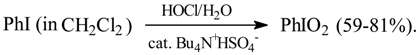Organic Iodine(I, III, and V) Chemistry: 10 Years of Development at the Medical University of Warsaw, Poland
Abstract
:Contents
- Introduction
- Syntheses of Aromatic Iodides
- Syntheses of (Dichloroiodo)arenes
- Syntheses od (Diacetoxyiodo)arenes
- Syntheses of Iodylarenes
- Syntheses of Diaryliodonium Salts
- Anion Metatheses in Diaryliodonium Halides
- Conclusions
1. Introduction
2. Syntheses of Aromatic Iodides
2.1. Our earlier results: aromatic iodides from aromatic organomercurials [22,23,24,25,26,27,28,29,30,31,32,33]
2.2. Aromatic Iodides from Some Highly Activated Aromatics with Lead(IV) Acetate as Oxidant [2]


2.3. Aromatic Iodides from Activated or Deactivated Arenes with Chromium(VI) Oxide as Oxidant [8]



2.4. Aromatic Iodides from Deactivated Arenes with Potassium Permanganate as Oxidant [11]


2.5. Aromatic Iodides from Activated and Deactivated Arenes with Various Brands of Manganese(IV) Oxide as Oxidants [11, 18d]


2.6. Aromatic Iodides from Activated and Deactivated Arenes with Sodium Periodate or Sodium Iodate as Oxidants [17]




2.7. Aromatic Iodides from Activated Aromatics Obtained by an Improved, Acid- catalyzed Iodinating Procedure with (Diacetoxyiodo)benzene as Oxidant [15]



2.8. Aromatic Iodides from Deactivated Aromatics with a Stable Urea•H2O2 Complex as Oxidant [98]

3. Syntheses of (Dichloroiodo)arenes
3.1. Biphasic Chlorination of Iodoarenes to (Dichloroiodo)arenes [12,18a, 64]
CaCl(OCl) + 2HCl (conc. aq.) → ↑Cl2 + CaCl2 + H2O (less effective)
3.2. Oxidative Liquid-Phase Chlorination of Iodoarenes to (Dichloroiodo)arenes [14,18a,b]


3.3. A One-Pot Method for Preparing (Dichloroiodo)arenes from Arenes [18c]

4. Syntheses of (Diacetoxyiodo)arenes
4.1. A Two-Step Conversion of Iodoarenes to (Diacetoxyiodo)arenes with Chromium(VI) Oxide as Oxidant [10]


4.2. (Diacetoxyiodo)arenes from Iodoarenes with Sodium Perborate Monohydrate as Oxidant [71]

4.3. (Diacetoxyiodo)arenes from Iodoarenes with Sodium Periodate as Oxidant [18e]

5. Syntheses of Iodylarenes

5.1. Early Results: Biphasic Oxidation of Iodobenzene to Iodylbenzene [64]
5.2. Iodylarenes from Iodoarenes with Sodium Periodate as Oxidant [18e]

6. Syntheses of Diaryliodonium Salts
6.1. Short-Cut Syntheses of Diaryliodonium Salts with Chromium(VI) Oxide as Oxidant [3, 4, 5, 6b, 9, 13]

6.2. Iodonium/Diiodonium Salts Derived from Various Tricyclic Carbo- and Heteroaromatic Systems [4, 5, 9]
6.3. Improved Syntheses of Some Diaryliodonium Salts from Symmetric Diarylmercurials and (Dichloroiodo)arenes (Willgerodt Method) [13]


6.4. Short-cut Syntheses of Diaryliodonium Bromides with Sodium Perborate as Oxidant [96]
- (1)
- ArI + NaBO3•H2O + 2Ac2O + 2H2SO4 → ArI(OSO3H)2 + [NaBO2] + 4AcOH
- (2)
- ArI(OSO3H)2 (not isolated) + Ar’-H (in excess) → Ar(Ar’)I+HSO4 - + H2SO4
- (3)
- Ar(Ar’)I+HSO4 - (not isolated) + aq. KBr (in excess) → ↓ Ar(Ar’)I+Br- + KHSO4 (Eq. 30)
7. Anion Metatheses in Diaryliodonium Halides
7.1. Oxidative Anion Metatheses in Crude Diaryliodonium Bromides [3]

7.2. Oxidative Anion Metatheses in Crude Diaryliodonium Iodides and Chlorides [6b]



8. Conclusions
References
- A list of our papers and Ph.D. theses on Organic Iodine(I, III and V) Chemistry:
- Krowczynski, A.; Skulski, L. p-Iodo-N-methylacetanilide and its Polyvalent Iodine Derivatives. Polish J. Chem. 1993, 67, 67–70. [Google Scholar]
- Krassowska-Swiebocka, B.; Lulinski, P.; Skulski, L. Aromatic Iodination of Activated Arenes With Lead Tetraacetate as the Oxidant. Synthesis 1995, 926–929. [Google Scholar] [CrossRef]
- Kazmierczak, P.; Skulski, L. A Short-Cut Synthesis of Diaryliodonium Bromides Followed by Oxidative Anion Metatheses. Synthesis 1995, 1027–1032. [Google Scholar] [CrossRef]
- Farhan, A.N.; Skulski, L. 2- and 2,8-Substituted Iodine(III) Derivatives of Dibenzofuran. Polish J. Chem. 1995, 63, 1663–1668. [Google Scholar]
- Farhan, A.N.; Skulski, L. Synthesis of N-acyl-3-(Br, Cl or NO2)-6-(aryliodonio)carbazole Bromides. Polish J. Chem. 1996, 70, 211–216. [Google Scholar]
- Two complementary papers: Farhan, A.N.; Kryska, A.; Skulski, L. Exemplary Oxidative and Evaporative Anion Metatheses in Potassium Halides. Bull. Polish Acad. Sci., Chem. 1996, 44, 201–203. [Google Scholar] Kazmierczak, P.; Skulski, L. Oxidative Anion Metatheses in Diaryliodonium Iodides and Chlorides. Bull. Chem. Soc. Jpn. 1997, 70, 219–224. [Google Scholar] [CrossRef]
- Abdo Nagi Farhan (Yemen), Ph.D. thesis entitled: “Syntheses of novel aromatic iodonium salts substituted with various carbo- and heterocyclic groups” [in Polish]; Fac. of Pharm., Med. Acad., Warsaw 1996, 146 pp. + Addenda, 173 refs. Defended on March 5, 1997.
- Lulinski, P; Skulski, L. The Direct Iodination of Arenes with Chromium(VI) Oxide as the Oxidant. Bull. Chem. Soc. Jpn. 1997, 70, 1665–1669, Erratum. See Ref. 11, p. 118. [Google Scholar]
- Farhan, A.N.; Skulski, L.; Kryska, A. 2- and 2,7-Substituted Iodonium/Diiodonium Salts Derived from Xanthene, Xanth-9-one, Fluorene, and Fluoren-9-one. Polish J. Chem. 1997, 71, 1236–1245. [Google Scholar]
- Kazmierczak, P.; Skulski, L. A Simple, Two-Step Conversion of Various Iodoarenes to (Diacetoxyiodo)arenes with Chromium(VI) Oxide as the Oxidant. Synthesis 1998, 1721–1723. [Google Scholar] [CrossRef]
- Lulinski, P; Skulski, L. Oxidative Iodination of Arenes with Manganese(IV) Oxide or Potassium Permanganate as the Oxidants. Bull. Chem. Soc. Jpn. 1999, 115–120. [Google Scholar]
- Krassowska-Swiebocka, B.; Prokopienko, G.; Skulski, L. Biphasic Chlorination of Iodoarenes to (Dichloroiodo)arenes. Synlett 1999, 1409–1410. [Google Scholar] [CrossRef]
- Skulski, L.; Wroczynski, P. Improved Syntheses of Some Diaryliodonium Salts from Symmetric Diarylmercurials and (Dichloroiodo)arenes (Willgerodt Method). Bull. Polish Acad. Sci., Chem. 1999, 47, 231–238. [Google Scholar]
- Kazmierczak, P.; Skulski, L.; Obeid, N. Oxidative Chlorination of Various Iodoarenes to (Dichloro-iodo)arenes with Chromium(VI) Oxide as the Oxidant. J. Chem. Res., Synop. 1999, 64–65. [Google Scholar] [CrossRef]
- Kryska, A.; Skulski, L. Improved, Acid-catalyzed Iodinating Procedures for Activated Aromatics with (Diacetoxyiodo)benzene as the Oxidant. J. Chem. Res., Synop. 1999, 590–591. [Google Scholar] and (M), 2501-2517. In its full-text version there is a review on the aromatic halogenation reactions with organic trivalent iodine reagents as the oxidants.
- Pawel Kazmierczak, Ph. D. thesis entitled: “New synthetic methods for preparing diaryliodonium salts, (diacetoxyiodo)arenes, and (dichloroiodo)arenes” [in Polish], Fac. of Pharm., Med. Acad., Warsaw 1999., 186 pp. + Addenda, 314 refs. Defended on December 8, 1999.
- Lulinski, P.; Skulski, L. Iodination of Both Deactivated and Activated Arenes with Sodium Periodate or Sodium Iodate as the Oxidants. Bull. Chem. Soc. Jpn. 2000, 73, 951–956. [Google Scholar] [CrossRef]
- Papers in press or in preparation for publication: Baranowski, A.; Plachta, D.; Skulski, L.; Klimaszewska, M. Liquid-Phase and Biphasic Chlorination of Some Iodoarenes to (Dichloroiodo)arenes with Sodium Peroxodisulfate as the Oxidant. J. Chem. Res., Synop. 2000, 435–437. [Google Scholar] [CrossRef] Obeid, N.; Skulski, L. Novel Oxidatixe, Liquid-Phase Chlorination Procedures for the Preparation of (Dichloroiodo)arenes from Iodoarenes. Polish J. Chem. 2000, 74, 1609–1615. [Google Scholar] (c)Lulinski, P.; Obeid, N.; Skulski, L. A One-Pot Method for Preparing (Dichloroiodo)arenes from Arenes. (d)Krassowska-Swiebocka, B.; Lulinski, P.; Skulski, L. Chemical Manganese Dioxide (CMD): Its Application to Oxidative Iodination of Benzene, Halobenzenes and Some Deactivated Arenes. (e)Kazmierczak, P.; Skulski, L.; Kraszkiewicz, L. A Facile Synthesis of (Diacetoxyiodo)arenes and Iodylarenes from Iodoarenes with Sodium Periodate as the Oxidant.
- Reviews on Aromatic Iodination: Roedig, A. Houben-Weyl, 4th ed.; Vol. V/4a, Mueller, E., Ed.; Stuttgart, 1960; pp. 517–678. [Google Scholar] Merkushev, E.B. Synthesis 1988, 923. March, J. Advanced Organic Chemistry, 4th ed.; New York, 1992; pp. 531–534. [Google Scholar] Grimmet, M.R. Adv. Heterocyclic Chem. 1993, 57, 291, ibid., 58, 271; ibid., 59, 245. Sainsburry, M. Halobenzenes. In Rodd’s Chem. Carbon Compounds, 2nd ed.; Sainsburry, M. Amsterdam, 1996; Vol. 3, Part 1; pp. 178–224. [Google Scholar] The Merck Index, 12th ed.; CD-ROM, Merck Co., 1998.
- Nesmeyanov, A.N.; Makarova, L.G. Organic Compounds of Mercury; Amsterdam, 1967. [Google Scholar] Makarova, L.G. Organometallic Reactions; Becker, E.I., Tsutsui, M., Eds.; Vol. 1, New York, 1970; pp. 119–348, Vol. 2, New York 1971, pp. 355-423. [Google Scholar] Mueller, E. (Ed.) Methoden der organischen Chemie (Houben-Weyl); Vol. 13(2b), Stuttgart, 1974.
- Varvoglis, A. The Organic Chemistry of Polycoordinated Iodine; Weinheim, 1992. [Google Scholar] Varvoglis, A. Hypervalent Iodine in Organic Synthesis; San Diego, 1997. [Google Scholar] Chemistry of Hypervalent Compounds; Akiba, K. (Ed.) New York, 1999; Chap. 11 and 12.
- Skulski, L.; Wroczynski, P. Polish J. Chem. 1982, 56, 975.
- Skulski, L.; Wroczynski, P. Khim. Geterotsikl. Soed. 1983, 543.
- Wroczynski, P.; Kujawa, A.; Skulski, L. Khim. Geterotsikl. Soed. 1985, 386.
- Khodeir, N.M.; Skulski, L.; Wroczynski, P. Bull. Polish Acad. Sci., Chem. 1986, 34, 443.
- Khodeir, N.M.; Skulski, L.; Wroczynski, P. Bull. Polish Acad. Sci., Chem. 1987, 35, 9.
- Skulski, L.; Kujawa, A.; Kujawa, T.M. Bull. Polish Acad. Sci., Chem. 1987, 35, 499.
- Skulski, L.; Kujawa, A.; Wroczynski, P. Khim. Geterotsikl. Soed. 1989, 249.
- Kandeel, E.; Skulski, L.; Wroczynski, P. Bull. Polish Acad. Sci., Chem. 1990, 38, 37.
- Niemyjska, M.; Skulski, L.; Zdrojewska, H. Bull. Polish Acad. Sci., Chem. 1990, 38, 41.
- Skulski, L.; Kujawa, A.; Wroczynski, P. Bull. Polish Acad. Sci., Chem. 1991, 39, 23.
- Baranowski, A.; Skulski, L. Bull. Polish Acad. Sci., Chem. 1991, 39, 29.
- Skulski, L.; Baranowski, A.; Lempke, T. Bull. Polish. Acad. Sci., Chem. 1991, 39, 459.
- Serguchev, Yu. A.; Davydowa, V.G.; Makhon’kov, D.I.; Cheprakov, A.V.; Beletskaya, I.P. Zh. Org. Khim. 1985, 21, 2010, J. Org. Chem. USSR 1985, 21, 1841.
- Shimizu, A.; Yamataka, K.; Isoya, T. Bull. Chem. Soc. Jpn. 1985, 58, 1611. [CrossRef]
- Grigor’ev, M.G.; Buketova, I. A.; Polevshchikov, P.F. Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 1987, 30, 22, Chem. Abstr. 1998, 109, 6142.
- Cainelli, G.; Cardillo, G. Chromium Oxidations in Organic Chemistry; Berlin, 1984. [Google Scholar]
- Chaikovskii, V.K.; Novikov, A.N. Zh. Prikl. Khim. 1984, 57, 134, Chem. Abstr. 1984, 100, 191452.
- Chen, J.-A.; . Lin, C.-S.; Liu, L.K. J. Chin. Chem. Soc. (Taiwan) 1996, 43, 95.
- Fieser, L.F.; Fieser, M. Reagents in Organic Synthesis; Vols. 1-18. Oxidation in Organic Chemistry; Wiberg, K.W. (Ed.) New York, 1965. Oxidation. Techniques and Application in Organic Synthesis; Augustine, R.L. New York, 1969. [Google Scholar]
- Galecki, J. Preparatyka Nieorganiczna; Warsaw, 1964; p. 439. [Google Scholar] Karyakin, Yu.V.; Angelov, I.I. Chistye Khimicheskie Reaktivy; Moscow, 1955; p. 333. [Google Scholar]
- Aoyama, T.; Sonoda, N.; Yamaguchi, M.; Toriyama, K.; Anzai, M.; Ando, A.; Schioiri, T. Synlett 1998, 35. see also Refs. 4, 5, 6, 7, 8 therein for their former works in which the CMD (Wako product) was used as the oxidant. Hirano, M.; Yakabe, S.; Hikamori, H.; Clark, J.H.; Morimoto, T. J. Chem. Res. Synop. 1998, (i) Part 1, p. 308; (ii) Part 2, p. 310; (iii) Part 3. 771.
- Fatiadi, A.J. Synthesis 1974, 229. see particularly Table 4 therein, p. 243. Pizey, J.S. Synthetic Reagents; Pizey, J.S. New York, 1981; Vol. 4, pp. 184–187, A brief review on the I2/H5IO6 reagent. [Google Scholar]
- Wirth, H.O.; Koenigstein, O.; Kern, W. Liebigs Ann. Chem. 1960, 634, 84.
- Keys, A.; Johnson, D. R.; Soulen, R. L. CHED Newsletter Fall 1994, Abstr. No.72. In Paper presented at a 208th National Meeting of the American Chemical Society, Washington, D.C., August 21-25, 1994. Brazdil, L.C.; Cutler, C.J. J. Org. Chem. 1996, 61, 9621. Brazdil, L.C.; Fitch, J.L.; Cutler, C.J.; Haynik, D.M.; Ace, E.R. J. Chem. Soc.,Perkin Trans. 2 1998, 933.
- Also polystyrene was previously iodinated with I2/I2O5 reagent, see: Yamada, Y.; Okawara, M. Macromol. Chem. 1972, 152, 153. Togo, H.; Abe, S.; Nogami, G.; Yokoyama, M. Bull. Chem. Soc. Jpn. 1999, 72, 2351 and 2354.
- Suzuki, H. Org. Synth. 1971, 51, 94, or Coll. Vol. VI, p.700.
- Aldrich Catalogue Handbook of Fine Chemicals 1999-2000.
- Chaikovskii, V.K.; Kharlova, T.S.; Filimonov, V.D.; Saryucheva, T.A. Synthesis 1999, 748.
- Togo, H.; Nogami, G.; Yokoyama, M. Synlett 1998, 534.
- McKillop, A.; Kemp, D. Tetrahedron 1989, 45, 3299.
- Ogata, Y.; Aoki, K. J. Am. Chem. Soc. 1968, 90, 6187.
- Niemyjska, M. unpublished results.
- Keefer, R.M.; Andrews, L.J. J. Am. Chem. Soc. 1958, 80, 277.
- Willgerodt, C. Tageblatt der 58. Vers. deutscher Naturforscher u. Aertzte; Strassburg, 1885. [Google Scholar]
- Willgerodt, C. Die organischen Verbindungen mit mehrwertigem Jod. Stuttgart, 1914. [Google Scholar]
- Lucas, H.J.; Kennedy, E.R. Org. Synth.; Coll. Vol. III, New York, 1955; p. 482. [Google Scholar]
- Taylor, R.T.; Stevenson, T.A. Tetrahedron Lett. 1988, 29, 2033.
- Zanka, A.; Takeuchi, H.; Kubota, A. Org. Process Res. Dev. 1998, 2, 270. [CrossRef]
- Toehl, A. Ber. dtsch. chem. Ges. 1893, 26, 2949.
- Karele, B. Ya.; Neiland, O. Ya. Latv. PSR Zinat. Acad. Vestis, Kim. Ser. 1970, 587. Chem. Abstr.1971, 74, 42033.
- Makhon’kov, D.I.; Cheprakov, A.V.; Beletskaya, I.P. Zh. Org. Khim. 1986, 22, 681.
- Koyuncu, D.; McKillop, A.; McLaren, L. J. Chem. Res., Synop. 1990, 21.
- Golinski, J.; Kazmierczak, P.; Skulski, L. communicated at a Meeting of the Polish Chemical Society, Krakow, September 4-7, 1991; Abstracts of Papers, (1). p. 25.
- Nekrasov, B. Textbook of General Chemistry; Moscow, 1969; p. 258. [Google Scholar]
- Morton, J.R.; Wilcox, H.W. Inorg. Synth.; Vol. 4, New York, 1953; p. 48. [Google Scholar]
- Muzart, J. Synthesis 1995, 1325. McKillop, A.; Sanderson, W.R. Tetrahedron 1995, 51, 6145. McKillop, A.; Sanderson, W. R. J. Chem. Soc., Perkin Trans. 1 2000, 471.
- Lu, C.-S.; Hughes, E.W.; Giguere, P.A. J. Am. Chem. Soc. 1941, 53, 1507.
- Stang, P.J.; Zhdankin, V.V. Chem. Rev. 1996, 96, 1123. Kitamura, T.; Fujiwara, Y. Org. Prep. Proced. Int. 1997, 29, 409. Varvoglis, A. Tetrahedron 1997, 53, 1179. Muraki, T.; Togo, H.; Yokoyama, M. Rev. Heteroatom Chem. 1997, 17, 213. Moriarty, R.M.; Prakash, O. Adv. Heterocycl. Chem. 1998, 69, 1. Varvoglis, A.; Spyroudis, S. Synlett. 1998, 221. [Google Scholar] Kirschning, A. J. Prakt. Chem./Chem.-Ztg. 1998, 340, 184. [CrossRef] Ochiai, M. Kikan Kagaku Sosetsu 1998, 34, 181. (in Japanese). Hypervalent Organic Compounds (book in Japanese); Anon.; Tokyo, 1998. Muraki, T.; Togo, H.; Yokoyama, M. J. Org. Chem. 1999, 64, 2883. Wirth, T.; Hirt, V.H. Synthesis 1999, 1271. 2nd Symposium on Iodine Utilization, Chiba, Japan, Oct. 16, 1999. Proceedings (FIU Rep. No. 2); see pp. 5-8 and 116-136..
- Kazmierczak, P.; Skulski, L. communicated at a Meeting of the Polish Chemical Society, Poznan, Sept. 23-26, 1996.
- Kryska, A.; Lechnio, J.; Skulski, L. communicated at an All-Polish Meeting of Medicine Students, Warsaw, April 15-17, 1999.
- Beringer, F.M.; Falk, R.A.; Karniol, M.; Lillien, J.; Masullo, G.; Mausner, M.; Sommer, E. J. Am. Chem. Soc. 1959, 81, 342.
- Hartmann, C.; Meyer, V. Ber. dtsch. chem. Ges. 1894, 27, 426. [CrossRef]
- Sharefkin, J.G.; Saltzman, H. Org. Synth. 1963, 43, 65.
- Banarjee, A.; Banarjee, G.C.; Bhattacharia, S.; Banarjee, S.; Sammadar, H. J. Indian Chem. Soc. 1981, 58, 605.
- Lucas, H.J.; Kennedy, E.R. Org. Synth. 1942, 22, 72.
- Ciustea, G.; Panescu, A. Rev. Chim. (Bucharest) 1969, 20, 216.
- Chaundhari, S.S. Synlett 2000, 278.
- Ireland, R.E.; Liu, L. J. Org. Chem. 1993, 58, 2899.
- Frigerio, M.; Santagostino, M.; Sputore, S. J. Org. Chem. 1999, 64, 4537.
- Meyer, S.D.; Schreiber, S.L. J. Org. Chem. 1994, 59, 7549.
- Bayraktaroglou, T.O.; Gooding, M.A.; Khatib, S.F.; Lee, M.; Kourouma, M.; Landot, R.G. J. Org. Chem. 1993, 58, 1264.
- Olah, G.A. Halonium Ions; New York, 1975. [Google Scholar]
- Koser, G.F. Halonium Ions. In The Chemistry of Functional Groups, Supplement D; Patai, S., Rappoport, Z., Eds.; New York, 1983; Chap. 25. [Google Scholar]
- Sandin, R.B. Chem .Rev. 1943, 32, pp. 249, pp. 266.
- Willgerodt, C. Ber. dtsch. chem. Ges. (a) 1897, 30, 56; (b) 1898, 31, 915.
- Freidlina, R. Kh.; Nesmeyanov, A.N. Compt .Rend. [Doklady] Acad. Sci. U.R.S.S. 1940, 29, 567, Chem. Abstr. 1941, 35, 3614.
- Beringer, F.M.; Lillien, I. J. Am. Chem. Soc. 1960, 82, 725.
- Neiland, O. Ya. Latv. PSR Zinat. Akad. Vestis, Kim. Ser. 1964, pp. 589., pp. 591.
- Beringer, F.M.; Chang, L.L. J. Org. Chem. 1972, 37, 1516.
- Beringer, F.M.; Briedley, A.; Drexler, M.; Gindler, E.M.; Lumpkin, C.C. J. Am. Chem. Soc. 1953, 75, 2708.
- van der Puy, M. J. Fluorine Chem. 1982, 21, 385.
- Kitamura, T.; Matsuyuki, J.; Taniguchi, H. Synthesis 1994, 147.
- Jurd, L. Austral. J. Sci. Res. 1949, 2[A], 595. Seikel, M.K. Org .Synth. 1944, 24, 47. Gaca, J. personal communication.
- Forster, M.O.; Schaeppi, J.H. J. Chem. Soc. 1912, 101, 382.
- Kryska, A.; Skulski, L. communicated at an Annual Meeting of the Polish Chemical Society, Lodz, Sept. 10-15, 2000.
- Lucas, H. J.; Kennedy, E. R.; Formo, M. W. Org. Synth. 1942, 22, 70.
- Kryska, A.; Lulinski, P.; Skulski, L. communicated at an Annual Meeting of the Polish Chemical Society, Lodz, Sept. 10-15, 2000.
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Skulski, L. Organic Iodine(I, III, and V) Chemistry: 10 Years of Development at the Medical University of Warsaw, Poland. Molecules 2000, 5, 1331-1371. https://doi.org/10.3390/51201331
Skulski L. Organic Iodine(I, III, and V) Chemistry: 10 Years of Development at the Medical University of Warsaw, Poland. Molecules. 2000; 5(12):1331-1371. https://doi.org/10.3390/51201331
Chicago/Turabian StyleSkulski, Lech. 2000. "Organic Iodine(I, III, and V) Chemistry: 10 Years of Development at the Medical University of Warsaw, Poland" Molecules 5, no. 12: 1331-1371. https://doi.org/10.3390/51201331