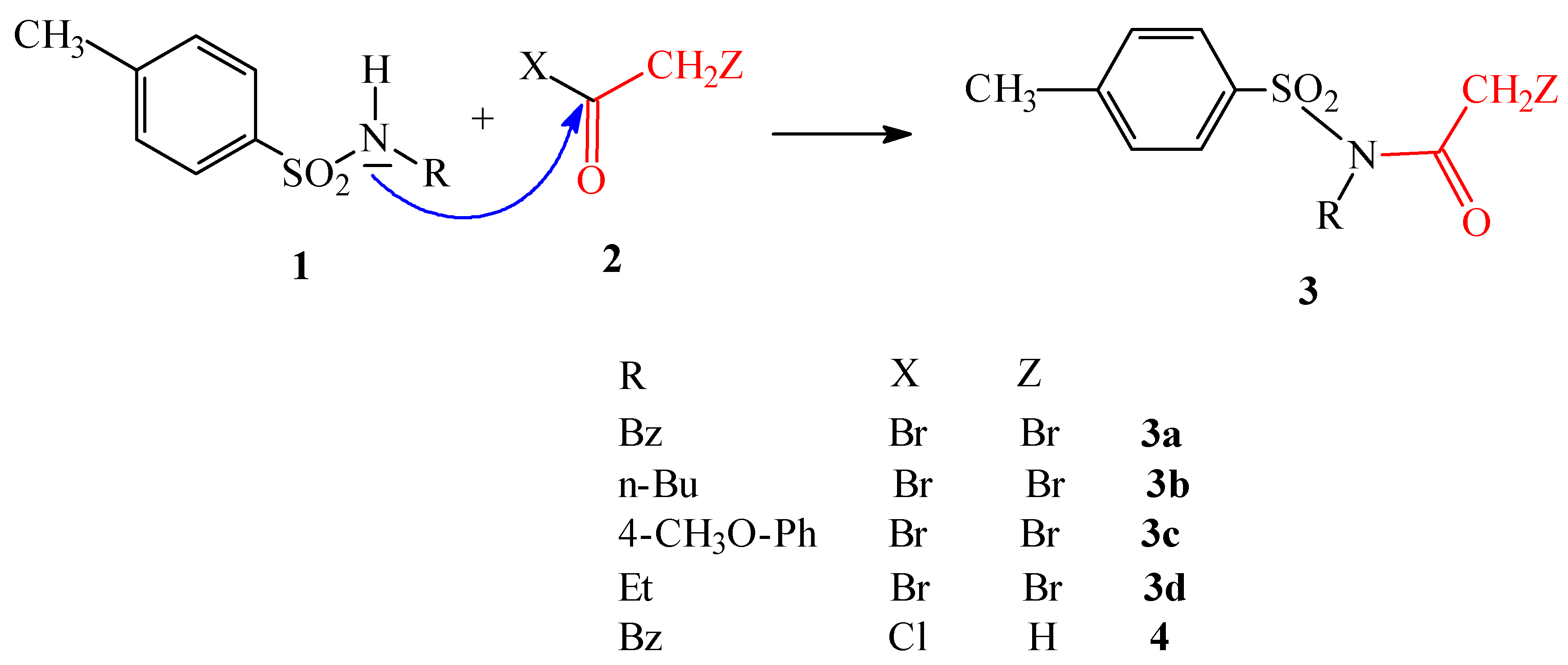
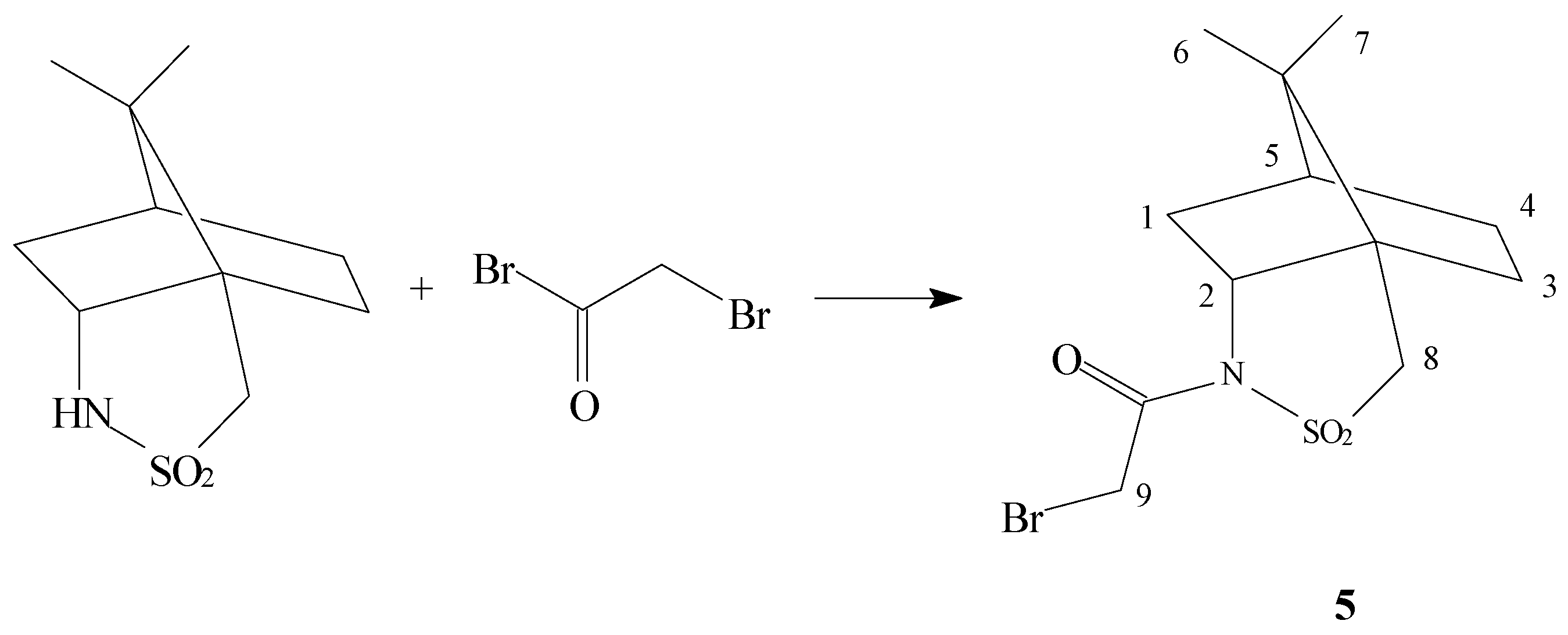
Discussion
With reference to described methods for the acylation of sulfonamides leading to sulfonylacetamides we were able to find papers which we could divide into two basic general groups.
The first approach uses the presence of bases in order to activate the relatively slightly nucleophilic nitrogen. Pyridine [
3,
4,
5] has been used to make mono- and diacetyl derivatives of primary aliphatic and aromatic amides even at very low temperatures
via the formation of a known pyridine - acetyl chloride complex. Additionally, pyridine helps in deprotonation. Sodium hydride [
6,
7,
8] has been used to make the sulfonamidic nitrogen atom more nucleophilic with the system Cu / Cu
2Cl
2 to prevent dimerization of the primary formed product in the case of the introduction of acryloyl chloride onto a sulfonamidic or amidic nitrogen atom. We tried to apply these methods to our system but none of them was successful.
The second approach uses a direct reaction between a N-alkyl sulfonamide and acyl halides [
9] both with and without the presence of glacial acetic acid. This was also found unsuccessfull. The direct reaction between a sulfonamide and acetic anhydride is described as well [
10].
Neither of the two approaches whether proceeding under microwave irradiation [
11], catalysed with a base [
12], or as uncatalysed reactions without solvent on a solid phase [
13] led in our case to the desired results.
Therefore we were forced to develop and introduce a new, more convenient way for their preparation. Our approach was based on the assumption that a Lewis acid such as ZnCl
2 would activate the carbonyl group in the bromoacetyl bromide molecule more than the α-carbon atom substituted with bromine towards nitrogen atom attack by interaction of the Zn atom of ZnCl
2. This is based on the fact that bromine attached to a C
sp3 carbon atom is several orders less reactive than one bound to carbonyl. Our expectation was shown to be correct and the bromoacetylium ion successfully attacked the relatively less nucleophilic sulfonamidic nitrogen to form a N-CO bond. Our prediction had been supported by an observation published in paper [
10] in which the nitrogen atom in the sulfonyl group readily underwent a reaction with acetic anhydride in the presence of anhydrous ZnCl
2 or without it to form acetylated sulfonamides.
Although Oppolzer´s work [
14] gives an example of introducing a chloroacetyl group into Oppolzer´s sultam, his approach requires the application of non-standard conditions (n-BuLi as a base in THF at –78°C) and because of this we decided to try to apply our ZnCl
2 technique.
Experimental
General
Melting points were determined on a Kofler hot-stage Rapido 79-2106 and are uncorrected. IR spectra were recorded on a FTIR ATI Mattson spectrophotometer in KBr pellets. NMR spectra were recorded on a BRUKER 500 (500/125 MHz) instrument in CDCl
3 with TMS as an internal standard (ppm, Hz). Signals in
13C NMR spectra were assigned by means of APT experiments. Signals in
1H NMR spectrum of compound
5 were elucidated using (H,H)-COSY spectrum and ACD/HNMR software. MS spectra were recorded on a FISONS INSTRUMENTS TRIO 1000 spectrometer in positive mode. TLC was carried out on Silufol plates (Kavalier, Czech Republic) in chloroform or in diethylether (compound
5) and detected in UV light (at 254 nm) or in iodine vapours (compound
5). (+)-2,10-bornanesultam and bromoacetyl bromide were products of Fluka. Bromoacetyl bromide was distilled under reduced pressure (34-35°C at 2 mm Hg) prior to use. Benzene or toluene were dried over NaH and distilled from it. Anhydrous ZnCl
2, a product of Lachema (Czech Republic), was fused in a stainless-steel cup, quenched and stored over P
4O
10. Preparation of N-alkyl sulfonamides is given in ref. [
1].
General method to prepare compounds 3a-3c and 4
1 equiv. of sulfonamide 1a-c was dissolved in hot benzene or toluene. To a warm solution 1.3 equiv. of bromoacetyl bromide (acetyl chloride in case of preparation 4) and a catalytic amount of anhydrous ZnCl2 were added. The suspension was heated under reflux, protected against air moisture with calcium chloride, till the speck of 1 disappeared completely. This took a few hours. The cooled brownish suspension was concentrated in vacuo, chloroform and water were added and the phases were separated. The organic phase was washed with water and then with an aqueous solution of Na2CO3. The chloroform phase was dried over anhydrous Na2SO4 and evaporated in vacuo. The crude products 3a-3c were purified by crystallization from ethanol.
N-Benzyl-N-(p-toluenesulfonyl) bromoacetyl bromide (3a)
Yield 58% of a white powder, m.p. 148-149°C. For C15H16NSO3Br calculated: 382.27 g.mol-1. EI-MS, m/z (%):384.0 (M++2, 3), 382.0 (M+, 3), 367.2 (5), 352.2 (7), 262.1 (27), 260.1 (8), 228.1 (23), 226.0 (25), 196.3 (11), 184.0 (15), 181.0 (12), 106.1 (100), 91.0 (84), 77.0 (16), 65.0 (27), 51.0 (12). IR: 2925, 2854, 1718 (ν C=O), 1565, 1545, 1496, 1496,1460, 1356 (ν SO2), 1159 (ν SO2), 1064, 1005, 922, 872, 823, 752, 665, 584, 548). 1H NMR spectrum: 2.41 s, 3H (CH3); 4.17 s, 2H (CH2); 5.05 s, 2H (CH2CO); 7.26-7.31 m, 7H (CH arom.); 7.65-7.66 AB quartet, 2H, J=8.3 Hz (CH arom.). 13C NMR spectrum: 21.53 (CH3); 29.10 (CH2); 50.10 (CH2CO); 127.69; 127.84; 128.63; 129.82 (4xCH arom.); 135.59; 145.34; 145.39 (3xCq arom.), 166.04 (CO).
N-(n-Butyl)-N-(p-toluenesulfonyl) bromoacetamide (3b)
Yield 48% of a snow-like crystalline substance forming long needles, m.p. 66-67°C. For C13H18O3SNBr calculated 348.29 g mol-1. EI-MS, m/z (%): 350 (M++2,1), 348 (M+,1), 184 (30), 178 (56), 176 (56), 155 (95), 152 (7), 150 (7), 139 (10), 122 (17), 91 (100), 78 (4), 65 (32); 57 (68), 42 (11), 41 (11). IR: 2964, 2933, 2866, 1720 (ν C=O), 1707, 1657, 1595, 1447, 1440, 1361 (ν SO2), 1157 (ν SO2). 1H NMR spectrum: 0.89 t, 3H, J1=7.4 (CH3); 1.30 q, 2H, J1=7.4 (CH2), 1.62 m, 2H (CH2); 2.43 s, 3H (CH3 arom.); 3.70 t, 2H, J2=7.7 (CH2); 4.30 s, 2H (CH2); 7.33 and 7.76 AB quartet, 4H, J3=8.2 Hz (CH arom.). 13C NMR spectrum: 13.70 (CH3); 20.12 (CH2); 21.80 (CH3); 29.64 (CH2); 31.40 (CH2); 47.79 (CH2); 127.77; 130,20 (2x CH arom.); 135.97; 145.54 ppm (2x Cq arom.); 165.99 ppm (CO).
N-(4-Methoxyphenyl)-N-(p-toluenesulfonyl) bromoacetamide (3c)
Yield 58% of a snow-like crystalline substance forming plain prismas, m.p. 138-139°C. For C16H16O4SNBr calculated: 398.29 g.mol-1. EI-MS: 400 (M++2, 1), 398 (M+, 1), 280 (8), 278 (21), 277 (75), 245 (4), 244 (22), 162 (12), 149 (52), 134 (23), 123 (27), 122 (100), 106 (18), 95 (19), 91 (43), 78 (17), 65 (25), 63 (10). IR: 2968, 2953, 2884, 1725 (ν C=O), 1709, 1656, 1595, 1462, 1440, 1362 (ν SO2), 1157 (ν SO2). 1H NMR spectrum: 2.66 s, 3H, (CH3); 4.03 s, 3H (OCH3); 5.09 s, 2H (CH2); 7.03 and 7.29 AB quartet, 4H, J1=8.85 (CH arom.); 7.49 and 7.91 (AB quartet, 4H, J2=8.1 (CH arom.). 13C NMR spectrum: 21.53 (CH3); 52.69 (CH2); 55.43 (OCH3); 114.45; 125.24; 127.38; 129.25 (4x CH arom.); 129.11; 136.09; 143.66; 157.88 (4x Cq arom.); 167.23 ppm (C=O).
N-Ethyl-N-(p-toluenesulfonyl) bromoacetamide (3d)
Yield 66% of a crystalline solid, m.p. 88.8-91.0°C. For C11H14BrNO3S calculated 320.20 g.mol-1. EI-MS: 322 (M++2, 19), 320 (M+, 20), 240 (22), 155 (18), 150 (61), 148 (66), 139 (13), 104 (18), 91 (100), 89 (13), 65 (44). IR: 3051; 2983; 2922; 1684 (ν C=O); 1593; 1362 (ν SO2); 1263; 1169 (ν SO2); 1068; 870. 1H NMR spectrum: 1.25 t, 3H, J=6.9 (CH3); 2.45 s, 3H, (CH3Ph-); 3.80 q, 2H, J=6.9 (CH2N); 7.35 and 7.80 AB quartet, 4H, J=8.4 (CH arom.). 13C NMR spectrum: 14.39 (CH3); 21.49 (CH3Ph-); 29.33 (CH2N); 42.88 (CH2Br); 127.50; 129.94 (2xCH arom.); 135.66; 145.28 (2xCq arom.); 165.52 (C=O).
N-Benzyl-N-(p-toluenesulfonyl) acetamide (4)
Yield 61% of a crystalline solid, m.p. 99.5-100.5°C. For C16H17O3NS calculated: 302.37 g.mol-1. EI-MS: 302 (M+,1), (12), 148 (88), 132 (7), 107 (15), 106 (100), 92 (15), 91 (86), 79 (13), 77 (15), 65 (21), 51 (7), 43 (35). IR: 3068; 2964; 2923; 2854; 1693 (ν C=O); 1597; 1496; 1458; 1348 (ν SO2); 1296; 1228; 1118 (ν SO2); 1124.3; 1087.6; 1024; 966; 837; 723; 650; 546. 1H NMR spectrum: 2.28 s, 3H (CH3); 2.40 s, 3H (CH3); 5.08 s, 2H (CH2); 7.25 and 7.61 AB quartet, 4H, J=8.2 (CH arom.); 7.28 - 7.38 m, 5H (CH arom.). 13C NMR spectrum: 21.38; 24.68 (2x CH3); 49.33 (CH2); 127.54; 127.78; 128.40; 129.56 (4x CH arom.); 136.41; 136.54; 144.72 (3x Cq arom.); 170,12 ppm (C=O).
General method to prepare compound 5
(+)-2,10-Bornanesultam (1,0 g, 4.65 mmol) was dissolved at room temperature in dry benzene prepared according to the method above to form a saturated solution. To this solution bromoacetyl chloride (1.22 g, 6.1mmol) and a catalytic amount of anhydrous ZnCl2 were added. The flask was then well sealed and held at room temperature for about 6 hours. Finally, the reaction mixture was refluxed with a condenser for 30 minutes when the colour of the mixture turned to orange. Solvent was removed in vacuo, then chloroform and water were added and the phases were separated. The working up procedure was the same as above. The crude product 5 was purified by crystallization from methanol.
N-Bromoacetyl-(+)-2,10-bornanesultam (5)
Yield 1.0 g (63% ). m.p. 115-117°C. For C12H17NO3SBr calculated 336.269 g.mol-1. EI-MS (m/z, %): 338.8 (M++2,80), 336.8 (M+, 60), 106.2 (7), 105.2 (13), 95.1 (48), 93.1 (100), 79.1 (72), 67.1 (46), 55.0 (22). IR: 2985 (ν CH); 2958 (ν CH); 2908 (ν CH); 1701 (ν CO); 1460; 1412; 1332 (ν SO2); 1207; 1132 (ν SO2); 1062; 1037; 985; 777; 692; 528.4. 1H NMR spectrum: 0.98 s, 3H (CH3); 1.16 s, 3H (CH3); 1.42 m, 2H (H-3); 1.91 m, 2H + 1H (H-1 +H-5); 2.10 m, 2H (H-4); 3.48 and 3.54 AB quartet, 2H, J=13.9 (H-8); 3.91 m, 1H (H-2); 4.21 and 4.33 AB quartet, 2H, J=13.05 (H-9); 13C NMR spectrum: 19.76 CH3; 20.64 CH3; 26.32 CH2; 27.42 CH2; 32.68 CH2; 37.83 CH2Br; 44.46 C-5; 47.77 C(CH3)2; 48.92 Cq; 52.61 C-8; 65.36 C-2; 164.39 C=O.