a difference between the solubility values in benzene of no more than two or three fold, apperently, as a consequence of the changes to molecular interactions within the crystals [
1].
As is evident from the solubility values of the etioporphyrin isomers the mutual position of the sub-stituents in the porphyrin macrocycle is one of the most important factors determining β-octalkylporphyrin solubility values. Etioporphyrin isomers without symmetry axis are most soluble [
2].
The solubility values of β-octalkylporphyrins in alcohols - methanol, ethanol, propanol, are far less than in benzene and essentially (by an order) depend on the porphyrin structure.
β-Octamethylporphyrin is an exception of all β-octalkylporphyrins. It is hardly soluble in the majority of organic solvents and has very low solubility values even in benzene.
Ms-alkylsubstitution generally increase the porphyrin solubility values in benzene in several times and in ethanol of approximately by an order [
3]. As a result the 5,15-dialkylsubstitution β-octalkylporphyrin solubility in benzene becomes 10
-2 mol/l. Sharp increase of the solubility values is observed for ms- methylsubstituted porphyrins to be compared with the ms-unsubstituted porphyrins futher increase of porphyrin solubility. Increasing the alkyl length up to C
2H
5 in 5,15-positions raise unevenly the β-octamethylporphyrin solubility making it commensurable with other 5,15-dialkyl-β-octalkylporphyrins.
For studied ms-substituted β-octamethylporphyrins maximum solubility value observed for 5,15-dipropylsubstituted porphyrin in benzene and 5,15-dibutylsubstituted porphyrin in ethanol. It should be noted that the influence of ms-alkyl substitution on the porphyrin solubility was investigated only for 5,15-substituted porphyrins. However, it may be supposed that the greater number of ms-alkyl substituents the more porphyrin solubility values. This supposition corroborates, for example, the fact that 5-hexylsubstitution in 3,7,13,17-tetramethyl-2,8,12,18-tetrabutylporphyrin increase its solubility in propanol in 4,5 times [
4] i.e. less than 5,15-substitution.
The data on solubility of N-alkylsubstituted porphyrins have appeared rather unexpected [
5]. Magni-fication of the porphyrin solubility at the expense alkyl group introducing directly into coordination center of the molecule depends greatly on the porphyrin nature. Because the given work is devoted to octalkylporphyrin solubility, we shall discuss the influence of N-methylsubstitution to β-octaethylporphyrin solubility.
Alkyl group introducing directly into coordination center of β-octaethylporphyrin increases macrocy-cle solubility in ten times. N-methyl-β-octaethylporphyrin is one of the most soluble porphyrins not only among alkylporphyrins but also among the porphyrins with any different structure (tabl.). The solubility of this porphyrin in ethanol is commensurable with the solubility of β-octaethylporphyrin in benzene.
Thus, the data of the Table confirm, that position of alkyl groups in the macrocycle of the porphyrin molecule render large influence on its solubility, allowing to vary the porphyrin solubility values in limits of several orders.
Experimental
The porphyrins (5,15-dimethyl-2,8,12,18-tetrabutyl-3,7,13,17-tetramethylporphyrin, 5,15-diethyl-2,8,12,18-tetrabutyl-3,7,13,17-tetramethylporphyrin, 5,15-dipropyl-2,8,12,18-tetrabutyl-3,7,13,17-tetramethylporphyrin, 5,15-dibutyl-2,8,12,18-tetrabutyl-3,7,13,17-tetramethylporphyrin) were synthe- sized by the method [
6]. The solvents cleared by known methods, the content of water in solvents did not exceed: in benzene - 0.01%, in ethanol - 0.03%. Solubility studied by the method of isothermal saturation with pectrophotometrical monitoring of concentration. The error of the method makes 5%.