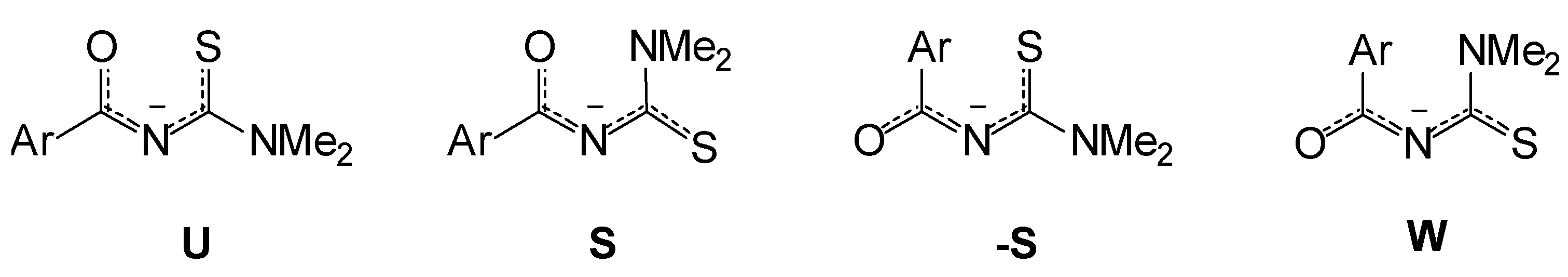
3,3-Dimethylacylthioureas: "S", "-S", "U" or "W" Conformation?
Abstract
:Introduction
Experimental
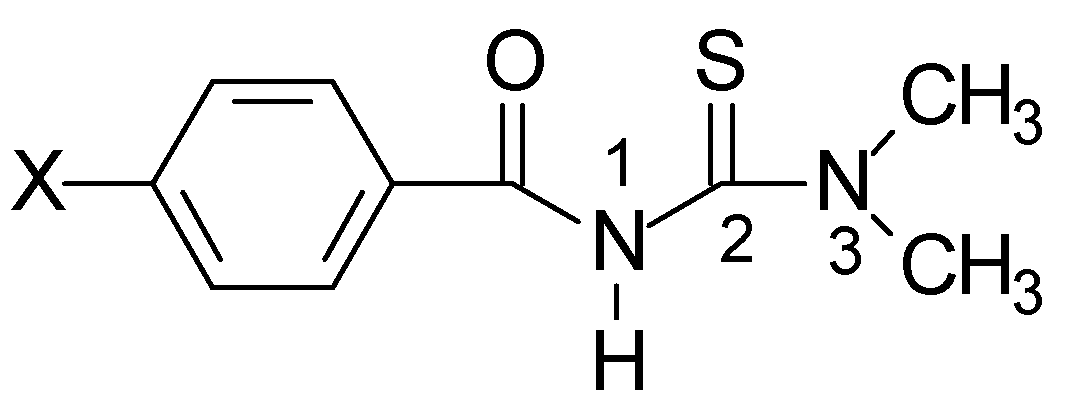
Results and Discussion
References and Notes
- Rodriguez, Y.; Macias, A. Chem. Het. 1987, 508.
- Plutin, A. Master Thesis. Universidad de la Habana, 1997. [Google Scholar]
- Sosa, M. Master Thesis. Universidad de la Habana, 1999. [Google Scholar]
Share and Cite
Sosa, M.; Piris, M.; Burton, G. 3,3-Dimethylacylthioureas: "S", "-S", "U" or "W" Conformation? Molecules 2000, 5, 445-446. https://doi.org/10.3390/50300445
Sosa M, Piris M, Burton G. 3,3-Dimethylacylthioureas: "S", "-S", "U" or "W" Conformation? Molecules. 2000; 5(3):445-446. https://doi.org/10.3390/50300445
Chicago/Turabian StyleSosa, M., M. Piris, and G. Burton. 2000. "3,3-Dimethylacylthioureas: "S", "-S", "U" or "W" Conformation?" Molecules 5, no. 3: 445-446. https://doi.org/10.3390/50300445


